Abstract
Zinc, copper, and selenium statuses were reported to be linked to the development of chronic diseases, especially coronary heart disease (CHD). Metabolic syndrome, a known CHD risk factor, was found to be highly prevalent in Lebanon. Nevertheless, no data are available on the statuses of plasma zinc, copper, and selenium, especially in terms of their relation to the components of the metabolic syndrome. A sample of 398 men and women aged 18–65 years was drawn from 23 health centers across Lebanon; anthropometric measurements and biochemical analyses of fasting plasma samples were performed. Subjects were found to have normal plasma statuses of copper and selenium but were at elevated risk of zinc deficiency. Plasma selenium levels correlated positively with all the components of the metabolic syndromes, while that of copper correlated only with total, high-density lipoprotein and low-density lipoprotein cholesterol. Plasma zinc did not correlate with any of the metabolic syndrome components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trace elements are essential nutrients for metabolism, growth, immunological, and neurological functions [1]. Copper is essential for many enzymes that catalyze oxidation–reduction reactions, detoxification, transport, production, and formation reactions [2]. Its deficiency may lead to arterial diseases, pigmentation loss, myocardial disease, and neurological effects. Copper’s richest sources are shellfish, organ meats, nuts, seeds, legumes, and grains [3]. The trace element zinc is required for more than 200 enzymes involved in acid–base balance, protein synthesis, reproduction, immunity, vision, and the nervous system [4]. Zinc is found in a wide range of foods especially in red meats, oysters, and shellfish. Its level in plants can be affected by the soil content of zinc, and its bioavailability is related to the phytate content of the food [5]. Regarding selenium, it was only until 1957 that the importance of this mineral in human nutrition was acknowledged [4]. Selenium is found as part of many proteins namely, selenoproteins [6], and was reported to have both antiviral and antioxidant effects [7]. Its deficiency is associated with the development of Keshan disease, a potentially fatal form of cardiomyopathy [8]. Studies have shown that the selenium content of food and its intake by people varies widely depending on the soil content of selenium [9].
Importance has been given to the study of copper, zinc, and selenium since their deficiencies has shown to be associated with the development of chronic diseases [10]. Epidemiological studies have demonstrated a positive association between low zinc and copper levels and increased risk of cardiovascular disease (CVD) [11]. Low urine selenium levels and low serum zinc, selenium, and copper/zinc ratio levels were reported in Saudi Arabian men with atherosclerosis [12]. Recently, copper, zinc, iron, and selenium levels were found to be related to the extent of myocardial damage [13]. Furthermore, a positive correlation between copper level and total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG) levels was reported [14]. However, studies on selenium have been inconclusive [15, 16].
The present study was designed to determine the plasma levels of zinc, copper, and selenium in Lebanon and their association with the components of the metabolic syndrome.
Materials and Methods
Study Design and Sample
This was part of a study related to the relation between diet and noncommunicable diseases. The sample study (n = 499) included 215 men and 284 women aged 18–65 years of age and of low to medium socio-economic status. Subjects were selected from the six districts (Muhafazats) across Lebanon (age and gender distribution of subjects was similar to that of the general population). Data collection, which took place between September 2003 and April 2004, included an interview, blood withdrawal, anthropometric measurements, and blood pressure. Sampling details have been reported previously [17].
Biochemical Analysis
Plasma samples were available for 398 subjects out of the 499, which represent about 80%, and this included 159 men and 239 women.
Fasting plasma samples were analyzed for total cholesterol, LDL-C, high-density lipoprotein cholesterol (HDL-C), TGs, and glucose as described previously [17]. For mineral assay, the entire plasma samples’ preparation was done by the method of microwave oven digestion (Milestone ETHOS PLUS, Sorisole, Italy). Copper and zinc concentrations were measured by atomic absorption spectrophotometry (SOLAAR, Atomic Absorption Spectrophotometer, Thermo Electron, Cambridge, UK). Selenium concentrations were determined using an atomic absorption spectrophotometer GF95 furnace (Model FS95).
Plasma statuses of zinc [5, 18], copper, and selenium [19] were presented according to different criteria.
Statistical Analysis
Data were presented as means and standard deviations (mean ± SD) and percentages. Various statistical testing using the STATA software including paired t testing, two-sample t tests with equal and unequal variances, analysis of variance, Pearson Chi-square testing, and correlation coefficients were performed. Statistical significance was considered at p < 0.05.
Results
Data related to sample characteristics and components of the metabolic syndrome were presented in a previous publication [17] and are summarized in Table 1. The percentage of women in the final sample was 60, and no difference in age was found between both genders. Mean body mass index (BMI) was significantly higher among men, in which 0.6% were underweight, 45.3% overweight, and 28.3% obese. Among women, 3.4% were underweight, 29.4% overweight, and 25.2% obese. The percentage of subjects with high waist circumference was 37.2, and this was similar between both genders.
Many alterations in lipid profile were observed. The percentage of subjects with high total cholesterol levels was 36.9, and this was higher among men compared to women. About one third of subjects were found to have high LDL-C levels, and the incidences among men were higher than that of women. The most pronounced difference between men and women was seen in hypertriglyceridemia, which was 56% in men and 22% in women. About half of the subjects were found to have low levels of HDL-C, and this was similar between both genders. Mean fasting plasma glucose of men was higher than that of women, and the percentage of subjects with elevated plasma glucose level was higher in men (28.3) compared to that of women (13.0). The percentage of subjects with elevated diastolic blood pressure (DBP) was 11.1, and this was similar between genders, but that of systolic blood pressure (SBP) was about 29 and was higher among men as compared to women. Similarly, a higher prevalence of hypertension was found among men (47.8%) compared to women (20.2%).
Table 2 summarizes the statuses of women and men according to the cut off points for each mineral. Mean plasma level of copper was 1.08 mg/L and that of women was significantly (p = 0.0001) higher than that of men. The percentage of subjects with low levels of plasma copper was about 4.5, and this was higher among men (7.5%) compared to women (2.5%), whereas the percentage of those with high levels of plasma copper was about 8.3, and this was higher among women (p = 0.001). It is worth noting that plasma levels of copper and selenium were normally distributed unlike that of zinc.
Plasma zinc concentration was 1.05 mg/L, and this was similar between genders. The percentage of subjects with low plasma zinc levels (using the IZiNCG [18] criteria, less than 0.7 mg/L for women and less than 0.74 mg/L for men) was 27, and this was slightly higher among men. However, 0.5% of subjects were found to have low plasma zinc levels when using a cutoff point of 0.5 mg/L. The percentage of subjects with high plasma zinc levels was about 10, and this was similar among genders.
Fasting plasma selenium concentration was 141.48 µg/L, and that of men was significantly higher (p = 0.0001) than that of women. All subjects were within the normal range; about 1.0% had low levels, and no subject was found to have high levels of selenium.
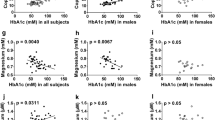
Table 3 shows the correlation between the different parameters and minerals’ concentrations. Plasma copper concentration was positively correlated with age (p = 0.003), as well as with total cholesterol (p = 0.004), HDL-C (p = 0.000), and LDL-C (p = 0.04). However, plasma zinc concentration did not correlate with any of the measured parameters. Plasma selenium concentration correlated positively with age (p = 0.01), waist circumference (p = 0.003), total cholesterol (p = 0.017), LDL-C (p = 0.019), TG (p = 0.001), glucose (p = 0.037), SBP (p = 0.000), and DBP (p = 0.0076). Nevertheless, a negative correlation was observed with HDL-C (0.005). In general, selenium seems to correlate with all the components of the metabolic syndrome. On the other hand, no correlation was observed between the plasma concentrations of the different minerals.
Discussion
To our current knowledge, this is the first study in Lebanon that documents plasma levels of copper, zinc, and selenium of adult population. Mean fasting plasma zinc level was found to be slightly higher than that of Canadians [20], Norwegians [21], and Germans [22]. Mean fasting plasma copper level was similar to that of Brazil, Japan, USA, Slovakia, and Australia [8]. Mean fasting plasma selenium level was slightly lower than that of Canada but was higher than the remaining countries [8].
Our results are in line with others, where plasma level of copper was higher in women [20], selenium higher in men [23, 24], and no gender difference for zinc [20]. Almost no subject was found to have plasma zinc levels below 0.5 mg/L; this may relate to a powerful homeostatic regulation by the body. However, the percentage (27.6%) of subjects with low level of plasma zinc according to the IZiNCG [5] criteria indicates that this population is at an elevated risk of zinc deficiency. The presence of infection is known to reduce plasma level of zinc, but this is not likely to be of major significance in the present study since other minerals (e.g., copper and selenium) are known to be similarly affected but their levels were not reduced. The status of zinc is known to be reduced in vegetarians [5], which are rare in Lebanon. However, a large percentage of energy consumption is derived from plant sources (cereals, legumes, and vegetables) [25], which are known to have a high phytate content. Bioavailability of zinc from the diet is highly dependent on the phytate/zinc molar ratio, where a high ratio is associated with a marked reduction in zinc absorption [5].
In line with our finding, a positive correlation between copper and age was reported by some [20], while others failed to find such a correlation [26]. Moreover, plasma copper levels of overweight and obese children and adolescent men were reported to be higher than that of controls [27], but in our study, the correlation between plasma copper and BMI failed to reach significance. Thus, the relation between plasma copper, age, and BMI is far from clear. In contrast to our finding, plasma copper level correlated negatively with total and LDL cholesterol in one study [27], and copper supplementation was reported to improve lipid profile of rats in another study [28].
Lebanese subjects were found to have a good status of selenium, and this may protect against the development of CVD, although there is controversy over the role of selenium in CVD [6]. Whatever, the protective effect of selenium was reported to be mediated via several mechanisms, including apoB production [29], immune function, arachidonic acid metabolism, and LDL-C oxidation [6]. The contribution of the antioxidant mechanism may not be of significant importance for the Lebanese subjects, who are likely to have a good antioxidant system through their adequate vitamin E status [30] and high consumption of citrus fruits (vitamin C). On the other hand, the relation between serum selenium levels and lipid profile is far from clear, although animal studies showed an improvement in lipid profile with increased selenium intake [31, 32]. In humans, the relationship between selenium and CHD is not clear despite its known role as an antioxidant. Furthermore, subjects with low selenium levels were reported to have an increased risk for ischemic heart diseases and myocardial infarction, and this was believed to be the result of a positive association between serum selenium and HDL-C [15, 33]. However, others failed to find such an association with HDL-C [34, 35] or total cholesterol [34]. A correlation between serum selenium and risk factors for CHD has been reported in adolescents [23], where a positive correlation was found with total cholesterol, HDL-C, non-HDL-C, and DBP. These relationships were reported to be influenced by the menopausal status, in which an association between serum selenium and HDL-C was found in premenopausal but not postmenopausal Japanese women. In postmenopausal women, erythrocyte selenium was negatively associated with total cholesterol and LDL-C [36]. However, in the present study, fasting plasma selenium concentrations correlated with all the components of the metabolic syndrome and several risk factors for CHD. Animal products are well known to be good sources of zinc, copper, and selenium. The failure of zinc and copper to exhibit a correlation with the components of the metabolic syndrome may indicate a minimal contribution of animal products to this process. Cereals can be a major source of selenium dependent on soil content, and their intake is known to contribute to about one third of energy intake in Lebanon [25]. Local productions of cereals contribute only to a small percentage of their total intake, although soil content of selenium in Lebanon was reported to be adequate [37]. The majority of cereals (wheat) are imported [36] from different countries. Increased consumption of cereals (carbohydrate) is known to affect both glucose and TG status and thus affecting other risk factors for CHD. Hence, the positive association between selenium and risk factors of CHD may be the result of increased cereals consumption (carbohydrate). In fact, plasma selenium levels correlated positively with energy and carbohydrate intake, while both zinc and copper correlated negatively (unpublished data).
In conclusion, subjects in our study were found to have a good status of copper and selenium but were at an elevated risk of zinc deficiency. Plasma zinc did not correlate with any of the metabolic syndrome components. Plasma selenium levels correlated positively with all the components of the metabolic syndromes, while that of copper correlated only with total cholesterol, HDL-C, and LDL-C.
References
Castillo-Durán C, Cassorla F (1999) Trace elements in human growth and development. J Pediatr Endocrinol Metab 12(5):589–601
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67S:952S–959S
Turnlund JR (2006) Copper. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ (eds) Modern nutrition in health and disease. 10th edn. Lippincott William & Wilkins, Baltimore, MD
Sauberlich HE (1999) Laboratory tests for the assessment of nutritional status. 2nd edn. CRC, Boca Raton, FL
Hotz C, Brown KH (2004) International Zinc Nutrition Consultative Group (IZinCG) technical document no. 1. Assessment of the risk of Zinc deficiency in populations and options for its control. Food Nutr Bull 25(Suppl 2):S94–S203
Alissa EM, Bahijri SM, Ferns GA (2003) The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med Sci Monit 9(1):RA9–RA18
Rayman MP (2002) The argument for increasing selenium intake. Proc Nutr Soc 61:203–215
Da Cunha S, Albanesi Filho F, Senra Antelo D, Miranda de Souza M (2003) Serum sample levels of selenium and copper in healthy volunteers living in Rio de Janeiro city. Sci Total Environ 301:51–54
Maksimovic ZJ, Djujic I (2003) Selenium research in Serbia, Yugoslavia. J Environ Pathol Toxicol Oncol 17:165–171
Kruse-Jarres JD, Rükgauer M (2000) Trace elements in diabetes mellitus. Peculiarities and clinical validity of determinations in blood cells. J Trace Elem Med Biol 14:21–27
Reunanen A, Knekt E, Marniemi J, Maki J, Maatela J, Aromma A (1996) Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr 50:431–437
Alissa EM, Bahjri SM, Ahmed WA, Al-ama N, Ferns GA (2006) Trace element status in Saudi patients with established atherosclerosis. J Trace Elem Med Biol 20(2):105–114
Altekin E, Çoker C, Rıza A, Şişman B, Önvural F, Kuralay Ö, Kırımlı (2005) The relationship between trace elements and cardiac markers in acute coronary syndromes. J Trace Elem Med Biol 18:235–242
Abiaka C, Olusi S, Al-Awadhi A (2003) Reference ranges of copper and zinc and the prevalence of their deficiencies in an Arab population aged 15–80 years. Biol Trace Elem Res 91(1):33–43
Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P (1982) Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet 2(8291):175–179 Jul 24)
Suadicani P, Hein HO, Gyntelberg F (1992) Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis 96(1):33–42
Sibai AM, Obeid OA, Batal M, Adra N, Dit El Khoury DT, Hwalla N (2008) Prevalence and correlates of metabolic syndrome in an adult Lebanese population. Prev Control (in press)
Gibson R (2005) Principles of nutritional assessment, 2nd edn. Oxford University Press, Oxford
Mahan LK, Escott-Stump S (2004) Food, nutrition and diet therapy, 11th edn. Saunders, Philadelphia, PA
Romero D, Henríquez Sánchez P, López Blanco F, Rodríguez E, Serra Majem L (2002) Serum copper and zinc concentrations in a representative sample of the Canarian population. J Trace Elem Med Biol 16:75–81
Caroli S, Alimonti A, Delle Femime P, Petrucci F, Senofonte O, Violante N, Menditto A, Morisi G, Menotti A, Falconieri E (1995) Role of Inductively coupled plasma atomic emission spectrometry in the assessment of reference values for trace elements in biological matrices. J Anal At Spectrom 7:859–864
Ruckgauer M, Klein J, Kruse-Jarres J-D (1997) Reference values for trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace Elem Med Biol 11:92–98
Spagnolo A, Morisi G, Marano G, Righetti G, Maietta A, Menotti A (1991) Serum selenium and precursors of cardiovascular risk factors in adolescents. Eur J Epidemiol 7(6):654–657
Safaralizadeh R, Kardar GA, Pourpak Z, Moin M, Zare A, Teimourian S (2005) Serum concentration of selenium in healthy individuals living in Tehran. Nutr J 14:4–32
FAO (2005) Food balance sheet, Lebanon 2005. Available at: www.fao.org. Accessed on November 28, 2007
Perrone L, Gialanella G, Moro R, Feng SL, Boccia E, Palombo G, Carbone MT, Di Toro R (1998) Zinc, copper and iron in obese children and adolescents. Nutr Res 18(2):183–189
Lima SC, Arrais RF, Sales CH (2006) Assessment of copper and lipid profile in obese children and adolescents. Biol Trace Elem Res 114(1–3):19–29
Galhardi CM, Diniz YS, Rodrigues HG et al (2005) Beneficial effects of dietary copper supplementation on serum lipids and antioxidant defenses in rats. Ann Nutr Metab 49(5):283–288
Dhingra S, Bansal MP (2005) Hypercholesterolemia and apolipoprotein B expression: regulation by selenium status. Lipids Health Dis 4:28
Obeid OA, Al-Ghali RM, Khogali M, Hwalla N (2006) Vitamins A and E status in an urban Lebanese population: A case study at Dar Al-Fatwa area, Beirut. Int J Vitam Nutr Res 76:3–8
Wojcicki J, Rozewicka L, Barcew-Wiszniewska B (1991) Effect of selenium and vitamin E on the development of experimental atherosclerosis in rabbits. Atherosclerosis 87:9–16
Mazur A, Nassir F, Gueux E, Moundras C, Bellanger J, Grolier P, Rock E, Rayssiguier Y (1996) Diets deficient in selenium and vitamin E affect plasma lipoprotein and apolipoprotein concentrations in the rat. Br J Nutr 76(6):899–907
Salonen JT, Solonen R, Seppanen K et al (1988) Relationship of serum selenium and antioxidants to plasma lipoproteins, platelets aggregability and prevalent of ischaemic heart disease in Eastern Finnish men. Atherosclerosis 70:155–160
Bukkens SG, de Vos N, Kok FJ et al (1990) Selenium status and cardiovascular disease risk factors in healthy Dutch subjects. J Am Coll Nutr 9:128–135
Jossa F, Trevisan M, Krogh V et al (1991) Serum selenium and coronary heart disease risk factors in southern Italian men. Atherosclerosis 87:129–134
Karita K, Yamanouchi Y, Takano T et al (2008) Associations of blood selenium and serum lipid levels in Japanese premenopausal and postmenopausal women. Menopause 15(1):119–124
Sillanpaa M, Jansson H (1992) Status of cadmium, lead, cobalt and selenium in soils and plants of thirty countries. FAO Soils Bulletin. Food and Agriculture Organization of the United Nations, Rome
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obeid, O., Elfakhani, M., Hlais, S. et al. Plasma Copper, Zinc, and Selenium Levels and Correlates with Metabolic Syndrome Components of Lebanese Adults. Biol Trace Elem Res 123, 58–65 (2008). https://doi.org/10.1007/s12011-008-8112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8112-0