Abstract
Breast cancer is a serious malignancy that has higher rate of morbidity and mortality. It has been known to affect the women indifferently. The lack and side effects in the current therapeutic modules result in the search of the wide treatment options including combinatorial treatment. The goal of this study was to investigate combinatorial anti-proliferative efficacy of biochanin A (BCA) and sulforaphane (SFN) against MCF-7 breast cancer cells. The study involves the utilisation of various qualitative techniques including cytotoxicity analysis (MTT), morphogenic analysis, AO/EtBr, DAPI, ROS, cell cycle, and cell migration analysis in order to examine the combinatorial efficacy of BCA and SFN in inducing the cell death. The results had shown that the cytotoxicity of BCA and SFN was found to be around 24.5 µM and 27.2 µM respectively, while the combination of BCA and SFN had shown an inhibitory activity at about 20.1 µM. And furthermore, AO/EtBr and DAPI had shown a profound increase in apoptogenic activity of compounds when treated in combination at lower dose. This apoptogenic activity may be attributed to the increased ROS production. Moreover, it has been shown that the BCA and SFN have been involved in the down-regulation of ERK-1/2 signalling pathway resulting in induction of apoptosis of cancer cells. Thus, our results had concluded that BCA and SFN co-treatment could be used as an efficient therapeutic target against breast cancer. Furthermore, in vivo efficiency by which the co-treatment induces apoptosis has to be deliberated further in near future to make their use commercially.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second largest cause of death. According to GLOBOCAN 2020, the International Agency for Research on Cancer had reported global predictions of cancer incidence and mortality to be around 19.3 million and 10 million in 2020 [1]. Among all cancers, breast cancer is the second most common cause of mortality among women around the world with about 2.1 million new cases and 627,000 deaths in 2018. Among the 2.3 million new cancer cases reported in 2020, breast cancer had overtaken lung cancer as the main cause of global cancer incidence, accounting for 11.7% of all cancer cases [1].
Breast cancer is a physiologically and clinically varied condition with numerous identified histological and molecular subtypes, each of them has its own causation, risk factor profiles, treatment responses and prognoses [2]. It has been known to affect the women indifferently. Exogenous hormone, genetic predisposition and variables altering endogenous hormone levels causing early menarche, later menopause, nulliparity, late age at first birth, having fewer children, shorter lactation periods, high mammographic breast density, anthropometric characteristics and benign breast diseases are all implicated in the development of breast cancer [3]. Also, dysregulation in the signalling pathways involving in the growth of the cells also has a pivotal role in cancer development.
Finding drugs that can modify specific targets has been a major focus of drug research in the cancer field. Despite the development of several novel medications with specific targets, resistance patterns, poor safety profiles and limited treatment efficacy are frequently reported. Owing to these problems, attempts have been undertaken to find combinatorial therapies using new medications and to generate various synergistic effects. This approach to drug design has been supported by successful clinical studies using multicomponent therapies [4].
Among various signalling cascades, mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) 1/2 signalling pathways have a significant role in delivering the extracellular signals to intracellular destinations. These extracellular signals are transduced by ERK/MAPK signalling cascade influencing the production of transcription factors that promote cytoskeleton deformation and increase tumour growth and metastasis [5]. Due to the pivotal role of ERK-1/2 signalling pathway, ERK disorders may result in cell injury and immoderate stimulation of upstream proteins and kinases which persuade various ailments [6]. Affliction in Ras-ERK-1/2 cascade is a key stimulus for the onset of many cancers. Thus, impeding the expression of this pathway can reduce tumour cell growth, migration and apoptosis [7].
Sulforaphane (4-methylsufinyl-3-butenyl isothiocyanate; SFN) is an isothiocyanate found especially in the seeds of Raphanus sativus [8]. It has been used to treat a wide range of human malignant tumours for a long time. ITCs proved to have a great deal of potential as anti-cancer agents in recent pharmacological studies against HCT-116, LoVo and HT-29 colon cancer cells in a dose-dependent manner [9]. SFN has been linked to a number of different anti-cancer effects, including the activation of apoptosis, arrest of cell cycle and prevention of angiogenesis and metastasis [10, 11]. Several studies have found that combining SFN with a traditional chemotherapy medication (such as cisplatin or doxorubicin) increases apoptosis.
Also, biochanin A (C16H1205), an isoflavone found in Trifolium pratense, is a distinctive anti-cancer drug target cancer cells while also disrupting various signalling pathways. Research works have shown that biochanin A can encourage the excretion of terminal cholesterol products and assist in lowering blood sugar and fat, blood pressure and liver tissue lipid levels [12]. It also possesses antiviral, antioxidant, anti-carcinogenic, anti-inflammatory and endothelial integrity and function protecting properties [13]. BCA shows potential as a phytochemical that suppresses breast cancer by stimulating the proliferation of oestrogen receptor-positive cells.
With all the above said reports, the present work focuses on examining the anti-proliferative efficacy of sulforaphane, biochanin A and their co-treatment in breast cancer cells (MCF-7) through various qualitative and quantitative analyses. The work also involves determining the molecular mechanistic approach behind the combinatorial efficacy of sulforaphane and biochanin A in apoptotic induction.
Materials and Methods
Maintenance of Cells
Breast cancer cells (MCF-7) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with L-glutamine and balanced salt solution (BSS) adjusted to contain glucose, Na2CO3, sodium pyruvate, non-essential amino acids, l-glutamine, HEPES and foetal bovine serum (GIBCO, USA). Penicillin and streptomycin (100 IU/100 µg) were also provided. The cells were maintained at 5% CO2 in a humidified CO2 atmosphere at 37 °C.
Cytotoxicity Assay
Cell viability was determined by using MTT assay after treatment with various concentrations of SFN and BCA along with selected concentrations of the combined drugs. MCF-7 and MCF-10A cells were trypsinised, seeded in a 96-well plate (1 × 104 cells/well) and allowed to adhere overnight. They were then treated with designated compounds over 24 h. Then, 10 µL of MTT was added to each well, and the plates were incubated for another 4 h at 37 °C. After removal of MTT containing medium, the formazan crystals formed were dissolved in 100 µL DMSO which was added to each well and the plates were agitated at 37 °C for 10 min before the absorbance of the coloured solution was measured at 550 nm using a microplate reader. The cell viability was then calculated.
Drug Interaction Analysis
Using CompuSyn software, the effectiveness of combinatorial treatments and their interactions was assessed (version 1.0). Combination index (CI) values below 1 suggest synergism, over 1 imply additive action and more than 1 show antagonistic behaviour between drugs.
Morphological Analysis
Morphological analysis was carried out to analyse the anti-apoptotic effects of the selected drug combination of BCA and SFN against MCF-7 cells. Cells were seeded in the 6-well culture plates and incubated for 24 h. After incubation, the cells were observed for alteration in their morphological changes with trinocular inverted phase contrast microscope (Lawrence & Mayo).
Fluorescence Microscopic Analysis
AO/EB staining was carried out to detect morphological evidences of apoptosis. The cells were treated with selected drug combination of BCA and SFN for 24 h. The cells were washed with PBS and 10 µL of acridine orange/ethidium bromide solution was added and incubated for 5 min. Then, the cells were washed with PBS and examined under fluorescence microscope with an excitation filter of 480 nm [14].
Nuclear Fragmentation Analysis
The MCF-7 cells were allowed to grow on a glass coverslip in a six-well plate and treated with selected drug combination of BCA and SFN for 24 h. The treated cells were fixed using methanol:acetic acid (3:1, v/v) and then washed with PBS. They were then subsequently stained using 10 µL DAPI (4′,6- diamidino-2-phenylindole) and kept in dark for 20 min followed by observation under a fluorescence microscope [15].
ROS Assay
MCF-7 cells (1 × 105/mL) were seeded to grow in a 6-well plate for 24 h. Following this, the cells were incubated with selected drug combination of BCA and SFN for 6 h. Then, the cells were subjected to 10 µM of DCFH-DA (2,7-dichlorofluorescein diacetate, HiMedia) staining at 37 °C for 20 min in order to measure the production of ROS in the BCA and SFN co-treated cells. Then, the collected cells were washed with PBS and were monitored under a fluorescent microscope [16].
Cell Cycle Analysis Assay
MCF-7 cells (1 × 105/mL) were grown in a 6-well plate for 24 h at 37 °C (5% CO2). Then, the medium was with selected drug combination of BCA and SFN for 24 h. Subsequently, the cells underwent 24 h of incubation and were collected by utilising trypsin followed by fixing in 70% ethanol and were stored at 20 °C for 1 h. The cells were suspended in 0.5 mL PBS supplemented with 50 mg mL−1 PI and 100 mg mL−1 RNase and were incubated for 30 min. Then, using a BD FACS flow cytometer, flow cytometry was done in duplicate, and from each sample, 10,000 events were taken and fluorescence signal intensity was documented and analysed by CellQuest and ModiFit.
Cell Migration Assay
A cluster suspension of MCF-7 cells was seeded in six-well plate containing medium. After 24 h of seeding, the inserts were removed and scratches with images were taken using a phase contrast inverted microscope. Furthermore, the A-549 cells were cultured for about 8 and 24 h. Cell migration in the MCF-7 cells was assessed by determining the cell-free surface at the commencement of the experiment (T0) and the surface concealed by the cells at the completion of the experiment with the help of the ImageJ software. The results are stated in each condition as wound closure percentage.
Protein Expression Analysis
In order to determine how the BCA and SFN combination affected the apoptotic and anti-apoptotic proteins in the treated and untreated cells, western blotting was used. The chosen medication combinations of BCA and SFN were used to treat the MCF-7 cells for 24 h in a 100-mm culture dish. The media was then removed, and the cells were repeatedly washed with PBS. The cells were then treated with lysis buffer for over 20 min. After that, the supernatant was centrifuged and used as protein extracts from the cells. On a 12% SDS-polyacrylamide gel electrophoresis, the exact same amount of protein was run from each concentration of the sample. The proteins were successively transferred to a nitrocellulose membrane, blocked for 1 h with 10% skim milk in water and then released. Primary antibodies against ERK-1/2, p-ERK-1/2 and β-actin were added at a v/v ratio of 1:1000 after washing with PBS containing 0.1% Tween-20. The primary antibodies were removed by washing following an overnight incubation at 4 °C, and the secondary antibodies were added. After 1 h of incubation at room temperature, the protein bands could be seen [16].
Statistical Analysis
All the experiments were done in triplicate. The data were analysed by SPSS software. The outcomes were recorded as mean ± SD. Statistical examination was achieved using GraphPad 5.0 (GraphPad Software, Inc.). A value of P < 0.05 was considered statistically significant.
Results
MTT Assay
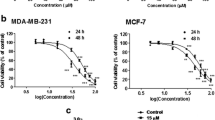
The goal of the study was to identify how well the BCA and SFN work together in breast cancer cells (MCF-7) and normal cells (MCF-10A). It was assessed by means of MTT analysis. The effects of BCA and SFN in breast cancer cells were examined in monotherapy as well as in combination. Compared to individual treatments and the control, we noticed a dose-dependent reduction in the cellular viability of treated cancer cells, which was also found to be more significant with the administration of combinations of drugs together. Table 1 and Fig. 1 show the viability of cells at the selected and treated dose, while the normal cells had not shown any significant changes in the viability of the compound-treated cells (Fig. 1).
Drug Interaction Analysis
In order to know about the drug interaction between the designated drugs (BCA and SFN), interaction analysis was done with the CompuSyn software. With the obtained results, the drug combination therapy (BCA and SFN) had shown synergistic effects which is responsible for the reducing the cell proliferation in MCF-7 cancer cells (Table 2).
Morphological Analysis
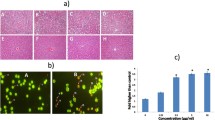
The morphological analysis of the MCF-7 cells was assessed with phase contrast microscope, and the efficiency of administration of compounds at different concentrations has been analysed. It has been seen from Fig. 2a to d that the control cells are found to be normal in their morphological appearance while treatment groups had undergone significant change in their morphology showing alteration including cell shrinkage, blebbing and detachment of cells from the surface. These changes were found to be more significant in the treatment groups with higher concentration, i.e. in a dose-dependent manner of combinatorial treatment. Thus, it has been evinced that BCA and SFN in combination had ameliorated the breast cancer with its anti-cancer activity in a dose-dependent manner.
AO/EtBr Staining
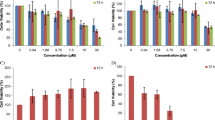
The alterations between viable and apoptotic cells can be detected by fluorescence microscopy following AO/EtBr and DAPI staining. AO/EtBr has been used to detect apoptotic bodies which have been occurred during the process of apoptosis. The co-treatment of MCF-7 cells with BCA and SFN had significantly increased the cellular death in a dose-dependent manner in comparison to control cells as shown in Fig. 3. The active live cells emit green fluorescence; pro-apoptotic and late apoptotic cells show yellowish orange fluorescence on treatment; and dead cells emit red colour. Thus, it has been displayed that the cell death was induced by BCA and SFN co-treated cells in a dose-dependent manner.
DAPI Staining
To observe the nuclear morphological changes following exposure to BCA and SFN, the cells were stained with DAPI. The result obtained from Fig. 4 depicts the nuclear fragmentation and condensation effect occurred due to the co-treatment of BCA and SFN. With an excitation wavelength of 488 nm, DAPI staining had shown reduced intensity in the control cells while the co-treatment cells have shown increased intensity signifying the effect of BCA and SFN in DNA fragmentation with dose-dependent manner. Thus, it has been proved that with the combined treatment of BCA and SFN, these effects have been concurrently increased as depicted by Fig. 4.
ROS Assay
In order to determine the ROS production in BCA and SFN co-treated MCF-7 cells, the cells were subjected to DCFH-DA staining as shown in Fig. 5. The BCA and SFN co-treatment had shown a profuse increase in ROS production in a dose-dependent way in the treated MCF-7 cells. This result indicates the apoptogenic activity of both BCA and SFN synergistically, activating mitochondrial dysfunction in the treated cells in a concentration-dependent manner.
Analysis of Cell Cycle
To explore the efficacy of compounds BCA and SFN in inhibition of cell cycle, this analysis was done. The treated cells were subjected to cell cycle analysis and the results are shown in Fig. 6. In the untreated control, the cells were distributed in the S and G1 phase of the cell cycle. Consequently, following the co-treatment BCA and SFN at different concentrations, the percentage of cells in the G0/G1 phase had underwent a significant reduction in the cell cycle at higher concentration (58.32%). These results depict the interference of the BCA and SFN in S-phase of the cell cycle which may lead to the apoptosis of cancer cells.
Cell Migration Assay
The migration and invasion abilities of MCF-7 cells on co-administration with BCA and SFN were measured with transwell migration assay. The transwell chambers are coated with Matrigel to detect cell invasion. It was found that the number of invading cells in the co-treatment group was significantly decreased compared to that in the control as depicted in Fig. 7. These results highlight the significant inhibition ability of BCA and SFN in co-treatment.
Protein Expression Analysis
In order to better understand the mechanisms underlying our above findings, we investigated the changes in expression of an important protein ERK-1/2 on the combinatorial treatment of SFN and BCA and the results has been depicted in Fig. 8. Our findings revealed a considerable dose-dependent down-regulation of ERK-1/2 protein in the combinatorial drug treated groups. Thus, the combined treatment of BCA and SFN on the MCF- 7 cells have synergistically inhibit the cell proliferation and promote apoptosis by inhibiting or deregulating the ERK-1/2 signalling pathway and therefore, this novel combination could be a promising potential therapeutic approach for breast cancer treatment.
Discussion
Breast cancer is an aggressive cancer that has a greater relapse incidence than other cancers in women and is resistant to chemotherapeutics. The development of breast cancer is a multi-step process involving multiple cell types, and its prevention remains challenging in the world [17]. In order to establish a viable treatment efficacy against breast cancer, many researchers are trying to comprehend its metabolic and molecular environment. One among the therapeutic approach is the use of phyto-based bioactive molecules with higher efficiency.
The isothiocyanate SFN has been identified as an effective chemotherapeutic agent and for treating breast cancer. Various studies demonstrated the molecular mechanisms induced by the SFN (isothiocyanate) in inducing apoptosis and inhibiting proliferation and migration in breast cancer [18]. In numerous tumours, including cervical cancer, epidermal squamous cell carcinoma, prostate cancer and pancreatic cancer, SFN coupled with cisplatin enhanced apoptosis and reduced cell proliferation. Also, it is an effective anti-cancer drug for the treatment of CRC in people based on these findings [19, 20]. The primary isoflavone found in red clover is biochanin A (Trifolium pratense). According to reports, it possesses anti-inflammatory, anti-carcinogenic and antioxidant benefits that are all good for your health. Moreover, it has been claimed to have tumour-suppressive properties in cases of gastrointestinal, prostate and breast malignancies [21].
The above findings were found to be correlated with our results when given as monotherapy. Our results also depict that though both BCA and SFN are known to have anti-carcinogenic properties individually [18, 21], their efficacy is slightly higher with lower concentration when given in a combination treatment. In the present study, it was demonstrated that the co-treatment with BCA and SFN synergistically enhanced the induction of apoptosis and inhibition of proliferation in MCF-7 cells in vitro in a lower concentration when compared to monotherapy.
Further fluorescent dyes like AO/EtBr and DAPI are also used to determine the manner of cellular death and cell morphological alterations produced by combined treatment of BCA and SFN. Apoptosis is essential for regulating equilibrium. Previous reports had highlighted the efficacy of epigallocatechin gallate and sulforaphane in inhibiting the ovarian cancer cells, while withaferin A and sulforaphane inhibit the breast cancer cells in a significant way [22]. Similarly, the epigallocatechin gallate and sulforaphane had found to inhibit the ovarian cancer cell growth by inhibiting the G2/M phase of the cell cycle by combinatorial approach [22]. Also, biochanin A and ginsenoside had synergistically reduced the growth of MDA-MB-231 and MCF-7 breast cancer cells [23].
Also, reactive oxygen species (ROS) plays an important role in tissue homeostasis, cellular signalling, differentiation and survival [24]. The results obtained from ROS assay showed that the MCF-7 cells co-treated with BCA and SFN at different concentration cells are known to produce increased number of ROS when compared to control group. The SFN and BCA are known to prevent cell migration which is the major threat for the spread of cancer to different parts of the body [25]. It has been reported that the biochanin A and ginsenoside had synergistically reduced the invasive ability of MDA-MB-231 and MCF-7 breast cancer cells in vitro, thus reducing its migration ability [23].
Among all signalling pathway, ERK signalling cascade plays an important role in various physiological functions in cells including survival, proliferation, differentiation, apoptosis and metabolism. ERK/MAPK signalling pathway is the core of signalling network involved in regulating cell growth, development and division [26]. Deregulation of the Ras/Raf/MEK/ERK cascade is a hallmark for driving tumorigenesis in many types of human cancers. Elevated ERK expression has been detected in various human tumours, such as ovarian, colon, breast and lung cancer. ERK/MAPK signalling pathway can promote the transformation of normal cells into tumour cells. Therefore, increased activation of ERK/MAPK signalling pathway may be closely related to the occurrence and development of tumours [27]. In previous studies, SFN and BCA are known to induce apoptosis by inhibiting ERK-1/2 pathway [28, 29].
Additionally, the combinatorial treatment had induced the down-regulation of expression of this pathway, which had inhibited the proliferation of cells significantly when compared to the monotherapy treatment [30]. Previous studies revealed that SFN and BCA are known to inhibit the ERK-1/2 pathway by reducing the H-Ras, p-Raf, p-MEK and p-ERK protein levels in the ERK cascade [28, 29]. In addition, BCA and SFN could block the Ras/Raf/MEK/ERK signalling pathways in human breast cancer cells [31]. Our results were found to be correlated with the above said findings, and further in vivo studies have to be done in order to explore them further.
Conclusion
In summary, this study had discussed the function of combined BCA and SFN in the suppression of cancer at lower concentrations. Our findings showed that in terms of reducing cell proliferation, promoting apoptosis and causing cell cycle arrest, the combination treatment (SFN and BCA) had the greatest impact, followed by compounds that were given singly and the control. Additionally, the down-regulation of important cell proliferation players like ERK-1/2 was enhanced by the combination treatments. Furthermore, future research should focus on understanding additional anti-cancer mechanisms in the control of metastasis and cancer stemness as well as the consumption of these combined phytodrugs for breast cancer patients.
Data Availability
Not applicable
References
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Nelson, H. D., Zakher, B., Cantor, A., Fu, R., Griffin, J., O’Meara, E. S., & Miglioretti, D. L. (2012). Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis a systematic review and meta-analysis. Annals of Internal Medicine, 156(9), 635–648. https://doi.org/10.7326/0003-4819-156-9-201205010-00006
Winters, S., Martin, C., Murphy, D., & Shokar, N. K. (2017). Breast cancer epidemiology, prevention, and screening. Progress in Molecular Biology and Translational Science, 151, 1–32. https://doi.org/10.1016/bs.pmbts.2017.07.002
Zhou, Q. M., Chen, Q. L., Du, J., Wang, X. F., Lu, Y. Y., Zhang, H., & Su, S. B. (2014). Synergistic effect of combinatorial treatment with curcumin and mitomycin C on the induction of apoptosis of breast cancer cells: A cDNA microarray analysis. International Journal of Molecular Sciences, 15(9), 16284–16301. https://doi.org/10.3390/ijms150916284
Braicu, C., Buse, M., Busuioc, C., Drula, R., Gulei, D., Raduly, L., … Berindan-Neagoe, I. (2019, October 1). A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers. Multidisciplinary Digital Publishing Institute (MDPI). https://doi.org/10.3390/cancers11101618
Khotskaya, Y. B., Holla, V. R., Farago, A. F., Shaw, M., Meric-Bernstam, K. R., F., & Hong, D. S. (2017, May 1). Targeting TRK family proteins in cancer. Pharmacology and Therapeutics. https://doi.org/10.1016/j.pharmthera.2017.02.006
Shah, S., Brock, E. J., Ji, K., & Mattingly, R. R. (2019, February 1). Ras and Rap1: A tale of two GTPases. Seminars in Cancer Biology. https://doi.org/10.1016/j.semcancer.2018.03.005
Beevi, S. S., Mangamoori, L. N., Subathra, M., & Edula, J. R. (2010). Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Foods for Human Nutrition, 65(3), 200–209. https://doi.org/10.1007/s11130-010-0178-0
Tian, G., Li, Y., Cheng, L., Yuan, Q., Tang, P., Kuang, P., & Hu, J. (2016). The mechanism of sulforaphene degradation to different water contents. Food Chemistry, 194, 1022–1027. https://doi.org/10.1016/j.foodchem.2015.08.107
Geng, Y., Zhou, Y., Wu, S., Hu, Y., Lin, K., Wang, Y., … Wu, W. (2017). Sulforaphane induced apoptosis via promotion of mitochondrial fusion and ERK1/2-mediated 26s proteasome degradation of novel pro-survival bim and upregulation of bax in human non-small cell lung cancer cells. Journal of Cancer, 8(13), 2456–2470. https://doi.org/10.7150/jca.19383
Liu, P., Atkinson, S. J., Akbareian, S. E., Zhou, Z., Munsterberg, A., Robinson, S. D., & Bao, Y. (2017). Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1α/VEGF signalling. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-12855-w
Li, Y., Yu, H., Han, F., Wang, M., Luo, Y., & Guo, X. (2018). Biochanin A induces S phase arrest and apoptosis in lung cancer cells. BioMed Research International, 2018. https://doi.org/10.1155/2018/3545376
Wu, Q., Wang, M., & Simon, J. E. (2003). Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. Journal of Chromatography A, 1016(2), 195–209. https://doi.org/10.1016/j.chroma.2003.08.001
Saranya, T., Kavithaa, K., Paulpandi, M., Ramya, S., Preethi, S., Balachandar, V., & Narayanasamy, A. (2020). Enhanced apoptogenesis and oncogene regulatory mechanism of troxerutin in triple negative breast cancer cells. Toxicology Research, 9(3), 230–238. https://doi.org/10.1093/TOXRES/TFAA029
Ramya, S., Paulpandi, M., Kavithaa, K., Saranya, T., Winster, H., Balachandar, V., & Narayanasamy, A. (2021). Fabatin-loaded silica nanoparticle-induced apoptosisviamitochondrial dysfunction: Targeting the PI3K/AKT molecular pathway as a therapeutic implication against triple negative breast cancer. New Journal of Chemistry, 45(38), 17847–17861. https://doi.org/10.1039/d1nj02922c
Kavithaa, K., Paulpandi, M., Ramya, S., Ramesh, M., Balachandar, V., Ramasamy, K., & Narayanasamy, A. (2021). Sitosterol-fabricated chitosan nanocomplex induces apoptotic cell death through mitochondrial dysfunction in lung cancer animal model: An enhanced synergetic drug delivery system for lung cancer therapy. New Journal of Chemistry, 45(20), 9251–9263. https://doi.org/10.1039/d1nj00913c
Sun, Y. S., Zhao, Z., Yang, Z. N., Xu, F., Lu, H. J., Zhu, Z. Y., … Zhu, H. P. (2017). Risk factors and preventions of breast cancer.International Journal of Biological Sciences. Ivyspring International Publisher. https://doi.org/10.7150/ijbs.21635
Pawlik, A., Wiczk, A., Kaczyńska, A., Antosiewicz, J., & Herman-Antosiewicz, A. (2013). Sulforaphane inhibits growth of phenotypically different breast cancer cells. European Journal of Nutrition, 52(8), 1949–1958. https://doi.org/10.1007/s00394-013-0499-5
Kerr, C., Adhikary, G., Grun, D., George, N., & Eckert, R. L. (2018). Combination cisplatin and sulforaphane treatment reduces proliferation, invasion, and tumor formation in epidermal squamous cell carcinoma. Molecular Carcinogenesis, 57(1), 3–11. https://doi.org/10.1002/mc.22714
Mi, L., Hood, B. L., Stewart, N. A., Xiao, Z., Govind, S., Wang, X., … Chung, F. L. (2011). Identification of potential protein targets of isothiocyanates by proteomics. Chemical Research in Toxicology, 24(10), 1735–1743. https://doi.org/10.1021/tx2002806
Moon, Y. J., Shin, B. S., An, G., & Morris, M. E. (2008). Biochanin A inhibits breast cancer tumor growth in a murine xenograft model. Pharmaceutical Research, 25(9), 2158–2163. https://doi.org/10.1007/s11095-008-9583-6
Sharma, M., & Tollefsbol, T. O. (2022). Combinatorial epigenetic mechanisms of sulforaphane, genistein and sodium butyrate in breast cancer inhibition. Experimental Cell Research, 416(1), 113160. https://doi.org/10.1016/j.yexcr.2022.113160
Ren, G., Shi, Z., Teng, C., & Yao, Y. (2018). Antiproliferative activity of combined biochanin A and ginsenoside Rh2 on MDA-MB-231 and MCF-7 human breast cancer cells. Molecules, 23(11). https://doi.org/10.3390/molecules23112908
Harris, I. S., & DeNicola, G. M. (2020, June 1). The complex interplay between antioxidants and ROS in cancer. Trends in Cell Biology. https://doi.org/10.1016/j.tcb.2020.03.002
Liu, F., Lv, R. Bin, Liu, Y., Hao, Q., Liu, S. J., Zheng, Y. Y., … Wang, M. (2020). Salinomycin and sulforaphane exerted synergistic antiproliferative and proapoptotic effects on colorectal cancer cells by inhibiting the pi3k/ akt signaling pathway in vitro and in vivo. OncoTargets and Therapy, 13, 4957–4969. https://doi.org/10.2147/OTT.S246706
Guo, Y., Pan, W., Liu, S., Shen, Z., Xu, Y., & Hu, L. (2020). ERK/MAPK signalling pathway and tumorigenesis (review). Experimental and Therapeutic Medicine, 19(3), 1997–2007. https://doi.org/10.3892/etm.2020.8454
Meloche, S., & Pouysségur, J. (2007, May 14). The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. https://doi.org/10.1038/sj.onc.1210414
Zhang, Y., Lu, Q., Li, N., Xu, M., Miyamoto, T., & Liu, J. (2022). Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. NPJ Breast Cancer, 8(1), 1–14. https://doi.org/10.1038/s41523-022-00402-4
Lai, X., Li, Y., & Gao, M. (2018). Biochanin A regulates the growth and migration of NSCLC through suppressing the VEGF/VEGFR2 signaling pathway. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. https://doi.org/10.3727/096504018x15321979274728
Lefloch, R., Pouysségur, J., & Lenormand, P. (2009, March 1). Total ERK1/2 activity regulates cell proliferation. Cell Cycle Cell Cycle. https://doi.org/10.4161/cc.8.5.7734
Dong, Q., Yang, B., Han, J. G., Zhang, M. M., Liu, W., Zhang, X., … Duan, S. F. (2019). A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Letters, 455, 60–72. https://doi.org/10.1016/j.canlet.2019.04.031
Author information
Authors and Affiliations
Contributions
Jutao Li and Junqin Xu have been involved in manuscript preparation and working. Yuxin Sun has been involved in working the scientific part of the manuscript. Ruolan Fu has been involved in manuscript preparation and working. Dan Ye has framed the work and revised the manuscript for submission.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Xu, J., Sun, Y. et al. An Insight on Synergistic Anti-cancer Efficacy of Biochanin A and Sulforaphane Combination Against Breast Cancer. Appl Biochem Biotechnol 196, 992–1007 (2024). https://doi.org/10.1007/s12010-023-04584-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04584-w