Abstract
Laccases are blue multicopper oxidases that oxidize a wide range of phenolic as well as non-phenolic substrates in the presence or absence of mediators. They occur in various species of bacteria, fungi, insects, and plants; bacterial laccases show high substrate specificity. Bacteria produce these enzymes either extracellularly or intracellularly and exhibit stability to a wide range of pH and temperature. Therefore, they are suitable for various industrial processes such as food, textile, and paper and pulp industry. They are also valuable for producing biofuels, pharmaceuticals, biosensors, and degradation of various environmental pollutants and xenobiotics compounds. Since bacterial laccases are more versatile in the sense of nutritional needs and ecological factors, their use can provide a promising solution to various problems related to industry and the field of biotechnology. However, there is a need for a thorough understanding of the chemistry and activity of bacterial laccases to enable their full potential use in bioremediation and biofuel production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases are copper-containing 1,4-benzenediol:oxygen oxidoreductases (EC 1.10.3.2) present in microorganisms, higher plants, bacteria, and insects [1]. These are glycosylated polyphenol oxidases which contain four copper ions in each molecule. Copper ions carry the oxidation of phenolics and non-phenolic compounds such as aromatic amines, diphenols, and aliphatic amines, along with the reduction of oxygen to water [2,3,4]. The bacterial laccase activity was detected for the first time in Azospirillum lipoferum isolated from the rhizosphere of rice in 1993 [5]. Various genera of bacteria such as Bacillus, Streptomyces, Klebsiella, Pseudomonas, Yersinia, Proteobacterium, and Marinomonas contain laccases as part of their metabolic processes [6]. These enzymes have also been reported in some bacteria of domain Archea, such as Haloferax volcanii [7]. The molecular weight of bacterial laccases ranges from 50–70 kDa, with most of them existing in the form of monomeric extracellular proteins, and many few occur as intracellular proteins [8, 9]. They are involved in pigmentation, toxin oxidation, morphogenesis, protection against UV and oxidizing agents [10]. Cot A protein of Bacillus subtilis is copper-dependent laccase present in the spore coat of bacterium. It is the first-ever laccase reported to be present in endospore coat and is involved in the production of brown melanin-like pigment and helps in protecting the bacterium from UV light and H2O2 [11].
The extraordinary stability and remarkable range of substrate specificity make them suitable for several biotechnological and industrial applications [12]. The substrate range includes aromatic amines, polyphenols, methoxy phenols, and various other inorganic compounds [13]. Laccase can efficiently oxidize these substrates by accepting electrons from molecular oxygen and producing phenoxy radicals that spontaneously rearrange to open their aromatic rings and thus enhance polymerization. These phenolic compounds are considered to be the possible substrates for laccases due to their low redox potentials (0.5–1.0 V). Additionally, the spectrum of substrates that laccase can oxidize can be considerably expanded by the application of low molecular weight redox mediators [14]. Table 1 illustrates the activity and stability of bacterial laccases on substrates with and without redox mediators.
These oxidoreductase enzymes have proficiency and are helpful in the biosynthesis of fibers, bio-detection, and environmental protection. At the industrial level, they are used in a variety of processes such as food improvement, bio-pulping in the paper industry, denim washing in the textile industry, and cosmetics manufacturing [23, 24]. Due to the high demand for efficient oxidation technologies, laccases have gained importance as biological oxidative agents and are beneficial for oxidizing toxic as well as non-toxic substrates. Laccase mediator systems also play role in the degradation of lignin, biosensor systems, biofuel synthesis, nano-biotechnological applications, pharmaceutical applications, and the bioremediation of toxic waste [25].
Properties of Laccases
Laccases are associated with a small set of enzymes also known as multicopper oxidases and they have the ability to produce a variety of extracellular enzymes such as manganese peroxidase, lignin peroxidase, and versatile peroxidases [26]. Laccases are monomer or homodimers of glycosylated proteins; bacterial laccases contain 10–25% of sugar residues compared to plants laccases that have higher contents of saccharides. Laccases have a wide range of redox potentials, ranging from 400 mV for plant laccases to 790 mV for certain fungal laccases. Laccases hold four copper ions per molecule, and mediators such as electron shuttle help them in oxidizing non-phenolic compounds [3, 27]. In addition, mannose is one of the significant carbohydrate components of laccases [11].
Laccases contain three types of copper prosthetic groups, which have been categorized on the basis of electron paramagnetic resonance signals and absorption spectra, namely type 1 copper (T1 Cu) is coordinated by two histidines, water molecules, and exhibit the first oxidation site, and shows absorption band at 600 nm in the UV–visible spectrum [28, 29]. T1 copper gives a blue shade due to the intense electronic absorbance of the cysteine-copper covalent bond [30]. Similar to T1 Cu, type 2 copper (T2 Cu) is also coordinated by two histidines and a water molecule. However, there does not exist any absorption band in the UV–visible spectrum but a parallel hyperfine coupling is recorded in EPR (A||= (105–201) × 10−4 cm−1). Moreover, type 3 copper (T3 Cu) carries two copper atoms: T3α Cu and T3β Cu. Both of these molecules are coordinated by three histidine residues. The T3 Cu is characterized by a broad absorption band at 330 nm ensuing from the hydroxyl bridge-to-metal (HO−–Cu) charge transition, but deficient in displaying EPR signals due to the anti-ferromagnetic coupling of two copper ions [14]. Usually, T2 and T3 copper sites are involved in the formulation of an oxygen-binding trinuclear cluster site (TNC) that is responsible for reducing oxygen to water. However, the T1 site functions by reducing substrate (electron donors) and transferring electrons to the T2–T3 copper clusters. In laccases, substrate oxidation at the T1 site is considered as a catalytic rate-limiting step, which is commonly controlled by the difference in the redox potential of this site and trinuclear site [31].
The entire laccase reaction entails a single electron (1e−) sequential oxidation of four reducing substrate molecules, followed by two double electron reduction of oxygen atoms into their respective H2O molecules. This reaction is usually catalyzed by the exchange of 4H+ equivalents. A laccase reaction is viewed structurally, mechanistically, and kinetically as two half-reactions that are joined by an internal electron transfer (IET) step and facilitated by the catalytic copper ions situated at the T1 Cu and T2 Cu/T3 Cu/T3 Cu trinuclear cluster (TNC) sites. Numerous sequence and metagenomic investigations have established that eleven (one Cys and ten His) residues composing the T1 copper and TNC laccase sites, and in general all multicopper oxidases (MCOs), explain their crucial involvement in the catalytic action of the enzyme. Similarly, other fully or highly conserved residues that play important roles in different catalytic steps involved in laccase action, such as the recognition and docking of reducing substrates, internal electron transfer (IET) from the T1 copper ion into the TNC site, and oxygen atom reduction at the TNC site have also been identified. These residues are typically found in close proximity to their respective areas of action, where they manifest as second sphere residues [32].
Three isozymes of laccases Lac I, Lac II, and Lac III have been discovered in bacteria, and studies show that laccase molecular weight varies in different organisms [11]. Bacterial laccases have a wider pH range as compared to fungal laccases, which make them efficient and more suitable for use in pulp processing in the paper industry and biobleaching in the textile industry [33]. Optimum temperature ranges from 30–50 °C, and they lose their activity completely at 60 °C [4]. The isoelectric point of laccases varies from 3 to 7, but the isoelectric point of plant laccase is as high as 9.0. The pH range for bacterial laccases is 3.0–9.0. However, Bacillus tequilensis SN4 laccase shows a maximum working temperature of 85 °C and a working pH of 8.0 [34,35,36]. They generate free radicals through the oxidation of substrate in a single electron response [4]. The major catalytic actions of laccase include reduction of type one copper by reducing substrate; electron transport among type 1, type 2, and type 3 copper; and reduction of final electron acceptor takes place which in this case is oxygen to form water at type 2 and type 3 copper sites. As mentioned earlier, T1 absorbs light in the range of 600 nm and is responsible for the blue color of the enzyme [4].
Bacterial laccases are easy to produce at the commercial level, and they show high resistance to inhibitory agents. Furthermore, they are more tolerant to a wide range of temperature and pH and offer a broad range of substrate specificity. The short generation time of bacteria makes it compatible to be used as a source of laccases at a commercial scale [9]. They are easy to clone and can be expressed in a variety of suitable hosts with suitable manipulation [37, 38].
Catalytic Properties of Bacterial Laccases
Fungal and bacterial laccases have been widely distinguished by numerous identifying approaches; most desirable is by employing substrates as well as inhibitors acting on them. Only brief data is present on bacterial laccase activity and their catalytic characteristics [39]. Marine Alteromonas sp. possessed both catalytic capacities of tyrosinase and laccase. The tyrosinase-related activities, cresolase, and catechol oxidase were selectively stimulated in the presence of small quantities of SDS in the assay mixture, and however, detergent did not enhance the oxidation of laccase substrates [40]. The presence of these two leading types of PPOs has been documented in certain fungi like Neurospora crassa and Agaricus bisporus [41] but not in prokaryotic cells, where laccases are uncommon [5]. The demarcation between tyrosinases and laccases in the bacterial kingdom may not be as obvious as suggested for PPOs derived from eukaryotic cells. The low molecular weight external fungal laccases have been described from Volvariella volvacea (58 kDa) [42] and Marasmius quercophilus (63 kDa) [43] compared to the internal bacterial laccases which have large molecular weight. Widely, fungal laccases have an acidic pH optimum, while most bacterial laccases are able to activate and are stable across larger pH (6.0–8.5) range [44, 45]. Bacterial laccase from γ-proteobacterium was stable in the pH range 4.0–9.0, but 70% stable at pH 3.0 and 10.6 even after 48 h of incubation at 37 °C. The enzyme was 100, 60, and 49% stable at pH 9.0 (Tris–HCl, 0.1 M), 10.6 (glycine–NaOH, 0.1 M), and 4.0 (citrate, 0.1 M), respectively after 60 days at 4 °C incubation; this stability of the enzyme might be because the organism did not create protease [44]. Metagenome-derived laccase showed optimal pH 9.0 for syringaldazine. Alkaline bacterial laccase (Lbh1) from B. halodurans C-125 showed a pH optimum of 7.5–8.0 using syringaldazine as a substrate [46]. The activity of bacterial laccases across a broader pH range might make them better as compared to fungal ones in industrial applications like biobleaching of paper pulp as well as dyestuffs processing [33].
Structural Classification of Bacterial Laccases
Laccases belong to the cupredoxin family, which contains many other oxidases such as manganese oxidase and ascorbate oxidase [47]. Cuperdoxin fold is the common signatory fold of this family. This fold is also known as the Greek-key motif, which consists of four beta-sheets arranged opposite to each other and are joined through hairpin loops. The first sheet is also directly connected with the fourth sheet through a long connection [48]. Depending upon the structure, bacterial laccases can be divided into two types: two-domain laccases and three-domain laccases [9].
Three-Domain Laccases
Endospore cotA laccase of Bacillus subtilis is a well-characterized three-domain laccase; therefore, it can be taken as a reference for understanding the structure of three-domain laccases. Cot A is a monomeric protein with a molecular weight of 65 kDa [49] and contains three cuperdoxin domains, as shown in Fig. 1. The primary domain (blue color) makes the N terminal of the protein and consists of 8 beta barrels which are connected with the first and second domain. Hydrogen bonding stabilizes the linkage among domains [48]. Domain two is located between domains 1 and 3; it consists of a comprehensive loop of a beta-barrel which is made up of 12 filaments. The small alpha-helical region connects domains 1 and 2, while domains 2 and three are connected with the help of a portion of the loop [50]. C terminal domain represented in the form of red color (Fig. 1) comprises of mono-nuclear copper center and helps to provide attachment point at T3 copper site [9]. It also provides an attachment site for the substrate near the T1 copper center, which is important for single-electron oxidation of the substrate. The highly compact structure and high amount of hydrophobic bonds of Cot A laccase make it thermostable [9].
Three-dimensional structure of CotA laccase from Bacillus subtilis retrieved from PDB: 3ZDW, it contains three domains. The first (N-terminal, domain I; in red color) cupredoxin-like domain of CotA (2–176 residues) appears in a somewhat distorted configuration. This N-terminal domain comprises of eight strands organized in β-barrel form and start with a coiled section (in yellow-colored sticks on the left side). The coiled section connects domains I and II. The second cupredoxin-like domain (domain II; in cyan color) with 183–340 residues carries α-helical fragment (in green colored sticks) connects domains I and II. Another large loop segment in domain II (in violet-colored sticks) connects domains II and III. The last C-terminal, domain III (369–501 residues) is represented in blue color. The copper atoms within the CotA structure are represented as light brown spheres and the mediator ABTS molecule close to the mono-nuclear copper center is shown in green color (modified from Enguita et al. [50])
Two-Domain Laccases
This class of laccases can be explained by using Streptomyces coelicolor laccase as a reference. These laccases consist of only two domains (Fig. 2) and lack a domain 2, which was responsible for connecting domain one with domain three in two-domain laccases [51]. Formation of trinuclear center becomes difficult in the absence of a domain; oligomerization is required in this case to form catalytic sites [9]. The formation of homotrimers takes place for the creation of a trinuclear center which is very important for the proper activity of laccases [52, 53]. Generally, laccases from different sources are divided into low redox potential and high redox potential laccases. Bacteria, plants, and insects contain low redox potential laccases, while high redox potential laccases commonly occur in fungi [54].
(a) Structure of two domains laccase from Streptomyces coelicolor PDB: 3CG8, where domain-I with 45–185 residues is shown in blue color and domain-II with 186–316 residues are shown in red color, while three trinuclear copper clusters (shown in brown spheres) are placed between domains I and II. (b) Enlarged image of two domains laccase from Streptomyces coelicolor is shown the additional helices and loops, and the major difference between two domain laccase, and (c) three-domain laccase from Bacillus subtilis (PDB ID: 3ZDW) is that mono-nuclear copper exist in domain III of three domain laccases as shown in figure (modified from Skálová et al. [51])
Production of Bacterial Laccases
Laccases are found in Gram-positive and Gram-negative bacteria inhabiting soil and aquatic environment. Laccase-producing bacteria belong to Phyla Alpha, Gemma Proteobacteria, Firmicutes, Cyanobacteria, Aquificae, Dinococcus, Thermus, and some members of domain Archea [49]. The occurrence of laccases have been reported in the following species, Bacillus subtilis [55], B. licheniformes [56], B. pumilus [57], Sinorhizobium meliloti [58], S. lavendulae [59], P. syringae [60], S. griseus [61], Escherichia coli [62], Thermus thermophilus [63], Oscillatoria boryana [64], Alteromonas sp. [40], and Haloferax volcanii [7]. The cellular location of laccases in bacteria varies from specie to specie; most of the natively expressed laccases in bacteria such as Bacillus subtilis [65] and Sinorhizobium meliloti [58] occurs intracellularly while few occurs extracellularly such as laccases of some bacilli and actinomycetes [66]. As the reaction of laccases produces toxic products, bacteria containing intracellular laccases have mechanisms to cope up with the toxicity [49]. Table 2 represents the applications of bacterial laccases.
Purification of Laccase from Different Sources
Laccase produced from S. psammoticus was partially purified by ammonium sulfate precipitation and immobilized in alginate beads by entrapment method using calcium and copper. Copper alginate beads retained 61% of laccase activity, compared to calcium alginate beads, which retained only 42.5% of laccase activity [74]. Without induction, Zhang et al. [75] produced laccase from Panus rudis in a specified shaken liquid culture; purified laccase enzyme had a molecular weight of 58 kDa with an isoelectric point of 3.5. McMahon et al. [34] purified laccase from cell extracts of soil bacterium P. putida F6 using a combination of anion exchange chromatography and gel filtration, and found laccase activity of 518 U mg−1 with a molecular mass of about 59 kDa. Suzuki et al. [59] identified a laccase from cell extracts of Streptomyces lavendulae REN (ST SL). On SDS-PAGE, the isolated enzyme appeared as a single protein band with a molecular mass of about 30 kDa. Da Cunha et al. [76] determined laccase activity spectrophotometrically using syringaldazine and observed an increase in absorbance when substrates were oxidized at room temperature. According to Jhadav et al. [77], purified laccase obtained from glucose and guaiacol medium demonstrated lower activity than its crude counterpart, and the efficiency of the purified extract was determined using SDS-PAGE. Diamantidis et al. [78] purified Azospirillum lipoferum laccase using dialysis and ammonium sulfate precipitation of the proteins from the supernatant. Laccase activity was detected in 30–60% saturated fractions with a molecular mass of approximately 60–70 kDa and an acidic isoelectric point (pI) of approximately pH 4.0 since laccases have been isolated and purified from various sources including fungi and plants [79]. Therefore, bacterial laccases carry several unique properties that are not characteristics of fungal and plant laccases such as stability at high temperature and pH.
Biotechnological and Industrial Applications
Although oxidation reactions are the biggest need of the industry, most of the conventional oxidation technologies are non-specific, produce undesirable side reactions, and involve the use of toxic compounds which are a potential hazard for the environment. These drawbacks of conventional chemical oxidation technologies have compelled industrialists and researchers to look for the use of enzymes in oxidation technologies. Enzymes are specific in their reaction and highly biodegradable, and require mild conditions for the reaction, which render them appropriate for use in industry as well as a research field. Due to the wide range of substrate specificity and no potential threat to the environment, laccases are seen as the best replacement for various chemical treatments [3]. Figure 3 elucidates the application of laccases. The following are the application of bacterial laccases in biotechnology and industry.
Food Industry
The quantity of phenolic content present in fruit juices greatly affects the quality of juices. High contents of phenols can change the color and taste of the juices, thus affecting the value. Darkening of these products takes place due to polyphenols; the use of laccases to remove phenol contents and avoiding chemical treatment such as the one which involves the use of activated carbon can help in maintaining color stability [80, 81]. Laccases can be beneficial for wine stabilization through controlling phenol contents in the wine [82]. White wine is also treated with laccases, and due to the excellent stability of bacterial laccases at low pH, inhibition of laccases with sulfites can be easily reversed. Wine treatment with laccases increases its shelf life and reduces the production cost [82, 83]. The presence of phenolic compounds such as proanthocyanidines causes the precipitation of protein and results in the formation of haze in beer; therefore, beer stabilization is also a very necessary step for enhancing shelf life [81]. Laccases are added at the end of the production process in order to increase the half-life of beer [82].
Laccases have also been reported to be used in baking due to their texture-improving properties. Their usage is beneficial for improving the consistency of the dough and enhancing the tensile strength of gluten structure [84]. A noticeable change in volume and softness of dough and transition in the structure of crumbs has been observed after adding laccases [81]. Laccases are also valuable for producing sugar beet pectin gelatin which is a gel formed by the oxidative cross-linking of ferulic acid. The oxidative process during the production of sugar beet pectin gelatin is carried out using oxidoreductases such as laccases and peroxidases. Laccases and peroxidases differ on the base of their electron acceptors; laccases require molecular oxygen as their terminal electron acceptor, while peroxidases require hydrogen peroxide (H2O2) as the final electron acceptor [84]. The gel which is obtained after the treatment with laccases is thermo-irreversible and can be used in luncheon meat and many other food products [83, 85].
Textile Industry
The textile industry makes a total of one-third of the dyestuff market, and it consumes a large volume of water and chemicals for the wet processing of textile [3]. The chemicals used in textiles are very diverse in nature and composition, ranging from inorganic compounds, polymers to organic compounds [86,87,88]. These dyes released from textile mills create a large volume of colored wastewater, and their inability to fade in the presence of water, light, and other chemicals makes them resistant to degradation [89]. Due to increased awareness about the environmental issues, new legislation requires textile industries to treat colored water before discharge [90].
The carcinogenic nature of several dyes such as benzidine raises concern about the environmental impact of these chemicals [91]. The wastewater from textile mills is usually treated through chemical or physical methods, which include irradiation, precipitation, ozonation, electrokinetic coagulation, use of activated carbon, and mixture of various gases [92]. Currently available methods for treating textile waste and dyes are ineffective and non-economical; therefore, the treatment method based upon the use of laccases provides promising solution due to their ability to degrade dyes of diverse chemical nature as well as synthetic dyes [93, 94]. Laccase-based hair dyes have also been introduced, which are less irritant and are easier to handle as compared to conventional dyes due to the replacement of H2O2 with laccases [95]. Laccases are also capable of enhancing dechlorination activity which causes the decrease in dissolved oxygen concentration [96].
Laccases are used in denim finishing for the removal of indigos in order to create abrasion effects on fabrics. However, fungal laccases such as the laccase from Trametes versicolor have proved to be more effective for this purpose [97]. Laccases are useful for the fixation of dyes on wool; this process is very economical at a large scale as less dye concentration is required and deep color fixation takes place [98]. Roving treatment of yarn under mild conditions enhances the regularity of yarn; use of laccases ensures desirable results with no impact on the integrity of the environment [23, 99]. Conventional anti-shrinking treatment for wool involves chlorination which has a hazardous impact on the eco-system; however, laccases use along with a mediator is a suitable method for preventing fabric shrinking [100]. Azo dyes make about half of the total synthetic dyes; laccases from bacterial sources such as Streptomyces have been reported to be used for the removal of azo dyes [101]. Bacterial laccases originated from Trametes versicolor [69], Stenotrophomonas maltophilia, and Streptomyces psammoticus are competent for the removal of a wide range of synthetic dyes [70].
Paper and Pulp Industry
Paper industries use the chlorination process for the delignification of wood and bleaching of paper, which is a pollution-generating process [102]. From the past few years, conventional chemical delignification and bleaching methods have been replaced by a safer method which includes the use of laccases from microbial sources. These enzymes not only help in effective delignification but also help in maintaining the integrity of cellulose [103] to get a better quality paper. As laccases cannot degrade lignin directly, various mediators are required for the proper activity of the enzymes. Therefore, during the process of delignification, the oxidation of the mediator is carried out first, and then, the oxidized mediator oxidizes the lignin in the wood [104]. Pre-bleaching of pulp with laccase reduces the need for hypochlorite by 10% and has been proved helpful in achieving as much brightness of resultant paper as achieved in the case of chemically treated pulped [105].
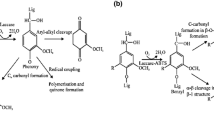
Laccases from bacterial sources such as P. stutzeri (soil bacterium) [67] and S. cyaneus [68] are used in the bleaching of eucalyptus pulp along with the use of HOBt (hydrobenzotriazole) and ABTS (2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) as mediators [72]. Lignocellulolytic based composite materials can be manufactured by using laccases for the adhesion of lignin fibers. The composite material prepared by enzymatic adhesion has good mechanical properties and does not require the use of toxic synthetic adhesives [106]. Recent studies show that the laccases can also be used to graft the various phenolic acids on the surface of eucalyptus kraft pulp fiber [107]. This ability of laccases can be helpful in the future for attaching versatile materials on the surface of fibers or composite materials to achieve hybrid materials with desirable properties such as hydrophobicity and charge [3]. The combination of xylanase and laccase can be used as effective tools for minimizing lignin and xylose contents from the pulp [108]. Both laccases and xylanases have a synergistic effect in enhancing the pulp quality [9]. Old newspapers and used papers can be recycled by using the combination of physical as well as enzymatic methods, while physical methods include sonication and microwaving [109]. Figure 4 represents the paper preparation process in which laccases were used.
Use of laccases in paper and pulp industry [105]
Pollution Degradation
The nature and catalytic activity of laccases make them compatible to be used in the biodegradation of various pollutants. Laccases from Klebsiella pneumonia are thermostable and pH stable; they can be used for degrading a wide range of dyes (such as brilliant blue X-BR, Congo red, malachite blue, bromophenol blue, etc.) produced during industrial processes under diverse pH ranges and temperature of 70 °C in a very short time of 90 min [6]. Bacillus pumilus Cot A laccase expressed in E. coli has been implemented for the degradation of a wide range of dyes, and results predict the higher discoloration yield for anthraquinonic and triphenylmethane dyes as compared to aromatic heterocyclic dyes [32]. Moreover, Cerrena sp. HYB07, a white-rot fungal strain is known to give a high yield of laccases with high specific activity and decolorizing ability under short production cycles. In this regard, Yang et al. [110] have investigated the application of a purified HYB07 laccase, in decolorization and detoxification of malachite green.
Laccases have also been reported to be helpful in aromatic xenobiotics and other pollutants found in industrial wastewater, contaminated soil, or water [31]. Laccases can inactivate contaminants either by complete degradation or by polymerization. Polymerization can be carried out among pollutants themselves or the copolymerization of pollutants with non-toxic substances. After polymerization, pollutants become insoluble in the water and can be easily removed through adsorption, sedimentation, and filtration [111]. Polycyclic aromatic hydrocarbons (PAHs) are ubiquitously present in the environment and are responsible for the contamination of air, soil, and water [112]. The low solubility and recalcitrant nature of PAHs render them resistant to degradation [31]. Therefore, the knowledge of microorganisms that exhibit a high potential to degrade PAHs is essential. Lignocellulolytic bacteria and fungi have the ability to degrade PAHs to achieve a safe level of PAHs in the environment. However, white-rot fungi have more advantages over lignocellulolytic bacteria due to their non-specific nature. Hence, white-rot fungi can help degrade a wide range of pollutants, including PAHs, to an undetectable level [31]. Wastewater from distilleries, beer mills, and olive mills contains an adequate amount of phenolic and non-phenolic compounds, which can pose harm to the environment; this waste can also be treated by using laccases from various microbial sources [31]. Laccases have also been proved useful in oxidizing the estrogenic hormones from sewage water [113]. Studies show that the laccases can mediate the coupling of 2,4,6-trinitrotoluene (TNT) to an organic soil matrix which can help in detoxifying ammunition residues [114].
Biofuels
Lignocellulolytic biomass is one of the most abundant biomass on earth, and recent studies have invented a way to produce bioethanol from lignocelluloses. However, the efficiency of this method depends upon the ease of hydrolysis of polysaccharides which depends upon the cost-effective treatment of lignocellulosic biomass to remove lignin. Lignin removal is an important step to make sugars freely available for the action of hydrolytic enzymes [115]. The use of laccases in degrading lignin is an important breakthrough in the biofuel industry, and they have been proved useful for not only removing lignin from lignocellulolytic biomass but also as biocatalysts for removing yeast growth inhibitors for further enzymatic action [116]. The use of laccases in corn stover hydrolysate has been reported to remove 84% of the phenolic content, and the addition of laccase before cellulose hydrolysis has been helpful in increasing the yield of bioethanol by 10% [117].
Cosmetics
Cosmetic and dermatological preparations containing laccases can be used for skin lightening purposes [118]. Furthermore, they also have application in hair bleaching and dying. The use of H2O2 in bleach and hair colors can have a damaging impact on the scalp as well as hair, so laccases are a safe replacement of H2O2 [119] as an oxidizing agent in hair dyes and bleaches. Laccases from bacterial sources such as actinomycetes (Thermobifida fusca) and basidiomycetes (Flammulina velutipes) have been tested for their oxidative potential and are widely used in hair colors [120, 121].
Biomedicines and Pharmaceuticals
Laccases can be used in biosensor systems due to their ability to reduce oxygen to water; consumption of oxygen during analyte oxidation can be a quantitative indicator for the laccase-based biosensors [115]. Laccase-based biosensors can be used in the food industry to detect the presence of polyphenols in fruits juices and beverages in order to monitor the quality of these products [122]. In the biomedical field, laccase biosensors have been developed to monitor the concentration of insulin, morphine, and codeine [123]. The incorporation of blood-tolerant laccases in self-powered and wireless medical devices and environmental sensors can provide a promising edge in creating high-tech devices for enhancing monitoring efficiencies [124]. In the pharmaceutical industry, laccases find applications in the development of antimicrobial and detoxifying agents. Anti-inflammatory agents, sedatives, anesthetic, and antibiotics are being synthesized by using laccases [125]. Anticancer drug actinocin has been made at the expense of laccases. This drug creates a hindrance in the growth of the tumor by interrupting the DNA transcription of tumor cells [126]. Laccases have also been proved efficient against reverse transcriptase of the human immunodeficiency virus (HIV) [127, 128] which have reported the anti-allergic activity of laccases.
Future Prospects and Conclusion
In conclusion, the current study summarizes the occurrence and molecular, biochemical, and structural features of the many bacterial laccases described to date. Use of homologous and heterologous expression systems, expression regulation, laccase mediators, immobilized enzyme for operational stability, and mutagenesis are the efficient ways that can be optimized and implemented in the future to get higher enzyme yield without increasing production cost to improve enzyme activity and stability. Laccase enzymes have the ability to work on a wide variety of substrates and detoxify a wide variety of contaminants, as well as the oxidation of hazardous compounds, making them valuable in the paper, pulp, and textile industries. However, one of the barriers to broad-scale laccase utilization is the inability to create huge quantities of the highly active enzyme at a cheap price. Recently, the utilization of low-cost sources for laccase manufacturing has been investigated. In this context, an emerging field in industrial wastewater treatment is harnessing its nutritional potential for laccase production. Apart from solid wastes, wastewater from the food processing sector has great potential. Secondly, laccase is involved in the carbon cycle and may aid in the degradation of a broad variety of xenobiotic or phenolic substances. The issue with laccase is that the enzyme has a poor substrate specificity and may possibly catalyze a large variety of reactions. According to some authors, the enzymatic oxidation of aromatic compounds may create by-products that convert blue laccase to yellow laccase (YL), which, unlike blue laccase, does not need a mediator to breakdown pollutants. As a result, further study in this area is required in the near future. Furthermore, despite numerous efforts to understand the role of laccase in the transformation of lignocelluloses, it is also unclear how significant a role laccase plays in lignin degradation, because in plant biomass it could be utilized as an enzymatic pretreatment method in cellulosic ethanol production.
References
Shraddha, Shekher, R., Sehgal, S., Kamathania, M. and Kumar. A. (2011). Laccase: Microbial sources, production, purification and potential biotechnological applications. Enzyme. Res. 2011.
Gianfreda, L., Xu, F., & Bollag, J. M. (1999). Laccases: A useful group of oxidoreductive enzymes. Bioremediation Journal, 3(1), 1–26.
Couto, S. R., & Herrera, J. L. T. (2006). Industrial and biotechnological applications of laccases: A review. Biotechnol Advances, 24(5), 500–513.
Singh, D., and Gupta, N. (2020). Microbial laccase: A robust enzyme and its industrial applications. Biologia.
Givaudan, A., Effosse, A., Faure, D., Potier, P., Bouillant, M. L., & Bally, R. (1993). Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of Azospirillumlipoferum. FEMS Microbiology Letters, 108(2), 205–210.
Liu, Y., Huang, L., Guo, W., Jia, L., Fu, Y., Gui, S., & Lu, F. (2017). Cloning, expression, and characterization of a thermostable and pH-stable laccase from Klebsiella pneumoniae and its application to dye decolorization. Process Biochemistry, 53, 125–134.
Uthandi, S., Saad, B., Humbard, M. A., & Maupin-Furlow, J. A. (2010). LccA, an archaeal laccase secreted as a highly stable glycoprotein into the extracellular medium by Haloferaxvolcanii. Applied and Environmental Microbiology, 76(3), 733–743.
Martins, L. O., Durao, P., Brissos, V., & Lindley, P. F. (2015). Laccases of prokaryotic origin: Enzymes at the interface of protein science and protein technology. Cellular and Molecular Life Sciences, 72(5), 911–922.
Chauhan, P. S., Goradia, B., & Saxena, A. (2017). Bacterial laccase: recent update on production, properties and industrial applications. 3 Biotech, 7(5), 1–20.
Singh, G., Bhalla, A., Kaur, P., Capalash, N., & Sharma, P. (2011). Laccase from prokaryotes: A new source for an old enzyme. Reviews in Environmental Science & Biotechnology, 10(4), 309–326.
Chandra, R., & Chowdhary, P. (2015). Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environmental Science. Processes & Impacts, 17(2), 326–342.
Bilal, M., & Iqbal, H. M. (2019). Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities–A review. International Food Research Journal, 123, 226–240.
Mehra, R., Muschiol, J., Meyer, A. S., & Kepp, K. P. (2018). A structural-chemical explanation of fungal laccase activity. Science and Reports, 8(1), 1–16.
Jones, S. M., & Solomon, E. I. (2015). Electron transfer and reaction mechanism of laccases. Cellular and Molecular Life Sciences, 72, 869–883.
Coria-Oriundo, L. L., Battaglini, F., & Wirth, S. A. (2021). Efficient decolorization of recalcitrant dyes at neutral/alkaline pH by a new bacterial laccase-mediator system. Ecotoxicology and Environmental Safety, 217, 112237.
Galai, S., Korri-Youssoufi, H., & Marzouki, M. N. (2014). Characterization of yellow bacterial laccase SmLac/role of redox mediators in azo dye decolorization. Journal of Chemical Technology and Biotechnology, 89, 1741–1750.
Taguchi, T., Ebihara, K., Yanagisaki, C., et al. (2018). Decolorization of recalcitrant dyes by a multicopper oxidase produced by Iodidimonas sp. Q-1 with iodide as a novel inorganic natural redox mediator. Scientific Reports, 8, 6717.
Afreen, S., Shamsi, T. N., Baig, M. A., Ahmad, N., Fatima, S., Qureshi, M. I., Hassan, M. I., & Fatma, T. (2017). A novel multicopper oxidase (laccase) from cyanobacteria: Purification, characterization with potential in the decolorization of anthraquinonic dye. PLoS One, 12(4), e0175144. https://doi.org/10.1371/journal.pone.0175144
Mishra, S. K., & Srivastava, S. K. (2016). Production of extracellular laccase from bacterial strain Bacillus subtilis MTCC 1039 using different parameter. Biosciences Biotechnology Research Asia, 13, 1645–1650. https://doi.org/10.13005/bbra/2312
Muthukumarasamy, N.P., Jackson, B., Joseph Raj, A., and Sevanan, M. (2015). Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochemistry research international. 765190.
Rajeswari, M., Vennila, K., & Bhuvaneswari, V. (2015). Optimization of laccase production media by Bacilllus cereus TSS1 using Box-Behnken design. International Journal of Chemistry and Pharmaceutical Sciences, 6(1), 95–101.
Solano, F., Garcia, E., Perez, D., & Sanchez-Amat, A. (1997). Isolation and characterization of strain MMB-1 (CECT 4803), a novel melanogenic marine bacterium. Applied and Environmental Microbiology, 63(9), 3499–3506.
Mojsov, K. D. (2014). Biotechnological applications of laccases in the textile industry. Savremene tehnologije, 3(1), 76–79.
Zheng, F., Cui, B. K., Wu, X. J., Meng, G., Liu, H. X., & Si, J. (2016). Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. International Biodeterioration & Biodegradation, 110, 69–78.
Zeng, S., Qin, X., & Xia, L. (2017). Degradation of the herbicide isoproturon by laccase-mediator systems. Biochemical Engineering Journal, 119, 92–100.
Xu, X., Xu, Z., Shi, S., & Lin, M. (2017). Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresource Technology, 241, 415–423.
Baiocco, P., Barreca, A. M., Fabbrini, M., Galli, C., & Gentili, P. (2003). Promoting laccase activity towards non-phenolic substrates: A mechanistic investigation with some laccase–mediator systems. Organic & Biomolecular Chemistry, 1(1), 191–197.
Claus, H. (2003). Laccases and their occurrence in prokaryotes. Archives of Microbiology, 179(3), 145–150.
Roberts, S. A., Weichsel, A., Grass, G., Thakali, K., Hazzard, J. T., Tollin, G., & Montfort, W. R. (2002). Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proceedings of the National academy of Sciences of the United States of America, 99(5), 2766–2771.
Ashraf, F., Irfan, M., Shakir, H. A., Ali, S., & Khan, M. (2020). An overview of production and industrial exploitation of bacterial laccases. Punjab University Journal of Zoology, 35(1), 147–156.
Madhavi, V., & Lele, S. S. (2009). Laccase: Properties and applications. Bio Resources., 4(4), 1694–1717.
Arregui, L., Ayala, M., Gómez-Gil, X., Gutiérrez-Soto, G., Hernández-Luna, C. E., de Los, H., Santos, M., Levin, L., Rojo-Domínguez, A., Romero-Martínez, D., Saparrat, M., Trujillo-Roldán, M. A., & Valdez-Cruz, N. A. (2019). Laccases: Structure, function, and potential application in water bioremediation. Microbial Cell Factories, 18(1), 200.
Singh, G., Sharma, P., & Capalash, N. (2009). Performance of an alkalophilic and halotolerant laccase from γ-proteobacterium JB in the presence of industrial pollutants. Journal of General and Applied Microbiology, 55(4), 283–289.
McMahon, A. M., Doyle, E. M., Brooks, S., & O’Connor, K. E. (2007). Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6. Enyzme and Microbial Technology, 40(5), 1435–1441.
Mongkolthanaruk, W., Tongbopit, S., & Bhoonobtong, A. (2012). Independent behavior of bacterial laccases to inducers and metal ions during production and activity. African Journal of Biotechnology, 11(39), 9391–9398.
Sondhi, S., Sharma, P., Saini, S., Puri, N., & Gupta, N. (2014). Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PloS One, 9(5), e96951.
Fernandes, T. A. R., da Silveira, W. B., Passos, F. M. L. and Zucchi, T.D. (2014). Laccases from Actinobacteria-What we have and what to expect. Advances in Applied Microbiology 2014.
Prins, A., Kleinsmidt, L., Khan, N., Kirby, B., Kudanga, T., Vollmer, J., & Le Roes-Hill, M. (2015). The effect of mutations near the T1 copper site on the biochemical characteristics of the small laccase from Streptomyces coelicolor A3 (2). Enyzme and Microbial Technology, 68, 23–32.
Sharma, P., Goel, R., & Capalash, N. (2007). Bacterial laccases. World. Journal of Microbiology and Biotechnology, 23, 823–832.
Sanchez-Amat, A., & Solano, F. (1997). A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochemical and Biophysical Research Communications, 240(3), 787–792.
Huber, M., & Lerch, K. (1987). The influence of copper on the induction of tyrosinase and laccase in Neurospora crassa. FEBS letters., 219(2), 335–338. https://doi.org/10.1016/0014-5793(87)80247-8
Chen, S. C., Ge, W., & Buswell, J. A. (2004). Molecular cloning of a new laccase from the edible staw mushroom Volvarielle volvacea: Possible involvement in fruit body development. FEMS Microbiology Letters, 230, 171–176.
Dedeyan, B., Klonowska, A., Tagger, S., Tron, T., Iacazio, G., Gil, G., & Petit, J. L. (2000). Biochemical and molecular characterization of a laccase from Marasmius quercophilus. Applied and Environment Microbiology, 66, 925–929.
Singh, G., Capalash, N., Goel, R., & Sharma, P. (2007). A pH-stable laccase from alkali-tolerant γ-proteobacterium JB: Purification, characterization and indigo carmine degradation. Enzyme and Microbial Technology., 41, 794–799.
Ye, M., Li, G., Liang, W. Q., & Liu, Y. H. (2010). Molecular cloning and characterization of a novel metagenome-derived multicopper oxidase with alkaline laccase activity and highly soluble expression. Applied Microbiology and Biotechnology, 87(3), 1023–1031.
Ruijssenaars, H. J., & Hartmans, S. (2004). A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Applied Microbiology and Biotechnology, 65, 177–182.
Gray, H. B., Malmström, B. G., & Williams, R. J. P. (2000). Copper coordination in blue proteins. JBIC Journal of Biological Inorganic Chemistry, 5(5), 551–559.
Enguita, F. J., Martins, L. O., Henriques, A. O., & Carrondo, M. A. (2003). Crystal structure of a bacterial endospore coat component: A laccase with enhanced thermostability properties. Journal of Biological Chemistry, 278(21), 19416–19425.
Janusz, G., Pawlik, A., Świderska-Burek, U., Polak, J., Sulej, J., Jarosz-Wilkołazka, A., & Paszczyński, A. (2020). Laccase properties, physiological functions, and evolution. International Journal of Molecular Sciences, 21(3), 966.
Enguita, F. J., Marçal, D., Martins, L. O., Grenha, R., Henriques, A. O., Lindley, P. F., & Carrondo, M. A. (2004). Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. Journal of Biological Chemistry, 279(22), 23472–23476.
Skálová, T., Dohnálek, J., Østergaard, L. H., Østergaard, P. R., Kolenko, P., Dušková, J., & Hašek, J. (2009). The structure of the small laccase from Streptomyces coelicolor reveals a link between laccases and nitrite reductases. Journal of Molecular Biology, 385(4), 1165–1178.
Machczynski, M. C., Vijgenboom, E., Samyn, B., & Canters, G. W. (2004). Characterization of SLAC: A small laccase from Streptomyces coelicolor with unprecedented activity. Protein Science, 13(9), 2388–2397.
Gunne, M., Höppner, A., Hagedoorn, P. L., & Urlacher, V. B. (2014). Structural and redox properties of the small laccase S sl1 from Streptomyces sviceus. FEBS Journal, 281(18), 4307–4318.
Munk, L., Sitarz, A. K., Kalyani, D. C., Mikkelsen, J. D., & Meyer, A. S. (2015). Can laccases catalyze bond cleavage in lignin? Biotechnology Advances, 33(1), 13–24.
Hullo, M. F., Moszer, I., Danchin, A., & Martin-Verstraete, I. (2001). CotA of Bacillus subtilis is a copper-dependent laccase. Journal of Bacteriology Research, 183(18), 5426–5430.
Koschorreck, K., Richter, S. M., Ene, A. B., Roduner, E., Schmid, R. D., & Urlacher, V. B. (2008). Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Applied Microbiology and Biotechnology, 79(2), 217–224.
Reiss, R., Ihssen, J., & Thöny-Meyer, L. (2011). Bacillus pumilus laccase: A heat stable enzyme with a wide substrate spectrum. BMA Biotechnology, 11(1), 1–11.
Castro-Sowinski, S., Martinez-Drets, G., & Okon, Y. (2002). Laccase activity in melanin-producing strains of Sinorhizobium meliloti. FEMS Microbiology Letters, 209(1), 119–125.
Suzuki, T., Endo, K., Ito, M., Tsujibo, H., Miyamoto, K., & Inamori, Y. (2003). A thermostable laccase from Streptomyces lavendulae REN-7: Purification, characterization, nucleotide sequence, and expression. Bioscience, Biotechnology, and Biochemistry, 67(10), 2167–2175.
Solano, F., Lucas-Elı́o, P., López-Serrano, D., Fernández, E., & Sanchez-Amat, A. (2001). Dimethoxyphenol oxidase activity of different microbial blue multicopper proteins. FEMS Microbiology Letters, 204(1), 175–181.
Endo, K., Hayashi, Y., Hibi, T., Hosono, K., Beppu, T., & Ueda, K. (2003). Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. Journal of Biochemistry, 133(5), 671–677.
Alexandre, G., & Zhulin, I. B. (2000). Laccases are widespread in bacteria. Trends in Biotechnology, 18(2), 41–42.
Miyazaki, K. (2005). A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles, 9(6), 415–425.
Palanisami, S., Saha, S. K., & Lakshmanan, U. (2010). Laccase and polyphenol oxidase activities of marine cyanobacteria: A study with Poly R-478 decolourization. World Journal of Microbiology & Biotechnology, 26(1), 63–69.
Martins, L. O., Soares, C. M., Pereira, M. M., Teixeira, M., Costa, T., Jones, G. H., & Henriques, A. O. (2002). Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of Bacillus subtilis endospore coat. Journal of Biological Chemistry, 277(21), 18849–18859.
Dubé, E., Shareck, F., Hurtubise, Y., Daneault, C., & Beauregard, M. (2008). Homologous cloning, expression, and characterization of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Applied Microbiology and Biotechnology, 79(4), 597–603.
Kumar, A., Vanamala, A. and Kumar, R. (2005). Exploration of bacterial laccase in Pseudomonas stutzeri and its application in bleaching the wood pulp: N6–008P. FEBS. J. 272.
Arias, M. E., Arenas, M., Rodríguez, J., Soliveri, J., Ball, A. S., & Hernández, M. (2003). Kraft pulp bio-bleaching and mediated oxidation of a non-phenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Applied and Environment Microbiology, 69(4), 1953–1958.
Romero, S., Blánquez, P., Caminal, G., Font, X., Sarrà, M., Gabarrell, X., & Vicent, T. (2006). Different approaches to improving the textile dye degradation capacity of Trametes versicolor. Biochemical Engineering Journal, 31(1), 42–47.
Niladevi, K. N., & Prema, P. (2008). Immobilization of laccase from Streptomyces psammoticus and its application in phenol removal using packed bed reactor. World Journal of Microbiology & Biotechnology, 24, 1215–1222. https://doi.org/10.1007/s11274-007-9598-x
Paszczynski, A., Pasti, M. B., Goszczynski, S., Crawford, D. L., & Crawford, R. L. (1991). New approach to improve degradation of recalcitrant azo dyes by Streptomyces spp. and Phanerochaete chrysosporium. Enzyme and Microbial Technology, 13(5), 378–384.
Held, C., Kandelbauer, A., Schröder, M., Cavaco-Paulo, A., & Gübitz, G. M. (2005). Biotransformation of phenolics with laccase containing bacterial spores. Environmental Chemistry Letters, 3(2), 74–77.
Bains, J., Capalash, N., & Sharma, P. (2003). Laccase from a non-melanogenic, alkalotolerant γ-proteobacterium JB isolated from industrial wastewater drained soil. Biotechnology Letters, 25(14), 1155–1159.
Naclerio, G., Falasca, A., Petrella, E., Nerone, V., Cocco, F. and Celico, F. (2010). Potential role of Bacillus endospores in soil amended by olive mill wastewater. 61(11), 2873-2879
Zhang, M., Wu, F., Wei, Z., Xiao, Y., & Gong, W. (2006). Characterization and decolorization ability of a laccase from Panus rudis. Enyzme and Microbial Technology, 39(1), 92–97.
Da Cunha, M. A. A., Barbosa, A. M., Giese, E. C., & Dekker, R. F. H. (2003). The effect of carbohydrate carbon sources on the production of constitutive and inducible laccases by Botryosphaeria sp. Journal of Basic Microbiology, 43, 385–392.
Jhadav, A., Vamsi, K. K., Khairnar, Y., Boraste, A., & Gupta, N. (2009). Optimization of production and partial purification of laccase by Phanerochaete chrysosporium using submerged fermentation. International Journal of Microbiology Research, 1, 9–12.
Diamantidis, G., Effosse, A., Potier, P., & Bally, R. (2000). Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biology & Biochemistry, 32, 919–992.
Shleev, S. V., Morozova, O. V., Nikitina, O. V., Gorshina, E. S., Rusinova, T. S., et al. (2004). Comparison of physico-chemical characteristics of four laccases from different basidiomycetes. Biochimie, 86, 693–703.
Ribeiro, D. S., Henrique, S. M., Oliveira, L. S., Macedo, G. A., & Fleuri, L. F. (2010). Enzymes in juice processing: A review. International Journal of Food Science, 45(4), 635–641.
Brijwani, K., Rigdon, A. and Vadlani, P.V. (2010). Fungal laccases: Production, function, and applications in food processing. Enzyme Research 2010.
Osma, J.F., Toca-Herrera, J.L. and Rodríguez-Couto, S. (2010). Uses of laccases in the food industry. Enzyme Research 2010.
Minussi, R. C., Rossi, M., Bologna, L., Rotilio, D., Pastore, G. M., & Durán, N. (2007). Phenols removal in musts: Strategy for wine stabilization by laccase. Journal of Molecular Catalysis. B, Enzymatic, 45(3–4), 102–107.
Mayolo-Deloisa, K., González-González, M., & Rito-Palomares, M. (2020). Laccases in food industry: Bioprocessing, potential industrial and biotechnological applications. Frontiers in Bioengineering and Biotechnology, 8, 222.
Norsker, M., Jensen, M., & Adler-Nissen, J. (2000). Enzymatic gelation of sugar beet pectin in food products. Food Hydrocolloids, 14(3), 237–243.
Mishra, G., & Tripathy, M. (1993). A critical review of the treatments for decolourization of textile effluent. Colourage, 40, 35–35.
Banat, I. M., Nigam, P., Singh, D., & Marchant, R. (1996). Microbial decolorization of textile-dyecontaining effluents: A review. Bioresource Technology, 58(3), 217–227.
Juang, R. S., Tseng, R. L., Wu, F. C., & Lin, S. J. (1996). Use of chitin and chitosan in lobster shell wastes for color removal from aqueous solutions. Journal of Environmental Science and Health, Part A, 31(2), 325–338.
Wesenberg, D., Kyriakides, I., & Agathos, S. N. (2003). White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnology Advances, 22(1–2), 161–187.
Kiro, K. (2013). Production and application of laccase in textile industry. Tekstilna industrija., 61(4), 11–15.
Baughman, G. L., & Perenich, T. A. (1988). Fate of dyes in aquatic systems: I solubility and partitioning of some hydrophobic dyes and related compounds. Environmental Toxicology and Chemistry, 7, 183–199.
Tavares, A. P., Cristóvão, R. O., Gamelas, J. A., Loureiro, J. M., Boaventura, R. A., & Macedo, E. A. (2009). Sequential decolourization of reactive textile dyes by laccase mediator system. Journal of Chemical Technology and Biotechnology, 84(3), 442–446.
Abadulla, E., Tzanov, T., Costa, S., Robra, K. H., Cavaco-Paulo, A., & Gübitz, G. M. (2000). Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Applied and Environment Microbiology, 66(8), 3357–3362.
Couto, S. R., & Sanromán, M. A. (2005). Coconut flesh: A novel raw material for laccase production by Trametes hirsuta under solid-state conditions: Application to Lissamine Green B decolourization. Journal of Food Engineering, 71(2), 208–213.
Roriz, M. S., Osma, J. F., Teixeira, J. A., & Couto, S. R. (2009). Application of response surface methodological approach to optimise Reactive Black 5 decolouration by crude laccase from Trametes pubescens. Journal of Hazardous Materials, 169(1–3), 691–696.
Novotný, Č, Erbanova, P., Cajthaml, T., Rothschild, N., Dosoretz, C., & Šašek, V. (2000). Irpex lacteus, a white rot fungus applicable to water and soil bioremediation. Appl. Microbiol. Biotechn., 54(6), 850–853.
Pazarlıoǧlu, N. K., Sariişik, M., & Telefoncu, A. (2005). Laccase: Production by Trametes versicolor and application to denim washing. Process Biochemistry, 40(5), 1673–1678.
Zille, A. 1996. Laccase reactions for textile applications, PhD Thesis, Universidade do Minho, Portugal.
Sharma, H. S. S., Whiteside, L., & Kernaghan, K. (2005). Enzymatic treatment of flax fibre at the roving stage for production of wet-spun yarn. Enzyme and Microbial Technology, 37(4), 386–394.
Lantto, R., Schönberg, C., Buchert, J., & Heine, E. (2004). Effects of laccase-mediator combinations on wool. Textile Research Journal, 74(8), 713–717.
Selvam, K., Swaminathan, K., & Chae, K. S. (2020). Production and industrial exploitation of bacterial laccases. Bioresource Technology, 35(1), 156.
Kuhad, R.C., Singh, A. and Eriksson, K.E.L. (1997). Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Biotechnology in the pulp and paper industry. 45–125.
Gamelas, J. A., Tavares, A. P., Evtuguin, D. V., & Xavier, A. M. (2005). Oxygen bleaching of kraft pulp with polyoxometalates and laccase applying a novel multi-stage process. Journal of Molecular Catalysis. B, Enzymatic, 33(3–6), 57–64.
Bourbonnais, R., Paice, M. G., Freiermuth, B., Bodie, E., & Borneman, S. (1997). Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Applied and Environmental Microbiology, 63(12), 4627–4632.
Virk, A. P., Sharma, P., & Capalash, N. (2012). Use of laccase in pulp and paper industry. Biotechnology Progress, 28(1), 21–32.
Hüttermann, A., Mai, C., & Kharazipour, A. (2001). Modification of lignin for the production of new compounded materials. Applied Microbiology and Biotechnology, 55(4), 387–394.
Lund, M., & Ragauskas, A. (2001). Enzymatic modification of kraft lignin through oxidative coupling with water-soluble phenols. Applied Microbiology and Biotechnology, 55(6), 699–703.
Valls, C., & Roncero, M. B. (2009). Using both xylanase and laccase enzymes for pulp bleaching. Bioresource Technology, 100(6), 2032–2039.
Virk, A. P., Puri, M., Gupta, V., Capalash, N., & Sharma, P. (2013). Combined enzymatic and physical deinking methodology for efficient eco-friendly recycling of old newsprint. PLoS One, 8(8), e72346.
Yang, J., Wang, Z., Lin, Y., et al. (2017). Immobilized Cerrena sp. laccase: Preparation, thermal inactivation, and operational stability in malachite green decolorization. Scientific Reports, 7, 16429. https://doi.org/10.1038/s41598-017-16771-x
Xu, F. (1999), Laccase, In Flickinger (M.C. and Drew, S.W. eds), Encyclopedia of bioprocess technology: Fermentation, biocatalysis Bioseparation, John Wiley and Sons Inc, New York, pp. 1545–1554.
Han, M. J., Park, H. T., & Song, H. G. (2004). Degradation of phenanthrene by Trametes versicolor and its laccase. Journal of Microbiology, 42(2), 94–98.
Tanaka, T., Tamura, T., Ishizaki, Y., Kawasaki, A., Kawase, T., Teraguchi, M., & Taniguchi, M. (2009). Enzymatic treatment of estrogens and estrogen glucuronide. Journal of Environmental Sciences, 21(6), 731–735.
Nyanhongo, G. S., Couto, S. R., & Guebitz, G. M. (2006). Coupling of 2,4,6-trinitrotoluene (TNT) metabolites onto humic monomers by a new laccase from Trametes modesta. Chemosphere, 64(3), 359–370.
Mate, D. M., & Alcalde, M. (2017). Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microbial Biotechnology, 10(6), 1457–1467.
Kudanga, T., & Le Roes-Hill, M. (2014). Laccase applications in biofuels production: Current status and future prospects. Applied Microbiology and Biotechnology, 98(15), 6525–6542.
Fang, Z., Liu, X., Chen, L., Shen, Y., Zhang, X., Fang, W., & Xiao, Y. (2015). Identification of a laccase Glac15 from Ganoderma lucidum 77002 and its application in bioethanol production. Biotechnology for Biofuels, 8(1), 1–12.
Golz-Berner, K., Walzel, B., Zastrow, L. and Doucet, O. (2004). Cosmetic or dermatological preparation with skin-lightening proteins. WO2004017931.
Morel, O. J., & Christie, R. M. (2011). Current trends in the chemistry of permanent hair dyeing. Chemical Reviews, 111(4), 2537–2561.
Saito, K. O., Ikeda, R., Endo, K., Tsujino, Y., Takagi, M., & Tamiya, E. (2012). Isolation of a novel alkaline-induced laccase from Flammulina velutipes and its application for hair coloring. Journal of Bioscience and Bioengineering, 113(5), 575–579.
Chen, C. Y., Huang, Y. C., Wei, C. M., Meng, M., Liu, W. H., & Yang, C. H. (2013). Properties of the newly isolated extracellular thermo-alkali-stable laccase from thermophilic actinomycetes, Thermobifida fusca and its application in dye intermediates oxidation. AMB Express, 3(1), 1–9.
Di Fusco, M., Tortolini, C., Deriu, D., & Mazzei, F. (2010). Laccase-based biosensor for the determination of polyphenol index in wine. Talanta, 81(1–2), 235–240.
Milligan, C., & Ghindilis, A. (2002). Laccase based sandwich scheme immunosensor employing mediatorless electrocatalysis. An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis, 14(6), 415–419.
Falk, M., Alcalde, M., Bartlett, P. N., De Lacey, A. L., Gorton, L., Gutierrez-Sanchez, C., & Shleev, S. (2014). Self-powered wireless carbohydrate/oxygen sensitive biodevice based on radio signal transmission. PloS One, 9(10), e109104.
Arora, D. S., & Sharma, R. K. (2010). Ligninolytic fungal laccases and their biotechnological applications. Applied Biochemistry and Biotechnology, 160(6), 1760–1788.
Burton, S. G. (2003). Laccases and phenol oxidases in organic synthesis-a review. Current Organic Chemistry, 7(13), 1317–1331.
Wang, H. X., & Ng, T. B. (2004). A novel laccase with fair thermostability from the edible wild mushroom (Albatrelladispansus). Biochemical and Biophysical Research Communications, 319(2), 381–385.
Roggen, E.L., S. Ernst, A. Svendsen, E.P. Friis and C. Von Der Osten. 2001. WO2001083559 A2.
Funding
This work was supported by grant no. 94/PAS from the Pakistan Academy of Science, Islamabad, Pakistan. Moreover, this work is carried out with the help of prestigious material of the libraries and special thanks to the Institute of Industrial Biotechnology, Government College University, Lahore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
We assure the integrity and quality of our research work. It is also stated that there is no plagiarism in this work and all points taken from other authors are well cited in the text. This study is completely independent and impartial.
Research Involving Human Participants and/or Animals
N/A. This research did not involve human participants and/or animals.
Informed Consent
N/A. This research did not involve human participants.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akram, F., Ashraf, S., Haq, I.u. et al. Eminent Industrial and Biotechnological Applications of Laccases from Bacterial Source: a Current Overview. Appl Biochem Biotechnol 194, 2336–2356 (2022). https://doi.org/10.1007/s12010-021-03781-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03781-9