Abstract
Oleoresin capsicum (OC) is an extract of chili pepper containing the active agent capsaicin. In this study, OC-loaded nanoemulsions were prepared by microfluidization and stabilized with sucrose monopalmitate (SMP) and lecithin. The difference in size and distribution of droplets determined the nanoemulsion behavior mainly due to the interaction of emulsifiers between oil and aqueous phase. The hydrophilic interaction between SMP and aqueous phase and the hydrophobic interaction between lecithin and oil phase were monitored with NMR relaxometry. OC nanoemulsion fabricated with SMP showed the best transparency with smallest droplet size (around 34 nm) and stable with glycerol after 28 days at ambient storage. Lecithin containing nanoemulsions showed improved bioactivity as showing antioxidant (0.82 mg DPPH/L) and antimicrobial (3.40 log for Escherichia coli and 4.37 log for Staphylococcus aureus) activity. Finally, results have important implications to determine the appropriate formulation conditions for OC with food-grade surfactants to be used in pharmaceuticals and food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chili peppers are members of the Capsicum spp. under Solanaceae family. Capsicum annuum, Capsicum frutescens, Capsicum chinense, Capsicum pendulum, and Capsicum pubescens are the commonly known species. For industrial applications, mostly C. annuum and C. frutescens are preferred. Spicy, pungent tastes of these two chili peppers come from the group of compounds known as capsaicinoids. This group includes dihydrocapsaicin, nordihydrocapsaicin, homocapsaicin, homodihydrocapsaicins, caprylic acid vanillyl amide, nonylic acid vanillyl amide, and decylicacidvanillyl amide. However, capsaicin and dihydrocapsaicin constitute 90% of the total capsaicinoids [1]. Binding of capsaicin to the transient receptor potential vanilloid (TRPV) ion channel receptor is the key mechanism to give its heat sensation. In pharmacological studies, capsaicin showed anti-cancer, analgesic, anti-inflammatory, and anti-obesity effects and bioactivity against respiratory and nervous system diseases in rodents and humans [2]. Besides, capsaicin is effective on microorganisms and was shown to decrease the action of Staphylococcus aureus, Salmonella typhimurium, Bacillus cereus, Listeria monocytogenes, and Helicobacter pylori [3].
Mostly extraction products or oleoresin forms of spices are used in food formulations to reflect the distinctive property of spices similar to those of the volatile oil form. However, both the oil and oleoresin forms are highly concentrated and can cause changes in the flavor, taste, or odor of the product [4]. Additionally, the dispersion and solubility of these lipophilic substances are low in water-based formulations. For chili peppers, oleoresin capsicum is the extraction product and widely used both as a colorant and flavoring in food formulations. In addition, oleoresin capsicum is a good source of capsaicinoids that contains mainly capsaicin.
In food applications, formulations usually contain a high amount of water that restricts the functionality of capsaicin. Therefore, increasing the solubility and diffusion of capsaicin is crucial to improve its functionality. Nanoemulsions are proven to be good delivery systems for lipophilic substances. They serve many advantages such as enhanced stability, efficient delivery, easy processing, and having a transparent or translucent appearance with small droplet sizes. The small droplet size of nanoemulsions is also directly related to the bigger surface area and improved functionality with flavor release [5]. Small amounts of nanoemulsions may be enough to give the same taste with higher quantities. Further, various food-grade ingredients can be present within nanoemulsion. Choi et al. [6] prepared oleoresin capsicum containing double- and triple-layer nanoemulsions by using alginate and chitosan biopolymers prepared with Tween 80. In a pharmacological study, nanoemulsions emulsified with a mixture of Tween 80 and Span 80 was used to improve the penetration of oleoresin capsicum through rat skin [7]. As a novel method, organogel-based nanoemulsions were used as delivery systems for capsaicin to reduce gastric mucosa irritation in rats. Capsaicin organogel was prepared with solubilization of capsaicin into medium-chain triacylglycerol by using heating and adding sucrose stearate to hot oil mixture. Afterwards, capsaicin-loaded organogel, emulsifier Tween 80, and water were finally homogenized to obtain a nanoemulsion [8]. These studies showed that synthetic emulsifiers like Tween 80 were abundantly used in nanoemulsion formulations. However, nanoemulsion formulations prepared with food-grade components should be developed due to label-friendly trend. Sucrose monopalmitate (SMP) and lecithin are two emulsifiers which are commonly used in food, cosmetic, and pharmaceutical industries due to being non-toxic, edible, and amphiphilic [9, 10]. Sucrose monopalmitate is a good emulsifier for oil-in-water nanoemulsions due to its high hydrophilic–lipophilic balance. A single fatty acid attached to sucrose unit gives hydrophilicity and water solubility to SMP [9]. Conversely, lecithin consists of two fatty acids attached to a phosphate group that has a relatively hydrophobic property with a low HLB value. Moreover, it is a natural emulsifier obtained from various oily seeds such as soybeans, sunflower, and rapeseed [11]. Both of them can form stable, small particle-sized nanoemulsions with a wide range of oil components that were shown in previous studies.
Therefore, in this study, the oleoresin capsicum nanoemulsions were formulated by physical treatments as heating or adding glycerol with food-grade emulsifiers, SMP, and lecithin. Their potential on emulsification, stability, and functionality such as antimicrobial and antioxidant activity were investigated. The information obtained from this study is expected to contribute to design food or other industrial nanoemulsions using oleoresin capsicum as multifunctional ingredient.
Materials and Methods
Materials
Oleoresin capsicum (OC) was purchased from a local food supplier (Alfasol, Gaziantep-Turkey) (SHU 1000 000). Soy lecithin was provided by Smart Kimya (Ankara, Turkey). Sucrose monopalmitate (SMP) was provided by Compass Foods Company (Singapore). Potassium phosphate monobasic anhydrous, sodium phosphate dibasic dihydrate, sodium acetate, ethanol, methanol, ethyl acetate, glacial acetic acid, 2, 2-diphenyl-2-picrylhydrazyl (DPPH), and pure capsaicin (≥ 95%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glycerol, Violet Red Bile Agar (VRBA), nutrient broth, and peptone from meat were supplied from Merck KGaA (Germany). Baird Parker Agar (BPA) with egg yolk tellurite was provided by Nisan Elektronik Ltd., Ankara, Turkey. Distilled water was used in all preparations of solutions and dispersions.
Capsaicin Content in Oleoresin Capsicum
The capsaicin content in oleoresin capsicum was determined by using HPLC system equipped with a Pursuit C18 column Microsorb MV C18 (4.6 × 250 mm, 5 mm) and UV–VIS (ProStar 330 PDA) detector. The mobile phase was a mixture of methanol/water (70:30 v/v). The flow rate was 0.8 mL/min for 15 min at ambient temperature and detection wavelength was 280 nm.
Solution Preparation
A phosphate buffer solution at pH 7.4 was prepared by dissolving 4.56 g of potassium phosphate monobasic anhydrous and 28.87 g of sodium phosphate dibasic dihydrate in 1000 mL of distilled water.
Nanoemulsion Preparation
The colloidal dispersions consisted of 2% (w/w) oil phase (oleoresin capsicum) and 98% (w/w) aqueous phase (buffer solution) which included 2% (w/w) surfactant (SMP or lecithin) and 0–50% (w/w) glycerol similar to previous studies of Qian and McClements [12] and Rao and McClements [13]. All components were weighed into a beaker and pre-homogenized using UltraTurrax (WiseTis Homogenizer; Witeg Labortechnik GmbH, Germany) at 15,000 rpm for 3 min. To investigate the effect of heating in the pre-homogenization period, the coarse emulsion was mixed with a magnetic stirrer and heated to 60 °C for 30 min. After cooled to ambient temperature, the resulting mixture was then homogenized with Ultraturrax at 15,000 rpm for 3 min. Then, the coarse emulsion was processed with microfluidization (Nano Disperser—NLM 100, South Korea) for five passes at 140 MPa pressure.
Determination of Particle Size
The mean particle sizes of nanoemulsions (surface area-based mean diameter, d3,2) were measured with laser diffraction technique (Malvern Mastersizer 3000; Malvern Instruments, UK) right after the preparation at 25 °C temperature. A little amount of nanoemulsion sample was dispersed in approximately 350 mL of continuously stirred distilled water until the obscuration reached 6–8%. This process helped to decrease the multiple scattering problem of nanoemulsions.
Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM) Analysis
The morphology of nanoemulsions prepared with SMP alone, lecithin alone, and distilled water was characterized using TECNAI G2 Spirit BioTwin transmission electron microscope (Philips-FEI, Eindhoven, Netherlands) operated at 80 kV with bright film-imaging mode. Five microliters of 100 times diluted sample was dropped onto 3 mm carbon film-coated copper grid and left dried at 25 °C for 3 h.
AFM image of the same nanoemulsion sample was taken by using Veeco Multimode V atomic force microscope (Veeco, Santa Barbara, CA) equipped with a j-type scanner (ca. 125 × 125 × 5 μm3 scan range) scanned with a tapping mode at a speed of 1 Hz. A 5-μL diluted sample was dropped onto smooth and dry glass surface that was dried in the air.
Turbidity Measurements
The absorbances of nanoemulsions were measured using a UV–Visible spectrophotometer (UV–VIS spectrophotometer, Optizen; Mecasys, Korea) at 600 nm for turbidity measurements right after preparation. The buffer solution was used as the blank.
NMR (Nuclear Magnetic Resonance) Experiments
The spin–spin relaxation times (T2) of nanoemulsions were measured using 0.5 T (22.35 MHz) benchtop system (SpinCore Technologies, Inc., Gainesville, USA) with operating conditions as Carr, Purcell, Meiboom, and Gill (CPMG) pulse sequence with a 90–180 pulse gap (τ) of 1.0 ms, spectral width of 300 kHz, 32 scans, repetition delay of 3 s, and number of echoes of 2500 at 25 °C temperature. Samples were placed in glass tubes with a 10-mm diameter.
Determination of Encapsulation Efficiency
The oleoresin capsicum retention in nanoemulsions was analyzed by using UV–visible spectrophotometer (UV–VIS spectrophotometer, Optizen; Mecasys, Korea) according to the method of Surassmo et al. [14] with slight modifications. A sample (0.3 mL) was added to 4.2 mL of ethyl acetate. The mixture was vortexed for 5 min, then centrifuged for 10 min at 2000 rpm. The collected supernatant was read by UV–VIS spectrophotometer at 451 nm (A1). Ethyl acetate was used as the blank. Efficiency was calculated using the following equation (Eq. 1):
where C1 was the free capsicum oleoresin amount (g) in which A1 values were converted to concentration by using the calibration curve prepared with only oleoresin capsicum.
Color Analysis
The color measurements were done with a benchtop spectrophotometer (model CM-5; Konica Minolta Inc., Japan) in terms of L* (brightness), a* (red/green ratio), and b* (yellow/blue ratio). Rectangular quartz cell was used with optical path of 10 mm. For zero calibration, distilled water was used as L*ref. = 100.0, a*ref. = 0.0, and b*ref. = 0.0.
Stability During Storage
Nanoemulsion samples were tested for 28 days after preparation to assess storage stability. Lecithin-containing nanoemulsions were kept at 4 °C and SMP-containing nanoemulsions were kept at 20 °C due to instability problems at 4 °C. During the storage period, particle size, NMR relaxometry, turbidity, and color measurements were conducted for all samples.
Antioxidant Activity—DPPH Assay
DPPH method was performed following the method of Wang et al. [15] with slight modifications. Each sample was dissolved in ethanol/acetic acid/water mixture (50:8:42, v/v) and vortexed for 1 min. Samples were filtered with a microfilter (0.45 μm Chromafil CA-45/25S; Düren). The same ethanol/acetic acid/water mixture (50:8:42 v/v) was used to dilute the filtered samples. Then, 0.1 mL of diluted sample was mixed with 3.9 mL methanol–DPPH solution (1:100) and left in the dark for 1 h at ambient temperature. The decrease in absorbance of samples at 517 nm was measured with UV–VIS spectrophotometer (A2). Pure methanol was used to make auto zero and 0.1 mL methanol–3.9 mL DPPH solution was used as blank (A1). The absorbance values (A1, A2) were converted to concentrations (C1, C2) using the standard curve prepared with DPPH solutions at 5, 10, 15, 20, and 25 ppm. Hence, activity was calculated as follows (Eq. 2):
Vsample and Vtotal represent the volume of capsaicin emulsion and total volume of the capsaicin emulsion and ethanol/acetic acid/water mixture in milliliters, respectively, and d represents the dilution rate.
Antimicrobial Activity
The antimicrobial activity of nanoemulsions was measured against two bacteria strains of Gram-positive Staphylococcus aureus (ATCC 43300) and Gram-negative Escherichia coli (ATCC 11229). The cultures were obtained from Public Health Institution of Turkey culture collection (Ankara, Turkey) and preserved in nutrient broths at 4 °C in the Department of Food Engineering, METU (Ankara, Turkey). The analysis was done according to the method of Zhang et al. [16] and Salvia-Trujillo et al. [17] with modifications. Bacterial strains were transferred to newly prepared nutrient broth separately and incubated at 37 °C with continuous agitation at 120 rpm for 18 h to adjust concentration up to 108–109 CFU/mL. Active cultures were collected by centrifugation at 3600×g for 10 min and washed twice with sterile saline (0.85% NaCl)–Tween 80 (0.1%) solution.
The test tube contained 0.5 mL of nanoemulsion, 4.5 mL of sterile phosphate buffered solution (PBS, pH 7.4), and aliquots of tested bacteria strain (1% v/v). This mixture was vortexed for 10 s and incubated 15 min at 37 °C for E. coli and 2 h at 35 °C for S. aureus. It was then diluted 105 times with sterile peptone water and 0.1 mL of diluent was spread on VRBA and Baird–Parker agar and incubated at 37 °C for 24 h and 35 °C for 48 h for E. coli and S. aureus, respectively. The tube containing nanoemulsion and PBS without inoculum and tube containing inoculum and PBS without nanoemulsion served as the negative and the positive controls. In addition, all nanoemulsion components were checked for antimicrobial activity.
Statistical Analysis
All measurements were performed in triplicate and reported as means and standard errors. Analysis of variance (ANOVA) was used to analyze significant differences at 5% significance level with the Tukey test by using Minitab (ver.16.2.0.0, Minitab Inc., UK).
Results and Discussion
Particle Size
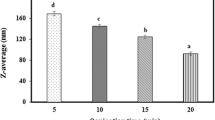
The d3,2 of nanoemulsions prepared with SMP and lecithin are shown in Fig. 1a. The d3,2 of nanoemulsions prepared with SMP was around 35 nm. Adding glycerol or heating the coarse emulsion significantly decreased the d3,2 from 39 up to 33 nm (p < 0.05). There are a number of other studies about SMP containing nanoemulsions showing similar results [18, 19]. In a study, Tween 80 and SMP were used with lemon oil and Tween 80 containing emulsions were shown to have larger mean particle sizes. This was associated with the hydrophilic head group of Tween 80 being bigger than that of SMP. Thus, after the oil and emulsifier were mixed, they formed bigger nanoemulsion droplets in the colloidal dispersion [9]. In contrast, significantly larger d3,2 was obtained with lecithin around 226 nm (p < 0.05, Fig. 1a). The primary reason behind this was that the highly polar environment due to water reduced the solubility of lecithin. Low solubility can contribute to the deposition of lecithin layer around oil droplet and further coalescence or flocculation resulting in bigger particles in the dispersion. To overcome this problem, adding alcohol is listed as one of the solutions [20]. It may be that emulsifiers with higher hydrophilic–lipophilic balance (HLB) values such as SMP are more likely to stabilize dispersed oil droplets in aqueous phase due to attached single fatty acid whereas low HLB numbered emulsifiers like lecithin tend to solubilize water in oil phase due to different numbers and types of fatty acids attached [18]. In addition, Ozturk et al. [10] found that to achieve a smaller particle size of nanoemulsions by using surfactants such as lecithin and quillaja saponin, a higher amount of lecithin (> 2% w/w) should be used relative to the quillaja saponin. Moreover, the addition of glycerol and combined effect with heating resulted in reduction in particle size of lecithin nanoemulsions. Glycerol is a viscous, water-soluble co-solvent and added to nanoemulsions to enhance physicochemical properties such as viscosity, refractive index, and solubility of components. The small droplets in an emulsion have high energies and instability problems may be observed due to an increase in droplet motions. However, changing the environment of the droplets can decrease the motions such as increasing the aqueous phase viscosity by adding glycerol. Furthermore, it may help the solubilization and absorption of surfactants to the interface and serve as another solution [21]. Similar to our results, it was observed that the smallest particle size of vitamin-E nanoemulsions was obtained after 40 and 50% (w/w) glycerol addition [22]. However, in our study, heating decreased the particle size by dissolving the surfactant and causing rearrangement of the surfactant on the surface. It was stated that surfactant head group was dehydrated during heating and droplet disruption became easier due to reduced interfacial tension [23]. In addition, TEM images confirmed the results that the particle sizes were around 200 and 100 nm for lecithin and SMP nanoemulsions, respectively, as shown in Fig. 1b and c. Besides, in the AFM images, relatively bigger particles were observed in lecithin nanoemulsions ranging between 96 nm and 1.07 μm compared to 20 nm to 1 μm for SMP nanoemulsions (Fig. 1d, e). The difference between the TEM and AFM results on particle sizes might be due to the tip broadening effect during the experiments on AFM device. This situation occurred when the tip was in contact with a sticky particle and this widened throughout the surface of the particle during measurement. Also, surface homogeneity of the nanoemulsion sample might have been lost during drying. In the same way, in their study, Salvia-Trujillo et al. [24] found higher particle size results with AFM than dynamic light scattering.
The particle size (d3,2) of oleoresin capsicum nanoemulsions prepared with SMP and lecithin (a). TEM images show nanoemulsions prepared with lecithin (b) and SMP (c). AFM images show nanoemulsions prepared with lecithin (d) and SMP (e). Error bars are standard errors from triplicates. Different letters above the bars in each column indicate significant differences in the mean of the sample (p ≤ 0.05). Small letters compare among SMP, and capital letters compare among lecithin
Turbidity
The turbidity of nanoemulsions was measured right after preparation, and results were given in Fig. 2. Lecithin nanoemulsions showed opaque and had higher turbidity values than SMP nanoemulsions. Similarly, Xue and Zhong [11] found that turbid thymol dispersions were obtained after using lecithin only as the emulsifying agent due to the low solubility of lecithin in aqueous solution. Conversely, almost transparent nanoemulsions were obtained by using SMP. Addition of SMP affected particle size distribution and resulted in monomodal distributed colloidal dispersions that were reflected in the appearance. A positive correlation of 0.98 was found between mean particle sizes and turbidity results of SMP nanoemulsions (p ≤ 0.05). Moreover, the effect of glycerol addition and heating was found to be significant (p ≤ 0.05). Conformational changes occurred within emulsifier molecule because of the changes in temperature. As the head group of nonionic emulsifier, SMP was dehydrated by heating in the aqueous phase, and the packing characteristics, oil–water solubility, the oil–water interfacial tension, and finally the stability of nanoemulsion were affected [21]. In addition, it is possible to think that the droplets gain kinetic energy and accelerating the transition of coarse emulsion to nanoemulsion by heating. Therefore, one would expect it to have transparent appearance. On the other hand, lecithin was found less affected by heating than nonionic SMP. Similarly, with our results, Kahlweit et al. [20] compared nonionic, anionic emulsifiers with lecithin to prepare microemulsions and found that the effect of temperature was less than nonionic and anionic emulsifiers. More transparent nanoemulsions were obtained with glycerol due to increase in the viscosity of the aqueous phase, reduction in droplet size, and a decrease in refractive index contrast between two phases as mentioned before [21, 23, 25].
Turbidity values of oleoresin capsicum nanoemulsions prepared with SMP and lecithin. Error bars are standard errors from triplicates. Different letters above the bars in each column indicate significant differences in the mean of the sample (p ≤ 0.05). Small letters compare among SMP, and capital letters compare among lecithin
Spin–Spin Relaxation Time (T 2) Measurements
The interaction of oil molecules with their surroundings in the aqueous phase is widely investigated with the complementary method of NMR relaxometry. Specifically, 1H NMR is well studied in the literature to determine the molecular structure of an emulsion or hydrogel systems via dynamics of protons. The protons within the sample have magnetic moments that align themselves with different directions under the external magnetic field. When the sample is removed from the magnetic field, the protons relax by turning back to their original state. Meanwhile, the decay of magnetization is expressed by a constant known as T2. This transverse magnetization is related to the energy transfer between neighboring spins and characterized by the spin–spin relaxation time constant (T2). Variations in water content, surrounding molecules, and physical state of water can be observed with NMR experiments as T2 [26]. In our study, the oil molecules of oleoresin capsicum were surrounded by the emulsifier and water molecules. In particular, local conformations of these ingredients changed when they were homogenized. For example, the T2 time of oleoresin capsicum was 77 ms whereas the T2 time of aqueous buffer solution without emulsifier was 2145 ms. Eventually, mobility and arrangement of oil molecule changed with the addition of emulsifier included in the water phase after homogenization [27]. Thus, the overall increase of T2 was observed changing from the highest of 1209.01 ms to the lowest of 488.28 ms after emulsification by using SMP and lecithin, respectively. There is a precise relationship between proximity of molecules and T2 [26]. The protons in oil molecules were in close proximity and thus T2 was the shortest. However, in the aqueous phase, the molecular movement of water was comparably slow along with the long T2. Therefore, in nanoemulsion, the proximity between oil molecules diminished with the attachment of emulsifier as neighboring molecules and the free motion of water molecules was restricted. In Fig. 3, it was seen that the signals (T2) from SMP nanoemulsions were higher than lecithin nanoemulsions. The hydrophilic SMP might be associated with the water phase and adsorbed on the oil surface whereas relatively hydrophobic lecithin might be associated with the oil phase and packed. These intermediate interactions resulted in longer T2 times for SMP and shorter T2 times for lecithin. Further, it was well correlated with the literature that the decrease in relaxation times with emulsifiers was due to changes in the local conformations of oil and water interfaces [28]. Moreover, distribution is related with the mobility and restricted mobility decreases T2 times [29]. In addition, the particle size is related to the interfacial area of the dispersion and larger droplets having lower interfacial area give shorter T2 times [30]. In that regard, it was observed that the T2 times of nanoemulsions at pH 7.4 was around 1091.48 ms (d3,2 = 39.63 nm) and 853.09 ms (d3,2 = 218 nm) for SMP and lecithin, respectively. A notable change observed in the presence of glycerol was the decrease in T2 times (p ≤ 0.05). In this regard, glycerol lowers the mobility of water molecules and samples showed faster relaxations (short T2).
T2 relaxation times of oleoresin capsicum nanoemulsions prepared with SMP and lecithin. Error bars are standard errors from triplicates. Different letters above the bars in each column indicate significant differences in the mean of the sample (p ≤ 0.05). Small letters compare among SMP, and capital letters compare among lecithin
Encapsulation Efficiency
The type and concentration of stabilizing agent of nanoemulsions, which are emulsifiers, are important parameters and significantly affect the encapsulation efficiency and the particle size. An emulsifier with good packing ability around a lipid core in a nanoemulsion gives higher encapsulation efficiency [31]. The results are given in Fig. 4. The maximum encapsulation efficiency for SMP was 71% (average 68.75%) whereas for lecithin it was 63% (average 59.5%). SMP showed better encapsulation behavior on the capsaicin. In the previous studies, retention efficiency of the bioactive components within emulsion increased as the particle size decreased. A possible reason for the observed relation was that decreasing the droplet surface diameter increased the interfacial area and supported even more homogenous dispersion [32]. Similar results were reported by Ozturk et al. [10] that interfacial layer of lecithin containing vitamin E nanoemulsion was flexible and could easily break. In addition, heating might enhance the solubility and conformation around the oil droplet and increased the efficiencies.
Encapsulation efficiencies of oleoresin capsicum nanoemulsions prepared with SMP and lecithin. Error bars are standard errors from triplicates. Different letters above the bars in each column indicate significant differences in the mean of the sample (p ≤ 0.05). Small letters compare among SMP, and capital letters compare among lecithin
Color
In L*, a*, and b* system, L* represents lightness, and a* and b* represent the color coordinates. + a is the red color and − a is the green color while + b is the yellow color and − b is the blue color [25, 33]. There were dramatic differences in the color results of nanoemulsions as given in Fig. 5a–c. The color of an emulsion is mainly determined by absorption of light through the droplets that change with the presence, concentration, and type of the chromophores [33]. Additionally, scattering the light, thus emulsion lightness could also be affected by geometry, composition, and microstructure of the emulsion [25]. At this point, small droplets scatter less light and lightness decreases. The opposite trend was observed in capsaicin nanoemulsions where L* values of SMP nanoemulsions (between 49.8 and 39.11) were significantly higher than lecithin nanoemulsions (between 7.65 and 3.43) (p ≤ 0.05). Besides, L* values of all nanoemulsions were found higher than oleoresin capsicum (L* = 0.18). More interaction with SMP and lecithin and the presence of glycerol samples result in loss of the initial dark color.
Color values L* (a), a* (b), and b* (c) of oleoresin capsicum nanoemulsions prepared with SMP and lecithin. Error bars are standard errors from triplicates. Different letters above the bars in each column indicate significant differences in the mean of the sample (p ≤ 0.05). Small letters compare among SMP, and capital letters compare among lecithin
Carotenoid pigments of capsanthin, capsorubin, and capsanthin 5,6-epoxide are responsible for the red, orange, and yellow color of nanoemulsion samples [34]. Oleoresin capsicum had a dark red color (almost black), whereas SMP and lecithin powders had white and yellow colors, respectively. However, the resulted colors of nanoemulsions were red-colored for SMP and yellow-colored for lecithin. As both the turbidity values and particle sizes of SMP nanoemulsions were lower than lecithin nanoemulsions, bright and red colored nanoemulsions were obtained. This situation reflected itself on the color values being higher. Negative correlations around 0.96 were found between mean particle sizes and L*, a*, and b* values of SMP nanoemulsions (p ≤ 0.05). In addition, opaque lecithin nanoemulsions had very low L*a*b* values as can be seen in Fig. 5. This was not surprising since these emulsions had large particle sizes. This is because the increased surface area of chromophores (capsaicin) covered with surfactants was higher in SMP nanoemulsions. The average a* values varied from 52.01 to 21.52 whereas the b* values ranged from 78.57 to 9.04 for SMP and lecithin nanoemulsions, respectively. The low a* (0.25) and b* (− 0.18) values of oleoresin capsicum disappeared in nanoemulsions.
Emulsion Stability
Nanoemulsions are designed for the commercial applications mostly such as food, beverage, or cosmetic products. Stability studies were carried out on the newly prepared capsaicin nanoemulsions. In fact, particle size measurements are good indicators of the stability and other methods such as turbidity and color analysis may be used for the same purpose. Relaxometry is used to point out the structural changes such as flocculation or aggregation similar to the turbidity analysis. Stability experiments showed that SMP nanoemulsions were less stable than lecithin nanoemulsions as shown in Table 1. After 28 days of storage, there was a visually observable settling in SMP nanoemulsions as seen in Fig. 6 (highlighted) and could not be further analyzed. On the other hand, glycerol containing SMP nanoemulsions showed good stability even after 28 days of storage. It was hypothesized that the structure enhancer glycerol could have retarded the coalescence by creating a barrier around the emulsifier–oil droplet and showed greater stability during storage. Larger particles are more prone to coalescence compared to small particles due to their higher Laplace pressure. Hydrodynamic interactions, colloidal forces, and surface charge become important for larger particles. During the storage time of 28 days, dramatic increases in the particle sizes of lecithin nanoemulsions were observed (p ≤ 0.05) whereas particle sizes of SMP nanoemulsions did not change significantly over time as shown in Table 1 (p > 0.05). As the particles get smaller, their energy becomes higher. However, in that period, small particles tend to reduce their energies with temperature drop around 4 °C during storage, and this might have driven the particles’ merging in lecithin nanoemulsions. Further coalescence could cause oiling-off, which can be defined as the formation of a layer of oil on top of the sample, but in these samples, particles are prone to coalescence locally and not ending with oiling-off. Overall, mean particle sizes and turbidity values increased while T2 and color values decreased significantly with time (p ≤ 0.05). The increase in the proximity of droplets resulted in faster relaxation by limiting the mobility of aqueous phase around the oil droplet [35]. Moreover, small particles are more prone to Ostwald ripening or coalescence rather than flocculation [36, 37]. Even though the particle size did not increase, merged particles resulted in an increase in turbidity values and decrease in T2 and color values of SMP nanoemulsions. Glycerol added to SMP nanoemulsions still looked transparent at the end of 28 days and might be considered as one of the most promising stable nanoemulsion formulations for further studies with oleoresin capsicum.
Antioxidant Activity—DPPH Assay
Okada et al. [38] found that phenolic OH group of capsaicin molecule was responsible for the antioxidant activity. Therefore, encapsulating the functional component, capsaicin, within nanoemulsion may help to maintain its antioxidant activity in water-based food systems. Besides, dispersion of oil phase and adsorption behavior of surfactant molecule within nanoemulsion may affect this antioxidant activity of capsaicin. Homogenization leads to reorientation of surfactant molecule to the water–oil interface, easing the formation of small oil droplets and dispersing them uniformly throughout the system [33]. DPPH experiment results showed that lecithin nanoemulsions had higher antioxidant activities than SMP nanoemulsions. In Fig. 7, the addition of glycerol decreased the antioxidant activity of pre-heated lecithin nanoemulsions. However, in SMP nanoemulsions heating or addition of glycerol did not affect the antioxidant activity significantly (p > 0.05). It was hypothesized that the solubilization of capsaicin was higher in lecithin nanoemulsion due to hydrophobic properties of lecithin. However, the decrease with the effect of glycerol and heating might come from the changing physical properties of oil–water interface and further the location of the oil droplets. It was found that antioxidant activity of emulsions was not related to the concentration of antioxidants, but their locations in emulsions may have originated from heterogeneity [39]. Overall, one of the issues that were obtained from these findings was the quantification of the antioxidant potential of capsaicin nanoemulsions prepared with surfactant SMP and lecithin by using the DPPH method. In that regard, capsaicin nanoemulsions under study exhibited good antioxidant properties and might be used effectively to control lipid oxidation.
The antioxidant activity of oleoresin capsicum nanoemulsions prepared with SMP and lecithin. Error bars are standard errors from triplicates. Different letters above the bars in each column indicate significant differences in the mean of the sample (p ≤ 0.05). Small letters compare among SMP, and capital letters compare among lecithin
Antimicrobial Activity
Since the main component in oleoresin capsicum was capsaicin, the amount of capsaicin in oleoresin capsicum was determined before testing the antimicrobial activity. It was found as 51.0650 ± 0.0919 mg/mL capsaicin in oleoresin capsicum. The antimicrobial activities of prepared nanoemulsions are shown in Fig. 8a and b. Both S. aureus and E. coli populations decreased after contact with nanoemulsions significantly (p ≤ 0.05). In lecithin nanoemulsions, the microbial reduction ranged from 2.84 to 3.40 log for E. coli within 15 min of contact time and from 3.57 to 4.37 log for S. aureus within 2 h of contact time. According to these results, S. aureus showed slightly higher reduction than E. coli. It is likely that phospholipid parts of membranes eased the entrance of lecithin-covered droplets which had also phospholipid parts that lead to a higher decrease on the cell hydrophobicity by capsaicin. Based on this explanation, higher antimicrobial activity was expected from these lecithin-containing nanoemulsions, but it was important to mention that all lecithin molecules could not integrate with capsaicin droplets. Gill et al. [40] stated that bacterial cells could be expected to repair themselves in nutrient-rich media such as lecithin. Thus, free lecithin molecules might have been used in repairing the damaged cell membrane by E. coli which might have limited the antimicrobial activity. Remarkably, the antimicrobial activities of SMP nanoemulsions were found much lower than lecithin nanoemulsions. The highest log reduction observed was 1.07 log for E. coli and 2.75 log for S. aureus with SMP nanoemulsions. One possible reason for differences in results may be that sucrose created proper growth conditions for the microorganisms and capsaicin droplets could not exhibit its hydrophobicity. At low concentrations, sucrose generally does not show the inhibitory effect as it is a carbohydrate source, and moreover, it is used to contribute to microorganism growth. On the contrary, higher concentrations of sucrose showed inhibitory activity due to water binding ability which limits the available water for the growth of microorganisms [41]. In addition, there is no significant difference between treatments of SMP nanoemulsions for S. aureus (p > 0.05) whereas individual or combined effect of glycerol and heating increased the log reduction for lecithin nanoemulsions. Conversely, glycerol addition expanded the log reduction to double for E. coli. It may be the case that in the presence of glycerol, the solubility and distribution of capsaicin increase within SMP containing aqueous phase. There is also another phenomenon that may relate to the difference in antimicrobial activity results as hydrophobicity differences between cell membranes of E. coli and S. aureus. In contrast to hydrophobic thick peptidoglycan layer of Gram-positive bacteria, there is an outer hydrophilic membrane of Gram-negative bacteria cell wall which shows resistance to pass lipophilic essential oils [42]. In the work conducted by Thomas et al. [43], the inhibitory effects of nisin on several Gram-positive and Gram-negative bacteria strains were explored. Sucrose fatty acid esters were used to enhance the incorporation of nisin in the study. It was observed that Gram-positive bacteria were affected more than Gram-negative ones. The same result was demonstrated in our study. Besides, SMP might have masked the antimicrobial activity of capsaicin while forming higher stability and appealing nanoemulsions. Therefore, the maximum inhibitory activity for all formulations among nanoemulsions was obtained by lecithin and glycerol containing heated nanoemulsions with 3.40 log reduction against E. coli whereas for S. aureus it was again with lecithin containing heated nanoemulsions with a value of 4.43 log.
Population decrease of Escherichia coli (a) and Staphylococcus aureus (b) after contact with oleoresin capsicum nanoemulsions. Population decrease means the ratio of survived microorganisms (CFU/mL) to inoculum culture (CFU/mL). Data are presented as mean ± standard error (p ≤ 0.05). ANOVA was conducted for each microorganism. Different letters above the bars in each column indicate significant differences in the mean of the sample (p ≤ 0.05). Small letters compare among SMP, and capital letters compare among lecithin
Conclusion
This study showed that oleoresin capsicum nanoemulsions with very small stable droplets can be obtained using sucrose monopalmitate as the surfactant in the presence of glycerol. The reduced droplet size with the changing refractive index after the addition of glycerol enhanced the transparent appearance and red color of SMP nanoemulsions. However, SMP nanoemulsions were unstable without glycerol when compared to nanoemulsions stabilized by lecithin. Although instability problems such as Ostwald ripening and coalescence caused droplet growth, lecithin nanoemulsions were stable after 28 days of storage at 4 °C. Heating helped to dissolve the lecithin, increased the interaction with oleoresin capsicum in the colloidal dispersion, and formed effective droplets resulting in higher antioxidant activity. Higher log reductions in E. coli and S. aureus populations were observed with lecithin nanoemulsions due to similarities between phospholipid parts of membranes and lecithin. Higher surfactant solubility in oil phase increased the bioactive capacities of the encapsulated component, whereas high surfactant solubility in aqueous phase increased the physical properties of oil-in-water nanoemulsion. NMR T2 relaxation results provided further support on the dispersion of oil phase or glycerol. Finally, by using the parameters obtained from this work, industrial-based packaging materials, biofilms, or pharmaceutical products could be designed as well as the research may be expanded to other surfactants, processing parameters, or pathogens to find the optimum formulations.
References
Al Othman, Z. A., Ahmed, Y. B. H., Habila, M. A., & Ghafar, A. A. (2011). Determination of capsaicin and dihydrocapsaicin in capsicum fruit samples using high performance liquid chromatography. Molecules (Basel, Switzerland), 16(10), 8919–8929. https://doi.org/10.3390/molecules16108919.
Rollyson, W. D., Stover, C. A., Brown, K. C., Perry, H. E., Stevenson, C. D., McNees, C. A., et al. (2014). Bioavailability of capsaicin and its implications for drug delivery. Journal of Controlled Release, 196, 96–105. https://doi.org/10.1016/j.jconrel.2014.09.027.
Dima, C., Coman, G., Cotarlet, M., Alexe, P., & Dima, Ş. (2013). Antioxidant and antibacterial properties of capsaicine microemulsions. 6th International Symposium Euro-Aliment 2013, October 3–5, 2013, Galati—ROMANIA, 37(1), 39–49.
Kanakdande, D., Bhosale, R., & Singhal, R. S. (2007). Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin and modified starch. Carbohydrate Polymers, 67(4), 536–541. https://doi.org/10.1016/j.carbpol.2006.06.023.
McClements, D. J. (2012). Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter, 8(6), 1719–1729. https://doi.org/10.1039/c2sm06903b.
Choi, A.-J., Kim, C.-J., Cho, Y.-J., Hwang, J.-K., & Kim, C.-T. (2011). Characterization of capsaicin-loaded nanoemulsions stabilized with alginate and chitosan by self-assembly. Food and Bioprocess Technology, 4(6), 1119–1126. https://doi.org/10.1007/s11947-011-0568-9.
Kim, J. H., Ko, J. A., Kim, J. T., Cha, D. S., Cho, J. H., Park, H. J., & Shin, G. H. (2014). Preparation of a capsaicin-loaded nanoemulsion for improving skin penetration. Journal of Agricultural and Food Chemistry, 62(3), 725–732. https://doi.org/10.1021/jf404220n.
Lu, M., Cao, Y., Ho, C.-T., & Huang, Q. (2016). Development of organogel-derived capsaicin nanoemulsion with improved bioaccessibility and reduced gastric mucosa irritation. Journal of Agricultural and Food Chemistry, 64. https://doi.org/10.1021/acs.jafc.6b01095, 23, 4735, 4741.
Rao, J., & McClements, D. J. (2012). Lemon oil solubilization in mixed surfactant solutions: rationalizing microemulsion & nanoemulsion formation. Food Hydrocolloids, 26(1), 268–276. https://doi.org/10.1016/j.foodhyd.2011.06.002.
Ozturk, B., Argin, S., Ozilgen, M., & McClements, D. J. (2014). Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural surfactants: Quillaja saponin and lecithin. Journal of Food Engineering, 142, 57–63. https://doi.org/10.1016/j.jfoodeng.2014.06.015.
Xue, J., & Zhong, Q. (2014). Blending lecithin and gelatin improves the formation of thymol nanodispersions. Journal of Agricultural and Food Chemistry, 62(13), 2956–2962. https://doi.org/10.1021/jf405828s.
Qian, C., & McClements, D. J. (2011). Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocolloids, 25(5), 1000–1008. https://doi.org/10.1016/j.foodhyd.2010.09.017.
Rao, J., & McClements, D. J. (2011). Food-grade microemulsions, nanoemulsions and emulsions: fabrication from sucrose monopalmitate & lemon oil. Food Hydrocolloids, 25(6), 1413–1423. https://doi.org/10.1016/j.foodhyd.2011.02.004.
Surassmo, S., Min, S.-G., Bejrapha, P., & Choi, M.-J. (2010). Effects of surfactants on the physical properties of capsicum oleoresin-loaded nanocapsules formulated through the emulsion–diffusion method. Food Research International, 43(1), 8–17. https://doi.org/10.1016/j.foodres.2009.07.008.
Wang, H., Gao, X. D., Zhou, G. C., Cai, L., & Yao, W. B. (2008). In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chemistry, 106(3), 888–895. https://doi.org/10.1016/j.foodchem.2007.05.068.
Zhang, H., Shen, Y., Weng, P., Zhao, G., Feng, F., & Zheng, X. (2009). Antimicrobial activity of a food-grade fully dilutable microemulsion against Escherichia coli and Staphylococcus aureus. International Journal of Food Microbiology, 135(3), 211–215. https://doi.org/10.1016/j.ijfoodmicro.2009.08.015.
Salvia-Trujillo, L., Rojas-Graü, M. A., Soliva-Fortuny, R., & Martín-Belloso, O. (2014). Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control, 37, 292–297. https://doi.org/10.1016/j.foodcont.2013.09.015.
Choi, S. J., Decker, E. A., Henson, L., Popplewell, L. M., Xiao, H., & McClements, D. J. (2011). Formulation and properties of model beverage emulsions stabilized by sucrose monopalmitate: influence of pH and lyso-lecithin addition. Food Research International, 44(9), 3006–3012. https://doi.org/10.1016/j.foodres.2011.07.007.
Henry, J. V. L., Fryer, P. J., Frith, W. J., & Norton, I. T. (2009). Emulsification mechanism and storage instabilities of hydrocarbon-in-water sub-micron emulsions stabilised with Tweens (20 and 80), Brij 96v and sucrose monoesters. Journal of Colloid and Interface Science, 338(1), 201–206. https://doi.org/10.1016/j.jcis.2009.05.077.
Kahlweit, M., Busse, G., & Faulhaber, B. (1995). Preparing microemulsions with lecithins. Langmuir, 11(5), 1576–1583. https://doi.org/10.1021/la00005a027.
Rao, J., & McClements, D. J. (2013). Optimization of lipid nanoparticle formation for beverage applications: influence of oil type, cosolvents, and cosurfactants on nanoemulsion properties. Journal of Food Engineering, 118(2), 198–204. https://doi.org/10.1016/j.jfoodeng.2013.04.010.
Saberi, A. H., Fang, Y., & McClements, D. J. (2013). Effect of glycerol on formation, stability, and properties of vitamin-E enriched nanoemulsions produced using spontaneous emulsification. Journal of Colloid and Interface Science, 411, 105–113. https://doi.org/10.1016/j.jcis.2013.08.041.
Wooster, T. J., Golding, M., & Sanguansri, P. (2008). Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir, 24(22), 12758–12765. https://doi.org/10.1021/la801685v.
Salvia-Trujillo, L., Rojas-Graü, M. A., Soliva-Fortuny, R., & Martín-Belloso, O. (2013). Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil–alginate nanoemulsions. Food Hydrocolloids, 30(1), 401–407 https://doi.org/10.1016/j.foodhyd.2012.07.004.
McClements, D. J. (2002). Theoretical prediction of emulsion color. Advances in Colloid and Interface Science, 97(1–3), 63–89. https://doi.org/10.1016/S0001-8686(01)00047-1.
Kirtil, E., & Oztop, M. H. (2016). 1H nuclear magnetic resonance relaxometry and magnetic resonance imaging and applications in food science and processing. Food Engineering Reviews, 8(1), 1–22. https://doi.org/10.1007/s12393-015-9118-y.
Jenning, V., Ma, K., & Gohla, S. H. (2000). Solid lipid nanoparticles (SLN™) based on binary mixtures of liquid and solid lipids: a 1 H-NMR study. International Journal of Pharmaceutics, 205(1-2), 15–21.
Le Botlan, D., Wennington, J., & Cheftel, J. C. (2000). Study of the state of water and oil in frozen emulsions using time domain NMR. Journal of Colloid and Interface Science, 226(1), 16–21. https://doi.org/10.1006/jcis.2000.6785.
Capitani, D., Segre, A. L., & Sparapani, R. (1991). Lecithin microemulsion gels: a NMR study of molecular mobility based on line widths. Langmuir, 7(2), 250–253.
Vermeir, L., Sabatino, P., Balcaen, M., Van Ranst, G., & Van Der Meeren, P. (2014). Food hydrocolloids evaluation of the effect of homogenization energy input on the enclosed water volume of concentrated W/O/W emulsions by low-resolution T2-relaxometry. Food Hydrocolloids, 34, 34–38. https://doi.org/10.1016/j.foodhyd.2013.01.024.
Mora-Huertas, C. E., Fessi, H., & Elaissari, A. (2010). Polymer-based nanocapsules for drug delivery. International Journal of Pharmaceutics, 385(1–2), 113–142. https://doi.org/10.1016/j.ijpharm.2009.10.018, 142.
Li, P.-H., & Lu, W.-C. (2015). Effects of storage conditions on the physical stability of d-limonene nanoemulsion. Food Hydrocolloids, 53, 1–7. https://doi.org/10.1016/j.foodhyd.2015.01.031.
McClements, D. J. (1999). Food emulsions: principles, practice, and techniques. CRC series in contemporary food science. (Vol. 10). https://doi.org/10.1016/S0924-2244(99)00042-4, 6-7, 241.
Hornero-Méndez, D., & Mínguez-Mosquera, M. I. (2001). Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika and red pepper oleoresins. Journal of Agricultural and Food Chemistry, 49(8), 3584–3588. https://doi.org/10.1021/jf010400l.
Kirtil, E., Oztop, M. H., Sirijariyawat, A., Ngamchuachit, P., Barrett, D. M., & McCarthy, M. J. (2014). Effect of pectin methyl esterase (PME) and CaCl2 infusion on the cell integrity of fresh-cut and frozen-thawed mangoes: an NMR relaxometry study. Food Research International, 66, 409–416. https://doi.org/10.1016/j.foodres.2014.10.006.
Li, P.-H., & Chiang, B.-H. (2012). Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrasonics Sonochemistry, 19(1), 192–197. https://doi.org/10.1016/j.ultsonch.2011.05.017.
McClements, D. J. (2012). Advances in fabrication of emulsions with enhanced functionality using structural design principles. Current Opinion in Colloid and Interface Science, 17(5), 235–245. https://doi.org/10.1016/j.cocis.2012.06.002.
Okada, Y., Tanaka, K., Sato, E., & Okajima, H. (2010). Kinetics and antioxidative sites of capsaicin in homogeneous solution. Journal of the American Oil Chemists' Society, 87(12), 1397–1405. https://doi.org/10.1007/s11746-010-1628-4.
Huang, S.-W., Frankel, E. N., Schwarz, K., & German, J. B. (1996). Effect of pH on antioxidant activity of α-tocopherol and Trolox in oil-in-water emulsions. Journal of Agricultural and Food Chemistry, 44(9), 2496–2502. https://doi.org/10.1021/jf960262d.
Gill, A. O., Delaquis, P., Russo, P., & Holley, R. A. (2002). Evaluation of antilisterial action of cilantro oil on vacuum packed ham. International Journal of Food Microbiology, 73(1), 83–92. https://doi.org/10.1016/S0168-1605(01)00712-7.
Marshall, D. L., & Bullerman, L. B. (1994). Antimicrobial properties of sucrose fatty acid esters. In C. C. Akoh & B. G. Swanson (Eds.), Carbohydrate polyesters as fat substitutes (pp. 149–168). New York: Marcel Dekker.
Donsì, F., & Ferrari, G. (2016). Essential oil nanoemulsions as antimicrobial agents in food. Journal of Biotechnology, 233, 106–120. https://doi.org/10.1016/j.jbiotec.2016.07.005.
Thomas, L. V., Davies, E. a., Delves-Broughton, J., & Wimpenny, J. W. (1998). Synergist effect of sucrose fatty acid esters on nisin inhibition of gram-positive bacteria. Journal of Applied Microbiology, 85(6), 1013–1022.
Acknowledgements
This study was funded by The Scientific and Technological Research Council of Turkey (TÜBİTAK) (grant number 214O436). COST Action CA 15209 European Network on Relaxometry is also acknowledged as some of the findings are discussed in the action’s network meetings and suggestions were taken into consideration in the final text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
In this study, principles of ethical and professional conduct have been followed.
Human and Animal Studies
This study does not involve research on human participants and/or animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Akbas, E., Soyler, U.B. & Oztop, M.H. Physicochemical and Antimicrobial Properties of Oleoresin Capsicum Nanoemulsions Formulated with Lecithin and Sucrose Monopalmitate. Appl Biochem Biotechnol 188, 54–71 (2019). https://doi.org/10.1007/s12010-018-2901-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2901-5