Abstract
Mesenchymal stem cells have been extensively used for cell-based therapies especially in neuronal diseases. Studies still continue to delineate mechanisms involved in differentiating mesenchymal stem cells into neuronal cells under experimental conditions as they have low mortality rate and hence, the number of cells available for experiments is much more limited. Culturing and differentiating of neuronal cell is more challenging as they do not undergo cell division thus, bringing them to differentiate proves to be a difficult task. Here, the aim of this study is to investigate whether Juglans regia L. (walnut oil) differentiates multipotent, C3H10T1/2 cells, a murine mesenchymal stem cell line, into neuronal cells. A simple treatment protocol induced C3H10T1/2 cells to exhibit a neuronal phenotype. With this optimal differentiation protocol, almost all cells exhibited neuronal morphology. The cell bodies extended long processes. C3H10T1/2 cells were plated and treated with walnut oil post 24 h of plating. The treatment was given (with walnut oil treated cultures with or without control cultures) at different concentrations. The cultured cells were then stained with cresyl violet acetate solution which was used to stain the Nissl substance in the cytoplasm of the induced neuronal culture. The results indicated that the C3H10T1/2 cells differentiated into neuronal-like cells with long outgrowths of axon-like structures able to take up the cresyl violet acetate stain indicating their preliminary differentiation into neuronal-like morphology with walnut oil treatment. Treating the mesenchymal stem cells can in future establish a cultured mesenchymal stem cell line as neuronal differentiating cell line model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryonic stem (ES) cells can be cultured to retain their ability contributing to all cell lineages. This implies that the cells can generate signals necessary to differentiate into various cell types. ES cells renew and differentiate indefinitely under appropriate conditions, and give rise to a wide range of mature cell types. Embryonic-derived mesenchymal stem cells (MSCs) constitute an unlimited source of differentiated cells, which can be used in pharmacological studies and medicine. MSCs, being multipotent cells, have the ability to self-renew, proliferate, and differentiate into a variety of cell types such as adipose tissue, tendon, stroma, osteocytes, and cartilages [1]. Having the ability to differentiate into various specialized cells, stem cells can be of great value under physiological or experimental conditions [2], and can overcome their commitment to differentiate into neuronal, cardiac, hepatocytes, and skeletal cells [3]. MSCs are relatively easily separated and grown in culture by many folds without losing their stem cell characteristics provided they are cultured under specific experimental conditions. MSCs are, therefore, of great importance in stem cell therapy with wide tissue distribution and multipotent differentiation. These properties suggest their critical role in injury healing especially when MSCs have the ability to differentiate into neural-like cells under in vitro and in vivo conditions [4–8]. MSCs being one of the two predominant non-hematopoietic stem cell types, display tremendous therapeutic properties including regenerative therapy for neurodegenerative disorders [9, 10]. The mesodermal MSCs are progenitors committed to differentiate into mesodermal lineage [11] and have also been induced using collagen scaffolds [12]. However, in addition to their differentiation to mesodermal lineage, their differentiation to neuro-ectodermal lineage elucidates their crucial role in neurogenesis [13, 14].
Since MSCs, have been given chemical induction using β-mercaptoethanol (BME), DMSO and butylated hydroxyanisole (BHA), growth factors, and 5-Aza-C [15], no study shows whether natural extracts have the capability to induce neuronal-like morphology in MSCs. The ability to produce in vitro cultures of neuronal cells is difficult and a fundamental task to understand the functioning of the nervous system. Their culturing is particularly challenging since mature neurons do not undergo cell division, and neuronal cell lines are difficult to culture and require utmost care. Many neuronal induction media can be used to differentiate and study neuronal cells. Preliminary evidence does highlight the ability of ES cells to differentiate into neuronal-like cells in vitro [16, 17]. However, it still remains enigmatic whether these cells can differentiate into the whole spectrum of functional neurons and glial cells.
Walnuts are the most widespread tree nuts in the world [18]. Being rich in α-linolenic acid (ALA; 18:3n23) and linoleic acid (LA; 18:2n26) as well as other polyphenolics, phytosterols, and micronutrients [19], walnuts play an important role in reducing inflammation and oxidative stress in the aging brain [20]. High compositions of n-3 and n-6 omega-3 fatty acids make walnuts as important nutritional factors for brain health. The extracts of walnut oil (WO) reduce the antioxidant production, and amyloid beta-protein (Aβ) induced cell death under in vitro conditions [21]. Neurons being sensitive in nature require neuroprotective effects against H2O2 production as well [22]. In the present study, we represent a possibility of MSCs to differentiate into neuronal differentiated cell line model using WO. However, it remains unclear whether these neuronal-like cells display functional activities which perform similar tasks to neurons and glial cells. Once differentiated and transplanted into rodent models exhibiting neuronal diseases, these MSC can be studied to survive, proliferate and migrate to the site of injury, and observed for their capability of improving functional recovery. Further purification of the neuronal cell progeny can be expanded for screening assays and drug discovery for neurological disorders. Therefore, WO properties and extracts can be used to provide a unique low-cost experimental model to study early steps of neuronal-like differentiation.
Materials and Methods
In vitro cell culture studies employing adherent monolayer culture of mouse mesenchymal stem cells (mMSC), C3H10T1/2, are cultured for continuous 8 days with respective doses of WO, RA with or without DMSO, and compared to the control (C) culture. Cresyl violet stain and reverse transcriptase-polymerase chain reaction (RT-PCR) analysis is used to further establish the differentiation of neuronal-like cells. All images are taken at×40/×10 with phase contrast microscope.
Cell Culture
C3H10T1/2 cells (National Centre for Cell Science, Pune) are cultured in DMEM (Cellgro) with 4.5 g/L glucose supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU/mL penicillin/100 μg/mL streptomycin (Sigma). Cells are kept in incubator at 37 °C and 5%/95% CO2 /air grown at 37 °C. After attaining 70% confluency, cells are treated for neuronal induction and further assessed for morphological changes, uptake cresyl violet staining, and RT-PCR analysis. The MSC characteristics of the cell lines are confirmed by flow cytometry prior to release by the repository cell.
Neuronal Induction
To induce neuronal differentiation, subconfluent cultures of murine C3H10T1/2 cell line are maintained and seeded into plastic culture flasks at a density of 50,000 cells/cm2, and maintained in DMEM/10% FBS. Prior to neuronal induction, fresh media is replaced. To initiate neuronal differentiation, cells are treated with WO at different concentrations (5, 10, 50 μL) with/without dimethylsulfoxide (DMSO) (Sigma).
Cresyl Violet Staining
Cresyl violet acetate staining is used to stain Nissl substances in the cytoplasm of neurons formed using formalin solution to identify the formation of neuropil in neuronal cells. Reagents are prepared with 10% formalin solution in Ca++/Mg++ free phosphate buffered saline. 0.5% of cresyl violet staining was prepared in 0.6% glacial acetate solution (further stored in dark bottle for future use). Treated cultured cells are removed from the incubator and growth medium is aspirated. The monolayer culture is gently washed twice with PBS. PBS is then aspirated and cells are fixed in formalin solution for 20 min at room temperature. Enough fixative is used as to cover the cell monolayer. After 20 min, the fixative is aspirated and the monolayer is gently washed twice with PBS. After washing, PBS is aspirated and staining solution is added to the cells. Enough solution is used as to cover the cell monolayer. Cells are then incubated for 30 min at room temperature. Staining medium is removed, and the cell monolayer is gently washed three times with PBS. To visualize the cells, cell monolayer is covered with PBS and evaluated. The staining results are viewed in bright field mode at ×10 or ×40 magnifications.
RNA Isolation and RT-PCR
Total RNA is isolated from WO treated C3H10T1/2 using TRIzol reagent (Invitrogen). RT-PCRs is carried out using SuperScriptIII One-Step RT-PCR kit (Invitrogen) according to manufacturer’s protocol in Master cycler gradient PCR (Eppendorf) machine. Primers (forward-F and reverse-R) for the respective genes are designed and procured from Genex, India (Table 1). The thermal cycler is programmed for cDNA synthesis followed by PCR amplifications. Amplification of murine nestin and β-actin reaction cycle consist of a 2-min denaturation at 95 °C, followed by 36 cycles at 94 °C for 30 s, 56 °C for 30 s, and 68 °C for 30 s. Amplified products are then resolved following electrophoresis on a 2% gel containing ethidium bromide (0.5 μg/mL). Results are evaluated by ImageJ (National Institute of Health, USA).
Statistical Analysis
Statistical analysis is performed using SPSS v14.0 software. Data was presented using unpaired Student’s t test as per the experimental design. The results were considered statistically significant at p < 0.05 where ‘*’ represents significance Test (WO) vs. Control (C).
Results
Experimental Procedure and Morphological Changes on Differentiation of C3H10T1/2 Neuronal Precursor Cell Differentiation
At day 0, C3H10T1/2 cells are plated at low density in a 6-well plate (5 × 103 cells per 10 cm dish) in DMEM medium containing FBS. Figure 1 shows the experimental model of the methodology employed. After 24 h, cells are observed to adhere to a monolayer and treated with WO at concentrations 5, 10, and 50 μL. Observations are made after every 24 h. Cultured cells are allowed to proliferate and spread progressively to form monolayer of differentiated cells. RA (positive control) and DMSO (negative control) treated cells show normal morphology; however, cells treated with WO show neuronal-like extensions with dendritic shape on day 3 (Fig. 2). Further, visual examination on the cells is performed. Cells treated with WO continue to differentiate neuronal phenotype with long neuronal processes till day 7–8 (Fig. 3a, b). However, the viability of neuronal cells decreases after day 7 where cell death is observed at day 9, which may be due to their inability to divide further. Therefore, it is observed that WO at a concentration of 10 μL exhibits more neuronal-like morphology as compared to the cells dosed with 5 μL of WO. This confirms that C3H10T1/2 induced with WO showed neuronal differentiation to which they start to die out after day 8. This further confirms that WO possesses properties to induce neuronal-like morphology in mouse MSCs.
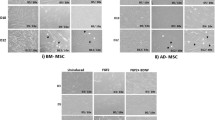
mMSC neuronal differentiation observation at day 1–3. Undifferentiated mMSC, control cells (C) (a, f, k); cells treated with DMSO (negative control) (b, g, l); cells treated with RA (c, h, m); cells treated with low and high dose concentration of WO, 5 μL (d, i, n) and 10 μL (e, j, o). Control cells show normal physiology between days 1–3, whereas cells treated with WO show formation of long extensive processes like neurons
a mMSC neuronal differentiation observation at day 4–8. Undifferentiated mMSC (C) (a, f, k, p); cells treated with DMSO (b, g, l, q); cells treated with RA (c, h, m, r); cells treated with low and high dose concentration of WO, 5 μL (d, i, n, s) and 10 μL (e, j, o, t). Neuronal cells surviving till day 8 of WO treatment (u, v). Death of neuronal cells on day 9 (x, y). b Detailed comparison of Control (a) vs. WO treated cells on day 7 (b)
Uptake of Cresyl Violet Stain in Differentiated Neuronal Cells
The differentiation of C3H10T1/2 into cells of neuronal lineages is accompanied by striking changes by day 3 as compared to the control cells with visible formation of dendritic and axonal-like structures. The differentiation of the neuronal cells is monitored over day 9; however, perfect morphology is actually observed on day 7. The cells were processed for cresyl violet acetate staining to observe and stain the presence of Nissl substance in the cytoplasm of the differentiated neuronal cells to confirm that the cells are indeed neuronal cells. The staining of the possibly differentiated neuronal-like cells show nuclei stain a light blue/violet color with Nissl bodies appearing dark violet with background remaining colorless (Fig. 4). Therefore, the results show uptake of staining solution by the presence of Nissl bodies in the neuronal-like cells.
Phase contrast image of differentiated mMSC into neuronal cells stained with cresyl violet. a Control cells showing no formation of Nissl granules and fail to uptake the stain. b RA treated cells also show no uptake of cresyl violet stain. Cells treated with WO show formation of Nissl’s granules after differentiation into neuronal cells (black arrows) (c, d)
Expression Levels of mRNA of Neuronal Specific Gene, Nestin, on Neuronal Cell Differentiation
In order to observe whether the neuronal-like cells, induced by WO, show expression of nestin, total RNA is isolated from control and WO induced the primary differentiation of MSCs from day 7. RT-PCR analysis is carried to identify neuronal marker, nestin, in order to confirm whether MSC’s showed differentiation to neuronal-like cells. Following reverse transcription, the products are PCR amplified employing primers specific to mice nestin gene. The electrophoretogram of the amplified products, shown in Fig. 5a demonstrates band intensification corresponding to mice nestin gene in the differentiated neuronal cells. The level of this amplification is quantified employing densitometric analysis of the nestin gene over the house keeping gene, β-actin (Fig. 5b). The results demonstrated that nestin expression was observed in WO treated cells at 10 μL as compared to the control and DMSO treated cells, which do not show to express nestin. Therefore, these results demonstrate that WO treatment at 10 μL significantly differentiated mesenchymal C3H10T1/2 cells into neuronal cells with nestin biomarker showing detectable mRNA expression levels.
a RT-PCR analysis of neuronal marker, nestin being expressed in WO treated cells confirming mMSC, C3H10T1/2, differentiated into neuronal-like cells. b Average densitometric quantification of three independent experiments. Each bar represents the mean ± SEM (n = 3) of the experiment done in duplicate. *p < 0.05, vs. C
Discussion
In this study, we aimed to investigate whether MSCs derived from mice bone marrow stem cells, C3H10T1/2, could differentiate into neuronal precursor cells by WO. By using the 10 μL of WO as alternate to neuronal growth or supplement, this protocol is performed in order to develop an in vitro cell-based model of neuronal cell lines which otherwise are expensive and difficult to culture. This experiment is expected to release further information that mice MSCs can be induced to neuronal cells and further WO properties can initiate gene expressions, which otherwise remain silent, necessary to induce neuronal differentiation. MSCs have ability to differentiate into neuronal cells which can be found in all parts of the brain [23]. Human MSCs have tendency to migrate and survive similarly to mice astrocytes when the cells are grafted in mice stratum [24]. However, upon grafting, they lose mesenchymal cell markers. Similarly here, the bone marrow mice mesenchymal stem cells had tendency to convert into neuronal-like cells upon induction with WO. It may be possible that the WO may be the induction medium in expressing gene regulatory network necessary for neuronal differentiation. Hence, the properties of WO can be further tested which stimulate neuronal differentiation. Many attempts have been brought into effect in making bone MSCs differentiate using RA and brain-derived neurotrophic factors into neurons and astrocytes [25]. Using various neuronal induction mediums can help produce neurons and study biomarkers to investigate neuronal differentiation from non-neuronal cell lines and further use them to assess ways to perform stem cell therapies. However, the mesenchymal stem cells were not able to respond to RA as neuronal induction medium. The ability of MSCs to differentiate into neuronal cells gives an advantage towards developing stem cell therapy. Since MSCs can give rise to various neurons, astroglia, and oligodendroglia, their differentiation through neurotrophic stimulation factors can significantly activate genes necessary for neuronal differentiations such as nestin, notch1, MAP2, SOX1, 2 & 9, Vimentin etc. Since characterization of neuronal stem cells and their isolation is proven to be difficult, in vitro stem cell differentiation can be initiated by synergistic stimulation by neurotrophic factors such as epidermal and fibroblast growth factor-2 so that these cells exhibit critical neuron stem cell markers. This study holds dual advantage in highlighting the role WO in inducing neuronal cell lines from mice MSCs expressing nestin and producing cost-effective neuronal cell line model for short-term studies. This further highlights potential of WO properties, and cloning of the neuronal cell lines using this medium of method. Another advantage of developing WO induced neuronal stem cell model is making drug screening and diagnosis easily available for studying various neurobiological pathways to study neurodegenerative diseases. Further subcloning of the differentiated neuronal cells using WO can initiate a pure neuronal stem cell line, and provide functional stability to the differentiated neurons. This can not only provide a cost-effective pure neuronal stem cell line, but can also increase their life span to more than 9 days in vitro. Although 7–8 days survival of WO induced neuronal cells can be used for short-term drug screening studies, further studies can also be initiated for analysis on their constant survival and immortalization which can be easily cryopreserved. Hence, this study provides a preliminary analysis showing potential of WO to have possible properties of inducing neuronal-like cells from MSCs. This neuronal progeny produced can also be enhanced for long-term culture: however, further analysis is required to assess their characteristics using electrophysiological measurements.
To make differentiated cells survive for a long duration also becomes one of the major areas to be studied. The differentiated cells can be made to survive and can be injected in mice models of neuronal diseases to study their ability to migrate and survive in the surroundings of the brain. Similar data has shown that bone marrow cells when injected into mouse peritoneum and these cells migrate to the brain and differentiate into neurons [26].
In conclusion, this is the first study which shows that WO has properties of inducing MSCs into neuronal-like cells which survive for almost 7–8 days expressing neuronal marker nestin. This method represents a renewable source of neurons that may significantly facilitate research on human neurogenesis and development of clinical neuronal transplantation and study various neurodegenerative diseases.
References
Mareschi, K., Novara, M., Rustichelli, D., Ferrero, I., Guido, D., Carbone, E., & Fagioli, F. (2006). Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Experimental Hematology, 34(11), 1563–1572.
Xie, W., Schultz, M. D., Lister, R., Hou, Z., Rajagopal, N., Ray, P., Whitaker, J. W., Tian, S., Hawkins, R. D., Leung, D., & Yang, H. (2013). Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell, 153(5), 1134–1148.
Grove, J. E., Bruscia, E., & Krause, D. S. (2004). Plasticity of bone marrow-derived stem cells. Stem Cells, 22(4), 487–500.
Song, S., Kamath, S., Mosquera, D., Zigova, T., Sanberg, P., Vesely, D. L., & Sanchez-Ramos, J. (2004). Expression of brain natriuretic peptide by human bone marrow stromal cells. Experimental Neurology, 185(1), 191–197.
Deng, W., Obrocka, M., Fischer, I., & Prockop, D. J. (2001). In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem. Biophys. Res., 282(1), 148–152.
Kim, B. J., Seo, J. H., Bubien, J. K., & Oh, Y. S. (2002). Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. Neuroreport, 13, 1185–1188.
Brazelton, T. R., Rossi, F. M., Keshet, G. I., & Blau, H. M. (2000). From marrow to brain: expression of neuronal phenotypes in adult mice. Science, 290(5497), 1775–1779.
Kopen, G. C., Prockop, D. J., & Phinney, D. G. (1999). Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proceedings of the National Academy of Sciences of the United States of America, 96(19), 10711–10716.
Kuci, S., Kuci, Z., Latifi-Pupovci, H., Niethammer, D., Handgretinger, R., Schumm, M., Bruchelt, G., Bader, P., & Klingebiel, T. (2009). Adult stem cells as an alternative source of multipotential (pluripotential) cells in regenerative medicine. Stem Cell Research & Therapy, 4(2), 107–117.
Chagastelles, P. C., Nardi, N. B., & Camassola, M. (2010). Biology and applications of mesenchymal stem cells. Science Progress. doi:10.3184/003685010X12708175591515.
García-Gómez, I., Elvira, G., Zapata, A. G., Lamana, M. L., Ramírez, M., Castro, J. G., Arranz, M. G., Vicente, A., Bueren, J., & García-Olmo, D. (2010). Mesenchymal stem cells: biological properties and clinical applications. Expert Opinion on Biological Therapy. doi:10.1517/14712598.2010.519333.
Ge, D., Song, K., Guan, S., et al. (2013). Culture and differentiation of rat neural stem/progenitor cells in a three dimensional collagen scaffold. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-013-0211-5.
Bae, K. S., Park, J. B., Kim, H. S., Kim, D. S., Park, D. J., & Kang, S. J. (2011). Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Medical Journal. doi:10.3349/ymj.2011.52.3.401.
Choong, P. F., Mok, P. L., Cheong, S. K., Leong, C. F., & Then, K. Y. (2007). Generating neuron-like cells from BM-derived mesenchymal stromal cells in vitro. Cytotherapy. doi:10.1080/14653240701196829.
Sanchez-Ramos, J. R. (2002). Neural cells derived from adult bone marrow and umbilical cord blood. Journal of Neuroscience Research, 69, 880–893.
Strübing, C., Ahnert-Hilger, G., Shan, J., Wiedenmann, B., Hescheler, J., & Wobus, A. M. (1995). Differentiation of pluripotent embryonic stem cells into the neuronal lineage in vitro gives rise to mature inhibitory and excitatory neurons. Mechanisms of Development, 53(2), 275–287.
Lee, S. H., Lumelsky, N., Studer, L., Auerbach, J. M., & McKay, R. D. (2000). Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nature Biotechnology, 18(6), 675–679.
Martínez, M. L., Labuckas, D. O., Lamarque, A. L., & Maestri, D. M. (2010). Walnut (Juglans regia L.): genetic resources, chemistry, by-products. Journal of the Science of Food and Agriculture. doi:10.1002/jsfa.4059.
Kitajka, K., Sinclair, A. J., Weisinger, R. S., Weisinger, H. S., Mathai, M., Jayasooriya, A. P., Halver, J. E., & Puskás, L. G. (2004). Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proceedings of the National Academy of Sciences of the United States of America, 101(30), 10931–10936.
Poulose, S. M., Miller, M. G., & Shukitt-Hale, B. (2014). Role of walnuts in maintaining brain health with age. The Journal of Nutrition, 144(4), 561S–566S.
Muthaiyah, B., Essa, M. M., Chauhan, V., & Chauhan, A. (2011). Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochemical Research. doi:10.1007/s11064-011-0533-z.
Heo, S. J., Cha, S. H., Kim, K. N., Lee, S. H., Ahn, G., Kang, D. H., Oh, C., Choi, Y. U., Affan, A., Kim, D., & Jeon, Y. J. (2012). Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2 -induced oxidative stress in murine hippocampal neuronal cells, HT22. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-012-9545-7.
Nivet, E., Vignes, M., Girard, S. D., Pierrisnard, C., Baril, N., Devèze, A., Magnan, J., Lanté, F., Khrestchatisky, M., Féron, F., & Roman, F. S. (2011). Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. The Journal of Clinical Investigation, 121(7), 2808–2820.
Eglitis, M. A., & Mezey, E. (1997). Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proceedings of the National Academy of Sciences of the United States of America, 94, 4080–4085.
Sanchez-Ramos, J., Song, S., Cardozo-Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., Freeman, T. B., Saporta, S., Janssen, W., Patel, N., & Cooper, D. R. (2000). Adult bone marrow stromal cells differentiate into neural cells in vitro. Experimental Neurology, 164, 247–256.
Mezey, E., Chandross, K. J., Harta, G., Maki, R. A., & McKercher, S. R. (2000). Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science, 290, 1779–1782.
Acknowledgements
The authors wish to thank the University Grants Commission (UGC), New Delhi for supporting the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Singh, V., Sherpa, M. “Neuronal-Like Differentiation of Murine Mesenchymal Stem Cell Line: Stimulation by Juglans regia L. Oil”. Appl Biochem Biotechnol 183, 385–395 (2017). https://doi.org/10.1007/s12010-017-2452-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2452-1