Abstract
Poly(ethylene glycol) (PEG 4000) and bovine serum albumin (BSA) were investigated with the purpose of evaluating their influence on enzymatic hydrolysis of sugarcane bagasse. Effects of these supplements were assayed for different enzymatic cocktails (Trichoderma harzianum and Penicillium funiculosum) that acted on lignocellulosic material submitted to different pretreatment methods with varying solid (25 and 100 g/L) and protein (7.5 and 20 mg/g cellulose) loadings. The highest levels of glucose release were achieved using partially delignified cellulignin as substrate, along with the T. harzianum cocktail: increases of 14 and 18 % for 25 g/L solid loadings and of 33 and 43 % for 100 g/L solid loadings were reached for BSA and PEG supplementation, respectively. Addition of these supplements could maintain hydrolysis yield even for higher solid loadings, but for higher enzymatic cocktail protein loadings, increases in glucose release were not observed. Results indicate that synergism might occur among these additives and cellulase and xylanases. The use of these supplements, besides depending on factors such as pretreatment method of sugarcane bagasse, enzymatic cocktails composition, and solid and protein loadings, may not always lead to positive effects on the hydrolysis of lignocellulosic material, making it necessary further statistical studies, according to process conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymatic hydrolysis (EH) is one of the main bottlenecks concerning technological use of lignocellulosic materials [1, 2]. Major problem is associated to production costs of lignocellulolytic enzymes and their efficiencies in converting feedstock into its respective monosaccharides [3, 4]. One reason is because hemicellulosic and lignin fractions hinder enzyme access to cellulose [5]. Also, lignin, besides representing a physical barrier for cellulase action, has been pointed as a pivotal factor responsible for non-productive bindings of enzymes, leading to reductions in hydrolysis yield, and therefore, requiring addition of greater amount of enzymes, which raises process costs [5–9].
Researches concerning pretreatment of lignocellulosic materials have been developed aiming at increasing both accessibility of cellulolytic enzymes and hydrolysis efficiency with the purpose of making the process viable for industry [10]. Also, with the objective of enhancing hydrolysis yield and reducing enzyme loadings, addition of supplements such as surfactants (tween 20 and tween 80), non-hydrolytic proteins (bovine serum albumin, BSA), and polymers (Poly(ethylene glycol), PEG, also used as surfactant) has been studied and their effects have been reported in literature [11–13]. Use and action of additives for improvement of cellulose conversion might involve the following mechanisms: (1) adsorption on lignin, minimizing non-productive bindings of cellulase; and (2) possible increase in enzymatic activities of cellulase and assistance in enzyme stabilization [5].
Positive effects regarding addition of these supplements for hydrolysis of different substrates such as newsprint [14], avicel, corn stover subjected to various pretreatment methods [15, 16], and bamboo [17] have been reported. Nevertheless, inhibitory effects due to addition of surfactants have also been observed in the hydrolysis of pure cellulose, while positive effects have been reported as being dependent on several factors, such as hydrolysis conditions, structural characteristics of cellulose, and the enzymatic cocktail used [18].
This work intends to study the addition of PEG 4000 and BSA in the enzymatic hydrolysis of pretreated sugarcane bagasse, evaluating influence of these supplements and their dependence on solid loading, enzyme cocktail, and its protein loading, as well as material composition.
Materials and Methods
Pretreatment
Sugarcane bagasse was subjected to different pretreatment techniques. For acid pretreatment, a dilute sulfuric acid solution (1.09 % v/v) with a solid to liquid ratio of 1:2.8 (w/v) was used, and the lignocellulosic biomass was subjected to 121 °C for 27 min, as described by Betancur and Pereira Jr. [19]. For alkaline pretreatment, sodium hydroxide 4 % (w/v) was added with a solid to liquid ratio of 1:20 and the material was submitted to 121 °C for 30 min, as established by Vásquez et al. [20]. The solid fraction obtained after acid pretreatment is denominated cellulignin (CL), while, after alkaline pretreatment, due to partial removal of lignin promoted by this process, it is named partially delignified cellulignin (PDCL) [21]. In natura sugarcane bagasse composition, as well as those of CL and PDCL, were determined according to Sluiter et al. [22].
Microorganism and Cellulases Production
Two different strains of filamentous fungus, Trichoderma harzianum IOC 3844 and Penicillium funiculosum ATCC 11797 were used. Cellulases production was carried out in a 10-L (nominal volume) bioreactor (Biostat B, B. Braun Biotech International, Germany) with 8 L (working volume) of Mandels and Weber medium [23] with optimum concentrations of nitrogen sources, as optimized by Maeda et al. [24] and Rocha et al. [25] for P. funiculosum and T. harzianum, respectively. A pre-inoculum, corresponding to 10 % of the working volume and to which 106 conidia/mL was inoculated, was added to the bioreactor. Pre-inoculum and cellulase production times were 30 and 42 h, respectively, for T. harzianum and 72 h for both steps for P. funiculosum. PDCL (15 g/L) was used as carbon source and the process was performed at 30 °C and pH 5.
After cellulase production, the medium was filtered through 0.2-μm porosity columns and concentrated using a membrane of 10 kDa with tangential-flow filtration in hollow fiber columns (QuixStand QSM-03SP bench top system, GE Healthcare) at room temperature.
Quantification of Enzymatic Activities
Filter paper (50 mg, Grade 1 Whatman Filter Paper), carboxymethyl cellulose sodium salt (ultra-low viscosity) 2 % w/v (CMC), avicel (cellulose microcrystalline) 2 % w/v, cellobiose 2 % w/v, and xylans from beechwood 2 % w/v were used as substrates for determination of FPase, CMCase, Avicelase, β-glucosidase, and xylanase activities, respectively.
FPase, CMCase, Avicelase, xylanase, and β-glucosidase activities were determined using the enzymes previously diluted and the respective substrates at 50 °C for 60 min for FPase quantification and for 15 min for the other activities. At the end of the reactions, total reducing sugars released during FPase, CMCase, avicelase, and xylanase determinations were quantified through 3,5-dinitrosalicylic acid (DNS) method [26], while glucose released during β-glucosidase activity quantification was determined through colorimetric method using glucose oxidase (Katal).
Total protein concentration was quantified using Bradford reagent (Bio-Rad, CA, USA) with BSA as standard [27].
Enzymatic Hydrolysis
In order to evaluate influence of PEG 4000 and BSA in cellulose conversion, several hydrolysis of sugarcane bagasse (CL and PDCL) were carried out at 50 °C and pH 5 for 48 h using enzymatic cocktail protein loadings of 7.5 and 20 mg/g cellulose and solid loadings of 25 and 100 g/L. Supplementation with PEG and BSA was done in a ratio of 1:1 with respect to the enzymatic cocktail protein loading.
Glucose and xylose release at the end of the process was analyzed using high-performance liquid chromatography (HPLC, Shimadzu, Refractive Index Detector 10, Hiplex-H column) with H2SO4 (0.5 mM) as the mobile phase at a flow-rate of 0.6 mL/min at 60 °C.
Synergism Degree Calculation
Considering synergism as the relationship between released glucose once substrate is hydrolyzed by the enzymatic cocktail along with the additive and the sum of released glucose when the enzymatic cocktail and the additives act individually, the degree of synergism was calculated according to the equation below:
Where: SD = synergism degree.
It must be noticed that the additives alone do not promote glucose release, being their terms equivalent to zero.
Results and Discussion
Pretreatment
Sugarcane bagasse was submitted to two different pretreatments, one of them with dilute acid with the purpose of solubilizing the hemicellulose fraction, reducing it from 25.2 to 14.6 % (w/w), and the other one with dilute acid followed by alkali solution (combined pretreatment) aiming at partial removal of lignin. The latter promoted a reduction in the obstruction represented by both physical barriers (hemicellulose and lignin), increasing enzymatic access to cellulose [6]. At the end of the consecutive pretreatments, hemicellulose was reduced from 25.2 to 12.5 %, while the lignin content decreased from 19.2 to 5.5 % (w/w), resulting in an increase of cellulose content from 34.7 to 67.1 % (w/w). Table 1 presents sugarcane bagasse composition after the different pretreatments.
Addition of Supplements on the Enzymatic Hydrolysis of Sugarcane Bagasse
Influence of Pretreatment Method and Enzymatic Cocktail
Enzymatic hydrolysis of sugarcane bagasse was performed with and without PEG 4000 and BSA supplementation for two distinct enzymatic cocktails produced by T. harzianum and P. funiculosum, each of them with different enzymatic activities (Table 2), for enzymatic cocktail protein and solid loadings equivalent to 7.5 mg/g cellulose and 25 g/L, respectively. Glucose released after 48 h of enzymatic hydrolysis with and without supplementation of the additives is presented in Fig. 1.
For CL hydrolysis using cellulase from T. harzianum, increases in glucose release were not observed with BSA and PEG 4000 supplementation. However, for PDCL hydrolysis, positive effects of these additives were noticed, promoting increases in glucose concentrations of 14 and 18 %, respectively. Positive effects were also observed when P. funiculosum cocktail was used for hydrolysis of both substrates, CL and PDCL. In these cases, hydrolysis efficiencies increased 16 and 28 % for CL and 15 and 10 % for PDCL with the addition of BSA and PEG 4000, respectively. Differences in the results might be related to distinct compositions of the enzymatic cocktails used and the synergism between these components in the hydrolysis process. Researches have indicated that the use of these supplements affects adsorption of cellobiohydrolases and endoglucanases, increasing the levels of free enzymes [28–32]. Börjesson et al. [8] proposed that the hydrophobic part of surfactants bind to the lignin, while the hydrophilic part acts as a steric hindrance, preventing the enzymes from adsorbing and binding unproductively to lignin, leading to more free enzymes that could adsorb to the cellulosic substrate. This situation enhances synergism among the enzymes, and hence, the glucose release.
Even though PDCL presents lower lignin levels, the supplementation loadings are calculated with respect to cellulose levels, which are higher for this material. Also, the action of these additives is not only related to the reduction of non-productive binding of the enzymes to lignin, but they also exhibit other properties. They have been pointed out due to their ability to improve the stability and activities of the enzymes [5, 12]. This, along with the structural modifications promoted by the pretreatments, could explain the increases of released glucose levels observed for this material.
In this study, for both cocktails, higher hydrolysis efficiencies were achieved when using PDCL as substrate, indicating that lignin presents negative effects on glucose release. Increments of up to 623 and 677 % in glucose release levels were achieved using T. harzianum and P. funiculosum, respectively, for PDCL hydrolysis when compared to CL hydrolysis.
Although studies have reported supplementation of PEG and BSA as strategies to reduce non-productive bindings of the enzymes to the lignin [16], problems related to presence of this component are not only associated to inactivation of the enzymes, but also to the physical barrier represented by lignin that hampers enzymatic access to cellulose [33], the reason why PDCL hydrolysis, along with BSA and PEG addition, which can interact with reduced lignin surface area, releases higher glucose concentrations, providing increased levels of hydrolysis efficiency. Hence, pretreatments, along with delignification, are of extreme relevance for a proper and efficient use of the lignocellulosic biomass [21]. Table 3 summarizes hydrolysis efficiencies for each situation studied.
Influence of Solid Loadings
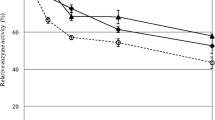
In order to evaluate the effect of the additives with respect to solid loadings, enzymatic hydrolysis of PDCL was carried out for two different solid concentrations (25 and 100 g/L) using the cocktail of T. harzianum. Figure 2 presents glucose release and hydrolysis efficiency after 48 h.
When raising solid loading from 25 to 100 g/L, an increase in the levels of glucose released was observed (7.5 to 25.5 g/L). On the other hand, hydrolysis efficiency was reduced from 40 to 34 %. This situation has been reported by previous studies, which explained this reduction due to several factors, such as lack of available water that leads to mass transfer problems, substrate and product inhibition, and low enzymatic accessibility to the substrate, among others [34]. Hence, to reach desirable efficiencies in these cases, it is necessary to increase enzymatic cocktail protein loading, which might make the process costly [5, 35].
Nevertheless, once BSA and PEG 4000 were supplemented, hydrolysis efficiencies for both solid loadings were maintained, achieving 46.2 and 45.6 % with BSA addition and 47 and 49 % with PEG 4000 addition, for 25 and 100 g/L of solids, respectively. These results denote that these supplements might play an important role in making it possible to use lignocellulosic biomass as a feedstock for the production of value-added products in a viable process.
Higher increments concerning released glucose were observed for the higher solid loading evaluated (100 g/L) once PEG and BSA were added. When increasing solid loadings, higher amounts of residual lignin are present due to material composition. This might be responsible for the formation of non-productive bindings of the enzymes, an effect that is minimized by the addition of the supplements. Also, at higher solid loadings, viscosity, a parameter that has been reported as an obstacle to biomass hydrolysis, is higher; this is ascribed to mass transfer issues. The viscosity-reducing effect of these additives, along with their ability to reduce formation of non-productive bindings of the enzymes to lignin, could explain the positive effects on glucose release at high solid loading [34].
This situation may have effects on the synergism between the enzymatic cocktail and the additives, which is shown in Fig. 3. For a solid loading equivalent to 100 g/L, a higher synergism degree was reached: 1.33 and 1.43 with BSA and PEG addition, respectively, against 1.14 and 1.18 for 25 g/L solid loading. In these conditions, BSA and PEG influence is more evident and their supplementation act more synergistically with the enzymatic cocktails.
Recent studies have reported that amphiphilic additives might increase the hydrolysis of xylans present in the hemicellulose fraction [5, 36]. Considering that T. harzianum cocktail, as reported in Table 1, presents high xylanase activity, the observed increases in glucose levels could be related to the hydrolysis of the hemicellulose fraction, which promotes an enhanced enzyme access to cellulose. Table 4 displays xylose concentration for T. harzianum with and without supplementation for the two solid loadings evaluated.
For 25 g/L solid loading, with BSA and PEG addition, increases of 17 and 20 % in xylose concentration, respectively, were reached, while for 100 g/L solid loading, increases were equivalent to 27 and 31 % with BSA and PEG supplementation, respectively.
Therefore, these supplements, as indicated, seem to act synergistically with cellulase and hemicellulase, mainly at high solid loadings, contributing to a more efficient use of the lignocellulosic biomass.
Influence of Enzymatic Cocktail Protein Loading
Influence of the enzymatic cocktail protein loadings with BSA and PEG addition in the hydrolysis of PDCL was also evaluated. Enzyme loading was increased from 7.5 to 20 mg/g of cellulose for a solid loading equivalent to 100 g/L. Figure 4 displays glucose release and hydrolysis efficiency after 48 h.
A 2.6-fold increase in the enzymatic cocktail, protein loading promoted an increase in glucose release from 26 to 48 g/L. For the highest loading (20 mg/g cellulose), once PEG 4000 and BSA were supplemented, in a 1:1 ratio (w/w) between enzymatic cocktail protein and additives, relevant increases in glucose levels were not observed, indicating that these substances are not always capable of enhancing hydrolysis yields. As reported by Eckard et al. [37], at high enzyme concentrations, the improving effect promoted by the addition of supplements can be masked, since a high-glucose release is expected, leaving no room for increases in the enzymatic hydrolysis yield. Hence, in order to better investigate and evaluate contribution of these additives to enzymatic hydrolysis of lignocellulosic materials, experimental design for specific process conditions (solid loading, enzymatic cocktails and their loadings, and type of material and supplements) ought to be carried out.
Conclusions
Increases in the hydrolysis efficiency of lignocellulosic biomass with the addition of both PEG 4000 and BSA showed to be dependent on material composition, enzymatic cocktail, and solid and protein loadings. Delignification process is extremely relevant for enhancing glucose release and better results for hydrolysis efficiency of PDCL were achieved when cocktails of the filamentous fungus T. harzianum and P. funiculosum were used. Increases in solid loading promoted higher synergism degrees among the cellulolytic enzymes and the additives; nevertheless, depending on the enzymatic cocktail protein loading and PEG and BSA concentration, these supplements may not contribute to increase hydrolysis yields.
References
Lynd, L. R., Laser, M. S., Bransby, D., Dale, B. E., Davison, B., Hamilton, R., Himmel, M., Keller, M., McMillan, J. D., Sheehan, J., & Wyman, C. E. (2008). How biotech can transform biofuels. Nature Biotechnology, 26(2), 169–172.
Ye, Z., & Berson, R. E. (2014). Factors affecting cellulose hydrolysis based on inactivation of adsorbed enzymes. Bioresource. Technol., 167, 582–586.
Alvira, P., Negro, M. J., & Ballesteros, M. (2011). Effect of endoxylanase and α-L-arabinofuranosidase supplementation on the enzymatic hydrolysis of steam exploded wheat straw. Bioresource. Technol., 102(6), 4552–4558.
Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A., & Blanch, H. W. (2012). The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnology and Bioengineering, 109(4), 1083–1087.
Yang, M., Zhang, J., Kuittinen, S., Vepsäläinen, J., Soininen, P., Keinänen, M., & Pappinen, A. (2015). Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone–butanol–ethanol fermentation. Bioresource. Technol., 189, 131–137.
Qing, Q., Yang, B., & Wyman, C. E. (2010). Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresource. Technol., 101(24), 9624–9630.
Kumar, L., Arantes, V., Chandra, R., & Saddler, J. (2012). The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresource. Technol., 103(1), 201–208.
Börjesson, J., Peterson, R., & Tjerneld, F. (2007). Enhanced enzymatic conversion of softwood lignocellulose by poly (ethylene glycol) addition. Enzyme. Microb Tech., 40(4), 754–762.
Arantes, V., & Saddler, J. N. (2010). Access to cellulose limits the efficiency of enzymatic hydrolysis: the role of amorphogenesis. Biotechnology for Biofuels, 3, 1–11.
Ravindran, R., & Jaiswal, A. K. (2016). A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresource. Technol., 199, 92–102.
Monschein, M., Reisinger, C., & Nidetzky, B. (2014). Dissecting the effect of chemical additives on the enzymatic hydrolysis of pretreated wheat straw. Bioresource. Technol., 169, 713–722.
Eriksson, T., Börjesson, J., & Tjerneld, F. (2002). Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme. Microb. Tech., 31(3), 353–364.
Menegol, D., Scholl, A. L., Fontana, R. C., Dillon, A. J. P., & Camassola, M. (2014). Increased release of fermentable sugars from elephant grass by enzymatic hydrolysis in the presence of surfactants. Energ. Convers. Manage., 88, 1252–1256.
Wu, J., & Ju, L. K. (1998). Enhancing enzymatic saccharification of waste newsprint by surfactant addition. Biotechnology Progress, 14(4), 649–652.
Kumar, R., & Wyman, C. E. (2009). Effect of additives on the digestibility of corn Stover solids following pretreatment by leading technologies. Biotechnology and Bioengineering, 102(6), 1544–1557.
Eckard, A. D., Muthukumarappan, K., & Gibbons, W. (2012). Pretreatment of extruded corn Stover with polyethylene glycol to enhance enzymatic hydrolysis: optimization, kinetics, and mechanism of action. Bioenergy Research, 5(2), 424–438.
Li, K., Wang, X., Wang, J., & Zhang, J. (2015). Benefits from additives and xylanase during enzymatic hydrolysis of bamboo shoot and mature bamboo. Bioresource. Technol., 192, 424–431.
Zhou, Y., Chen, H., Qi, F., Zhao, X., & Liu, D. (2015). Non-ionic surfactants do not consistently improve the enzymatic hydrolysis of pure cellulose. Bioresource. Technol., 182, 136–143.
Betancur, G. J., & Pereira Jr., N. (2010). Sugar cane bagasse as feedstock for second generation ethanol production: part I: diluted acid pretreatment optimization. Electronic Journal of Biotechnology, 13(3), 10–11.
Vásquez, M. P., da Silva, J. N. C., de Souza Jr, M. B., & Pereira Jr., N. (2007). Enzimatic hydrolysis optimization to ethanol production by simultaneous saccharification and fermentation. Appl. Biochem. Biotech., 137, 141–154.
Barcelos, C. A., Maeda, R. N., Betancur, G. J. V., & Pereira Jr., N. (2013). The essentialness of delignification on enzymatic hydrolysis of sugar cane bagasse cellulignin for second generation ethanol production. Waste and Biomass Valorization, 4(2), 341–346.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D. (2008). Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure, 1617.
Mandels, M., & Weber, J. (1969). Production of cellulases. Advances in Chemistry Series, 95, 391–413.
Maeda, R. N., da Silva, M. M. P., Santa Anna, L. M. M., & Pereira Jr., N. (2010). Nitrogen source optimization for cellulase production by Penicilliumfuniculosum, using a sequential experimental design methodology and the desirability function. Appl. Biochem Biotech, 161(1–8), 411–422.
Rocha, V. A. L., Maeda, R. N., Santa Anna, L. M. M., & Pereira, N. (2013). Sugarcane bagasse as feedstock for cellulase production by Trichoderma harzianum in optimized culture medium. Electronic Journal of Biotechnology, 16(5), 1–1.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Brethauer, S., Studer, M. H., Yang, B., & Wyman, C. E. (2011). The effect of bovine serum albumin on batch and continuous enzymatic cellulose hydrolysis mixed by stirring or shaking. Bioresource. Technol., 102(10), 6295–6298.
Palonen, H., Tjerneld, F., Zacchi, G., & Tenkanen, M. (2004). Adsorption of Trichoderma Reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. Journal of Biotechnology, 107(1), 65–72.
Ooshima, H., Sakata, M., & Harano, Y. (1986). Enhancement of enzymatic hydrolysis of cellulose by surfactante. Biotechnology and Bioengineering, 28(11), 1727–1734.
Sipos, B., Dienes, D., Schleicher, Á., Perazzini, R., Crestini, C., Siika-Aho, M., & Réczey, K. (2010). Hydrolysis efficiency and enzyme adsorption on steam-pretreated spruce in the presence of poly(ethylene glycol). Enzyme. Microb. Tech., 47(3), 84–90.
Chia-wen, C. H., Cannella, D., Jørgensen, H., Felby, C., & Thygesen, L. G. (2015). Cellobiohydrolase and endoglucanase respond differently to surfactants during the hydrolysis of cellulose. Biotechnology for Biofuels, 8(1), 1–10.
Saini, J. K., Patel, A. K., Adsul, M., & Singhania, R. R. (2016). Cellulase adsorption on lignin: a roadblock for economic hydrolysis of biomass. Renew. Energ. doi:10.1016/j.renene.2016.03.089.
Modenbach, A. A., & Nokes, S. E. (2012). The use of high-solids loadings in biomass pretreatment—a review. Biotechnology and Bioengineering, 109(6), 1430–1442.
Lou, H., Wang, M., Lai, H., Lin, X., Zhou, M., Yang, D., & Qiu, X. (2013). Reducing non-productive adsorption of cellulase and enhancing enzymatic hydrolysis of lignocelluloses by noncovalent modification of lignin with lignosulfonate. Bioresource. Technol., 146, 478–484.
Li, Y., Ge, X., Sun, Z., & Zhang, J. (2015). Effect of additives on adsorption and desorption behavior of xylanase on acid-insoluble lignin from corn stover and wheat straw. Bioresource. Technol., 186, 316–320.
Eckard, A. D., Muthukumarappan, K., & Gibbons, W. (2013). A review of the role of amphiphiles in biomass to ethanol conversion. Applied Sciences, 3(2), 396–419.
Acknowledgments
The authors would like to thank the Brazilian Petroleum Company (PETROBRAS) and Universidad de Costa Rica for scholarship and other financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Méndez Arias, J., de Oliveira Moraes, A., Modesto, L.F.A. et al. Addition of Surfactants and Non-Hydrolytic Proteins and Their Influence on Enzymatic Hydrolysis of Pretreated Sugarcane Bagasse. Appl Biochem Biotechnol 181, 593–603 (2017). https://doi.org/10.1007/s12010-016-2234-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2234-1