Abstract
The effect of the culture medium pH (3.5–6.0) on the carotenoid and lipid (as fatty acids) production by the yeast Rhodosporidium toruloides NCYC 921 was studied. Flow cytometry was used to evaluate the yeast’s physiological response to different culture medium pH values. The yeast biomass concentration and lipid content were maxima at pH 4.0 (5.90 g/L and 21.85 % w/w, respectively), while the maximum carotenoid content (63.37 μg/g) was obtained at pH 5.0. At the exponential phase, the yeast cell size and internal complexity were similar, at different medium pH. At the stationary phase, the yeast cell size and internal complexity decreased as the medium pH increased. At the exponential phase, the proportion of cells with polarized membranes was always high (>80 %) but at the stationary phase, the proportion of yeast cells with depolarized membranes was dominant (>65 %) and increased with the medium pH increase. The results here reported may contribute for yeast bioprocesses optimization. For the first time, multiparameter flow cytometry was used to evaluate the impact of medium pH changes on the yeast cell physiological status, specifically on the yeast membrane potential, membrane integrity, cell size and internal complexity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms can accumulate intracellular lipids that can be converted into biodiesel. Indeed, microbial biofuels do not show the inconveniences of biofuels obtained from food agriculture cultures (first-generation biofuels, 1G), such as the competition with food supply, arable land, and potable water. Oleaginous microbes such as microalgae and yeasts can be used as biodiesel feedstock. Indeed, these microorganisms grow faster than crop cultures and their cultivation is non-seasonal. Among oleaginous microorganisms, yeasts have a few advantages over bacteria, molds, and algae due to their higher growth rate, biomass and lipid productivities [1]. Moreover, biodiesel production from yeasts is of particular interest for countries located at higher latitudes, where the solar irradiance is lower than in countries closer to the tropics, wherein autotrophic microalgae may be more suitable.
Microbial biofuels are a renewable source of energy, do not contain sulfur, and are highly biodegradable [2]. So far, the major obstacle for commercialization of biofuels obtained from microbes is still the high production cost involved. Therefore, it is crucial to explore approaches to reduce the costs of microbial biofuel production processes. One possible way is the coproduction of high value-added products from the microbial biomass. Indeed, most of the published studies focusing on microbial biofuels describe the production of only one microbial biofuel. This means that the other available and valuable remaining microbial products or components in the microbial biomass are undervalued and lost. In fact, some microbial producers, beyond biofuels, may also produce intracellular high value-added products such as carotenoids, polyunsaturated fatty acids (PUFAs), fine carbohydrates, etc.
The yeast Rhodosporidium toruloides NCYC 921 (formerly Rhodotorula glutinis NRRL Y-1091) is an oleaginous microorganism that, beyond lipids, also produces carotenoids with commercial interest for food industry [3]. It is known that microbial synthesis of lipids and carotenoid is strongly dependent on the growth conditions, particularly medium pH [4]. In addition, if the yeast biomass, carotenoids, and lipids are produced under different optimal medium pH, it is crucial to know the optimal pH for each yeast product. Therefore, the study of the influence of the medium pH on the lipids and carotenoid production by yeasts is an important step to coproduce these products, before the process scale-up step.
However, most of the works that reported the effect of medium pH on the growth, lipid and/or carotenoid production by yeasts used traditional analytical methods for monitoring the yeast growth, such as optical density and dry cell weight, which do not give any information on the cell physiology [5–9]. Moreover, gravimetric extraction methods for lipid and carotenoid quantification are also used but are time-consuming, use high amounts of toxic organic solvent, and require significant amounts of biomass for carotenoid extraction and quantification [10, 11]. Importantly, carotenoid analysis usually takes a few days, and sometimes, data is only available after the cultivation is over, too late to introduce changes to the process control strategy [3].
Flow cytometry is a powerful technique that can be used to at-line monitor cell functions and microbial products during the yeast growth [3]. It also allows understanding the interaction of the microorganisms with the process environment and cell survival mechanisms when grown under adverse conditions. This information, when obtained in real time, allows changing the process control strategy during the time course of the cultivation, in order to achieve higher product yields and productivities.
The present work studied the effect of the initial medium pH on R. toruloides NCYC 921 growth, lipid and carotenoid production, using multiparameter flow cytometry to evaluate the carotenoid content, yeast cell size, internal complexity, and cell viability, when grown under different medium pH.
As far as the authors are aware, such approach has never been reported before.
Materials and Methods
Microorganism
The yeast R. toruloides NCYC 921 was purchased from the National Collection of Yeast Cultures (Norwich, UK). The strain was stored on slants of Malt Extract Agar, at 4 °C.
Growth Conditions
For inoculum preparation, R. toruloides yeast cells from one slant grown for 72 h at 30 °C were transferred to the growth medium with the following composition (g/L) [12]: CaCl2.2H2O, 0.134; MgSO4, 0.73; Na2HPO4, 2; KH2PO4, 7; yeast extract, 0.5; (NH4)2SO4, 1; glucose, sterilized separately, was added to the medium at a final concentration of 35 g/L. After 1 day of growth (exponential growth phase), this culture 2.5 % (v/v) was used to inoculate 1 L baffled shake flasks containing 200 mL of sterile culture medium. The initial medium pH was adjusted to 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0, before inoculation, since the yeast may produce lipids and carotenoids under a wide medium pH range [6]. The cultures were incubated in an orbital incubator shaker (INFORS HT Unitron Plus AJ252, Switzerland) at 30 °C and 150 rpm. R. toruloides shake flask growth was monitored for 72 h, by measuring the biomass concentration by optical density [3]. The yeast cell metabolic state was evaluated during the exponential and stationary phases, in terms of membrane potential and integrity, by flow cytometry.
After 72 h of growth, the lipidic storage materials (total fatty acids (TFAs) and total carotenoids (TCs)) were analyzed in R. toruloides cells, as they are usually produced during the stationary phase [10]. TC content was assessed by flow cytometry. The culture was then centrifuged and the pellet was stored at −18 °C for further analyses of TFA by gas-liquid chromatography. All the experiments were conducted with two independent replicates.
Analytical Methods
The analytical methods used in this work were previously described by Freitas et al. [3]. The lipids were evaluated as total TFA since the lipidic saponifiable fraction is the one that is used for biodiesel purposes. Flow cytometry was used to evaluate the carotenoid content and cell viability as above described. Cell viability was assessed detecting changes in the yeast cytoplasmic membrane potential and integrity. R. toruloides subpopulations analyzed by flow cytometry were identified according to Freitas et al. [3]. The advantages of multiparameter flow cytometry over the more conventional analytical techniques such as the traditional carotenoid extraction are well documented [3]. Briefly, by detecting the yeast cell autofluorescence, it is possible to quantify the yeast carotenoid content, and by using a mixture of the fluorescent dyes propidium iodide (PI) (P-1304, Molecular Probes, Carlsbad, CA, USA) and 3,3-dihexylocarbocyanine iodide (DiOC6(3)) (D-273, Molecular Probes, Carlsbad, CA, USA), it is possible to detect R. toruloides membrane integrity and membrane (mitochondrial and cytoplasmic) potential changes during the yeast growth.
It has been reported that bacteria, yeast, and microalgae can be detected from the background on the basis of their intrinsic light-scattering properties in forward angle light scatter (FS) and right angle light scatter (SS) [13, 14]. FS is measured in the plane of the laser beam and gives relative information on cell size. SS is measured at 90° to the laser beam and can provide information on cell granularity (or internal complexity) [14]. In the present work, light scatter measurements of R. toruloides cells were also carried out at the exponential and stationary phases.
Results and Discussion
Biomass, TC, and TFA
Figure 1a shows R. toruloides NCYC 921 profiles during the experiments carried out at different initial medium pH. The medium pH during the yeast growth decreased (Table 1), as described by Braunwald et al. [15] for R. glutinis ATCC 15125.
a Rhodosporidium toruloides biomass profiles for different initial medium pH. Biomass values represent the average value of two independent replicates with a standard deviation lower than 10 % (n = 2); b R. toruloides biomass and TFA and TC productivities at different initial medium pH, Px Biomass productivity; PTC Total carotenoid productivity; PTFA Total fatty acid productivity
The maximum biomass concentration (5.90 g/L) was obtained for the experiment carried out at pH 4.0. The specific growth rate attained its maximum (0.109/h) at pH 4.0 and decreased as the medium pH increased (Table 1). The maximum biomass productivity followed the same trend, achieving a maximum of 0.08 g/L/h. The maximum TC content (≈63 μg/g) and concentration (296.19 μg/L) were attained at higher medium pH values of 4.5 (Table 1). The total TFA content, concentration, and productivity followed the same trend as the biomass profiles increased up to pH 4.0, attaining 21.85 % (w/w dry cell weight), 1.24 g/L and 0.02 g/L/h, respectively, decreasing as the medium pH increased. The maximum TC concentration obtained in this work (296.19 μg/L) was lower than that reported by Braunwald et al. [14] (1247 μg/L) in a study where R. glutinis ATCC 15125 was cultivated in a growth medium with different carbon to nitrogen ratios. However, the lipid (as TFA) concentration reported in this work (1.24 g/L) was higher than the one reported by these authors (0.5 g/L).
The maxima biomass and TC and TFA productivities attained under different medium pH are depicted in Fig. 1b. It is clear that the maxima biomass and TFA productivities were found at the same pH (4.0), while the maximum TC productivity was observed at medium pH of 4.5.
Yoon and Rhee [12] and Pan et al. [16] also studied R. toruloides NCYC 921 (former R. glutinis NRLL Y-1091) growth and lipid production at a medium pH of 5.5. However, the results here reported demonstrated that the optimal medium pH for biomass and lipids (as TFA) is different from the optimal pH for carotenoid production. This observation is supported by previous work [3] that showed that fatty acids are growth-associated compounds, while carotenoids are mixed compounds, being produced during the exponential and stationary phase. Luo et al. [8] studied the effect of pH (4.0–7.0) on Saccharomyces cerevisiae biomass and β-carotene production and observed that initial higher medium pH favored cell growth, while the β-carotene content increased alone with the decrease of initial medium pH, indicating that a relatively low pH is beneficial for β-carotene formation, the opposite observed for R. toruloides NCYC 921 used in this work. The optimal pH difference for biomass, fatty acid, and carotenoid production observed for this yeast may be used to improve the lipid and carotenoid productivities, as already studied by Dias et al. [17].
Fatty Acid Composition
The yeast fatty acid composition obtained at different medium pH was also evaluated (Table 2). Overall, the major fatty acids were oleic acid (18:1ω9), linoleic acid (18:2ω6), stearic acid (18:0), and palmitic acid (16:0). The yeast fatty acid profile varied slightly at different medium pH values. Similar observations were noted for R. glutinis IIP-30 fatty acid composition at a pH range of 3–6 [5] and Candida sp. 107 fatty acid profile at a pH range of 3.5 to 7.5 [18]. Saturated fatty acid (SFA) percentages did not show a consistent tendency as the medium pH increased. The palmitic acid (16:0) percentage oscillated with the pH increase, the behenic acid (22:0) percentage decreased at the medium pH range of 4.0–5.5, and the lignoceric acid (24:0) increased as the pH increased at 4.5, decreasing thereafter until the pH of 6.0. A slight increase in the monounsaturated fatty acid (MUFA) percentage (18:1ω9, oleic acid) was observed, at the pH range of 4.0–5.5, attaining about 41 % of TFA. The polyunsaturated fatty acid (PUFA) percentages (18:2ω6 and 18:3ω3) decreased at the pH range of 4.5–5.5.
For user acceptance, yeast lipids as biodiesel will need to comply with existing standards. In the European Union, separate standards exist for biodiesel intended for vehicle use (European Standard EN 14214). Among the unsaturated fatty acids, special attention should be taken in relation to the linolenic methyl ester (18:3 ω3) and polyunsaturated methyl ester (≥4 double bonds) content. As these compounds increase the biodiesel susceptibility to oxidation, the EN 14214 specifies limits of 12 and 1 %, respectively. From Table 2, it can be seen that in all cases, the percentages of linolenic acid (18:3ω3) percentage was always lower than 12 % and no PUFA with more than three double bonds were detected. Therefore, the yeast fatty acid composition obtained in all experiments was suitable for biodiesel purposes, according to EN 14214.
Yeast Physiological Response
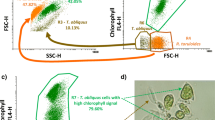
The membrane integrity and potential were also detected during the exponential and stationary phases (Fig. 2a, b). It can be seen that, during the exponential phase (Fig. 2a), the proportion of intact cells with polarized membranes was high (subpopulation B > 80 %, Fig. 2a (III)), while the proportion of stressed subpopulations (A and C) was always low (A + C < 20 %, Fig. 2a (III)). The density plots concerning the experiments at pH 4.0, 4.5, 5.0, 5.5, and 6.0 showed no substantial changes in the subpopulations profiles during the exponential phase (not shown for clarity).
a Flow cytometric analysis of R. toruloides cells taken from the exponential phase, stained with PI and DiOC6 (3). For clarity, only the density plots concerning the yeast cells grown at extreme pH values (3.5 and 6.0) are shown. I Density plot concerning the yeast cells grown at pH 3.5, showing the subpopulation percentages; II Density plot concerning the yeast cells grown at pH 6.0, showing the subpopulations percentages; III Effect of the initial medium pH on the subpopulations percentage, at the exponential phase. b Flow cytometric analysis of R. toruloides cells taken from the stationary phase, stained with PI and DiOC6 (3). IV Density plot concerning the yeast cells grown at pH 3.5, showing the subpopulation percentages; V Density plot concerning the yeast cells grown at pH 6.0, showing the subpopulation percentages; VI Effect of the initial medium pH on the subpopulation percentage, at the stationary phase. The subpopulations were identified according to Freitas et al. [3]. Subpopulation A corresponds to cells with intact and depolarized membranes; subpopulation B corresponds to intact cells with polarized membranes; subpopulation C corresponds to cells with permeabilized membranes. For III and VI, subpopulation values represent the average of two independent replicates with a standard deviation lower than 10 % (n = 2). I, II, IV, and V represent one of the replicates
On the contrary, during the stationary phase (Fig. 2b), the effect of pH on the yeast metabolic state, measured as membrane potential and integrity, was notorious (Fig. 2b (IV, V, VI)) as the subpopulation composed of cells with intact but depolarized membranes (subpopulation A) became dominant (A > 65 %, Fig. 2b (VI)) This may due to the fact that the yeast cells, when experiencing adverse conditions such as nutrient deprivation at the stationary phase, are more vulnerable to stress conditions [19]. In addition, a progressive increase in the proportion of subpopulation A was observed as the pH increased from 3.5 to 6.0 (Fig. 2b (IV, V, VI)). The density plots concerning the experiments conducted at pH of 4.0, 4.5, 5.0, 5.5, and 6.0 showed the same trend (not shown for simplicity). The proportion of cells with permeabilized membrane was always low (<10 %).
Despite the high proportions of cells with depolarized membranes (subpopulation B) observed during the stationary phase, R. toruloides NCYC 921 cells were able to synthesize carotenoids during this phase (Table 1). This observation is supported by previous work [3] that demonstrated that, during stationary phase, despite having their membrane potential affected, R. toruloides NCYC 921 cells could maintain their enzymatic activity and produce high amounts of carotenoids.
The yeast cell size and internal complexity detected during the growth at different medium pH are shown in Fig. 3. Again, the cell size and internal complexity (given by the FS and SS values, respectively, measured by flow cytometry) did not change significantly during the exponential phase (Fig. 3a (I, II, III, IV, and V) as the FS and SS signals almost remained constant (FS ≈ 750; SS ≈ 190) for all the experiments conducted at pH from 3.5 to 6.0 (Fig. 3a (V); only the FS and SS histograms concerning the experiments conducted at pH 3.5 and 6.0 during the stationary phase are shown, for clarity).
R. toruloides forward angle light scatter (FS) and right angle light scatter (SS) signals detected by flow cytometry, at the exponential phase (a) and stationary phase (b). For clarity, only the histograms concerning the yeast cells grown at extreme pH values (3.5 and 6.0) are shown. I FS signal histogram (number of events versus FS signal) for the yeast cells taken from the exponential phase, growing at pH 3.5. II SS signal histogram (number of events versus SS signal) for the yeast cells taken from the exponential phase, growing at pH 3.5. III FS signal histogram (number of events versus FS signal) for the yeast cells taken from the exponential phase, growing at pH 6.0. IV SS signal histogram (number of events versus SS signal) for the yeast cells taken from the exponential phase, growing at pH 6.0. V Effect of the initial medium pH on the FS and SS values, at the exponential phase. VI FS signal histogram (number of events versus FS signal) for the yeast cells taken from the stationary phase, growing at pH 3.5. VII SS signal histogram (number of events versus SS signal) for the yeast cells taken from the stationary phase, growing at pH 3.5. VIII FS signal histogram (number of events versus FS signal) for the yeast cells taken from the stationary phase, growing at pH 6.0. IX SS signal histogram (number of events versus SS signal) for the yeast cells taken from the stationary phase, growing at pH 6.0. X Effect of the initial medium pH on the FS and SS values, at the stationary phase. For V and X, the FS and SS values represent the average of two independent replicates with a standard deviation lower than 10 % (n = 2). Histograms I, II, III, IV, VI, VII, VIII and IX represent one of the replicates
At the stationary phase (Fig. 3b), a notorious decrease in the yeast cell size and internal complexity was observed as the medium pH increased from 3.5 to 6.0 (Fig. 3b (VI, VII, VIII, IX, X)), attaining the lowest values at pH 6.0 (FS ≈ 520; FS ≈ 160, Fig. 3b (VIII, IX, and X).
As far as the authors know, there are no reports on the effect of pH on the yeast cell size and internal complexity. Weisse and Stadler [20] investigated the effect of pH on cell volume of freshwater ciliates and found that in some ciliate species, the cell volume was significantly affected by medium pH changes, decreasing their size as the external medium pH increased. The authors attributed the ciliate inability to regulate cell size as an indicator of pH stress. In the same way, the yeast cell size decrease with the medium pH increase may be an indicator of yeast cell stress response, observed at the stationary phase.
In fact, this response was coincident with the increase in the proportion of cells with depolarized membranes (subpopulation B), as the medium pH increased, although this was not entirely expected. In fact, according to Plasek et al. [21] and Plasek et al. [22], low medium pH induced the yeast plasma membrane depolarization, as the proton flux across the membrane is reduced. Mitochondrial membrane also depolarizes as the external pH decreased [23]. The opposite was observed in the present work, which was attributed to the medium pH variation (pHinitial − pHfinal) that cells were exposed during the growth, at higher medium pH. Indeed, from Table 1, it can be seen that the pH variation was more pronounced for the experiments carried out at higher initial medium pH than those carried out at lower initial pH. In the latter case, cells would have adapted to lower initial medium pH, from the begging of the growth. At higher initial medium pH, the yeast cells would have experienced an abrupt medium pH drop during the growth, which might have induced the massive membrane depolarization observed at the stationary phase.
Conclusion
The work here reported contributes for a better understanding of the medium pH effect on the yeast carotenoid, lipid production, and stress response. This approach may also simulate the pH gradients that occur in large-scale yeast cultivations, in which inefficient mixing often results in stagnant regions often present in large-scale processes, which reduces the process yield. Understanding the cellular mechanisms as a response to such environments may contribute to enhance the yeast bioprocesses.
References
Li, Q., Du, W., & Liu, D. (2008). Perspectives of microbial oils for biodiesel production. Applied Microbiology and Biotechnology, 80(5), 749–758.
González-Delgado, Á. D., & Kafarov, V. (2011). Microalgae based biorefinery: issues to consider. Ciencia, Tecnologia y Futuro, 4, 5–22.
Freitas, C., Nobre, B., Gouveia, L., Roseiro, J., Reis, A., & Da Silva, T. L. (2014). New at-line flow cytometric protocols for determining carotenoid content and cell viability during Rhodosporidium toruloides NCYC 921 batch growth. Process Biochemistry, 49, 554–562.
Yong-Hong, L. I., Bo, L., Zong-Bao, Z. H. A. O., & Feng-Wu, B. A. I. (2006). Optimization of culture conditions for lipid production by Rhodosporidium toruloides. Chinese Journal of Biotechnology, 22, 650–656.
Johnson, V., Singh, M., Saini, V. S., Sista, V. R., & Yadav, N. K. (1992). Effect of pH on lipid accumulation by an oleaginous yeast: Rhodotorula glutinis lIP-30. World Journal of Microbiology and Biotechnology, 8, 382–384.
Latha, B. V., Jeevaratnam, K., Murali, H. S., & Manja, K. S. (2005). Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-PDY from natural source. Indian Journal of Biotechnology, 4, 353–357.
Ferrao, M., & Garg, S. (2012). Shake flask optimization of β-carotene production in Rhodotorula graminis RC04). African Journal of Biotechnology, 11, 11431–11437.
Luo, H., Niu, Y., Duan, C., Su, H., & Yan, G. (2013). A pH control strategy for increased β-carotene production during batch fermentation by recombinant industrial wine yeast. Process Biochemistry, 48, 195–200.
Naghavi, F. S., Hanachi, P., & Saboora, A. (2014). Effect of temperature, pH and salinity on carotenoid production in Rhodotorula mucilaginosa. Research in Biotechnology, 5, 01–04.
Saenge, C., Cheirsilp, B., Suksaroge, T., & Bourtoom, T. (2011). Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultures in palm oil mill effluent and application of lipids for biodiesel production. Biotechnology and Bioprocess Engineering, 16, 23–33.
Schneider, T., Graeff-Hönninger, S., French, W., Hernandez, R., Hernandez, R., Merkt, N., Claupein, W., & Pham, M. (2013). Lipid and carotenoid production by oleaginous red yeast Rhodotorula glutinis cultivated on brewery effluents. Energy, 61, 34–43.
Yoon, S., & Rhee, J. (1983). Lipid from yeast fermentation: effects of cultural conditions on lipid production and its characteristics of Rhodotorula glutinis. Journal of the American Oil Chemistry Society, 60, 1281–1286.
Da Silva, T. L., Feijão, D., & Reis, A. (2010). Using multi-parameter flow cytometry to monitor the yeast Rhodotorula glutinis CCMI 145 batch growth and oil production towards biodiesel. Applied Biochemestry and Biotechnology, 162, 2166–2176.
Hewitt, C. J., & Nebe-Von-Caron, G. (2004). The application of multi-parameter flow cytometry to monitor individual microbial cell physiological state. Advance Biochemistry Engineering/Biotechnology, 89, 197–223.
Braunwald, T., Schawemmlein, L., Graeff-Hönninger, S., Franch, W., Hernandez, R., Holmes, W., & Claupein, W. (2013). Effect of different C/N ratios on carotenoid and lipid production by Rhodototrula glutinis. Applied Microbiology and Biotechnology, 97(14), 6581–6588.
Pan, J., Kwak, M., & Rhee, J. (1986). High density cell culture of Rhodotorula glutinis using oxygen-enriched air. Biotechnology Letters, 8, 715–718.
Dias, C., Sousa, S., Caldeira, J., Reis, A., & Da Silva, T. L. (2015). New dual-stage pH control fed-batch cultivation strategy for the improvement of lipids and carotenoids production by the red yeast Rhodosporidium toruloides NCYC 921. Bioresource Technology, 189, 309–318.
Hall, M. J., & Ratledge, C. (1977). Lipid accumulation in an oleaginous yeast (Candida 107) growing on glucose under various conditions in a one and two-stage continuous culture. Applied and Environmental Microbiology, 33, 577–584.
Freitas, C., Nunes, E., Passarinho, P., Reis, A., & Da Silva, T. L. (2012). Effect of acetic acid on Saccharomyces carlsbergensis ATCC 6269 batch ethanol production monitored by flow Cytometry. Applied Biochemistry and Biotechnology, 168, 1501–1515.
Weisse, T., & Stadler, P. (2006). Effect of pH on growth, cell volume, and production of freshwater ciliates, and implications for their distribution. Limnology and Oceanography, 51(4), 1708–1715.
Plášek, J., Gášková, D., Lichtenberg-Fraté, H., Ludwig, J., & Höfer, M. (2012). Monitoring of real changes of plasma membrane potential by diS-C3(3) fluorescence in yeast cell suspensions. Journal of Bioenergy and Biomembranes, 44, 559–569.
Plášek, J., Gášková, D., Ludwig, D., & Höfer, M. (2013). Early changes in membrane potential of Saccharomyces cerevisiae induced by varying extracellular K+, Na+ or H+ concentrations. Journal of Bioenergy and Biomembranes, 45, 561–568.
Gášková, D., Brodská, B., Holoubek, A., & Sigler, K. (1999). Factors and processes involved in membrane potential build-up in yeast: diS-C3(3) assay. International Journal of Biochemistry and Cell Biology, 31, 575–584.
Funding
The present work was supported by FCT (Fundação para a Ciência e a Tecnologia) (also supported by FEDER funding through COMPETE—Programa Operacional Factores de Competitividade) and was carried out within the project FCOMP-01-0124-FEDER-019317 (ex-PTDC/AAC-AMB/116594/2010 entitled “CAROFUEL—New process for a sustainable microbial biodiesel production: The yeast R. glutinis biorefinery as a source of biodiesel, biogas, and carotenoids.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dias, C., Silva, C., Freitas, C. et al. Effect of Medium pH on Rhodosporidium toruloides NCYC 921 Carotenoid and Lipid Production Evaluated by Flow Cytometry. Appl Biochem Biotechnol 179, 776–787 (2016). https://doi.org/10.1007/s12010-016-2030-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2030-y