Abstract
Mixture of brown rice and rice bran fermented with Aspergillus oryzae, designated as FBRA, has been reported to reveal anti-carcinogenic and anti-inflammatory effects in rodents. Then, to test its potential anti-cancer activity, the aqueous extract was prepared from FBRA powder, and the effect of this extract on human acute lymphoblastic leukemia Jurkat cells was directly examined. The exposure to FBRA extract reduced the cell viability in a concentration- and time-dependent manner. The reduction of the cell viability was accompanied by the DNA fragmentation, and partially restored by treatment with pan-caspase inhibitor. Further studies showed that FBRA extract induced the cleavage of caspase-8, -9, and -3, and decreased Bcl-2 protein expression. Moreover, the expression of tBid, DR5, and Fas proteins was enhanced by FBRA extract, and the pretreatment with caspase-8 inhibitor, but not caspase-9 inhibitor, restored the reduction of the cell viability induced by FBRA extract. These findings suggested that FBRA extract could induce the apoptotic death of human acute lymphoblastic leukemia cells probably through mainly the death receptor-mediated pathway and supplementarily through the tBid-mediated mitochondrial pathway, proposing the possibility that FBRA was a potential functional food beneficial to patients with hematological cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is one of the most popular grains, and widely utilized as a staple food in Asia region. Brown rice, whole-grain before polish rice, is known to contain a large amount of nutrients, such as vitamins, minerals, and dietary fiber. Dietary fiber is a non-digestible food ingredient for modulating the growth and activity of intestinal microbiota, and contributes to the well-being of humans and animals. Therefore, brown rice is acknowledged as a prebiotic and has been reported to improve the physical conditions of the experimental animals [1–3]. Additionally, brown rice has previously been shown to contain several biological active components beneficial to human health. For instance, ferulic acid has been shown to cause an anti-inflammatory effect by scavenging reactive oxygen species and inhibiting nuclear translocation of NF-κB [4]. Furthermore, γ-orizanol has been reported to have the activities of both lowering plasma non-HDL-C levels and raising plasma HDL-C levels in hypercholesterolemic animals [5]. Moreover, phytic acid has also been reported to have an antioxidative activity [6]. Therefore, brown rice could be speculated as a promising material that provides health benefits. These bioactive components are found to be rich in rice bran, which consists of a husk and a germ part of brown rice. Therefore, rice bran is also considered of great value to be consumed for human wellness. However, almost all of it is discarded as an agro-industrial waste, the rest of which is used for producing rice-oil or soil improvement materials.
“Fermented brown rice and rice bran with Aspergillus oryzae”, which was abbreviated to FBRA, is a processed food manufactured by fermenting the mixture of brown rice and rice bran to improve their digestibility with A. oryzae. A. oryzae is known to contain several kinds of enzymes metabolizing carbohydrates and proteins, thereby producing a variety of functional substances during the fermentation of various grain. For example, kojic acid has been reported to have a tyrosinase inhibitory activity [7]. The amounts of antioxidants contained in soybean have been shown to increase during fermentation [8, 9]. On the other hand, rice bran enzymatic extract has been shown to improve the pathologic conditions of metabolic syndrome by attenuating dyslipidemia, hypertension, and insulin resistance [10]. Therefore, FBRA was expected to be a promising functional food beneficial to human health. Practically, FBRA has previously been shown to prevent the induction of acute colitis by dextran sulfate sodium through the modification of colonic microbiota [11, 12], and also reported to suppress the development of acute hepatitis in Long-Evans Cinnamon rats [13]. Furthermore, FBRA has been shown to prevent chemical and inflammation-related carcinogenesis in various organs of rodents [14–21]. However, potentially active components of FBRA have been still unidentified, and possible mechanisms underlying their biological effects have not yet been fully understood.
The cells damaged by mechanical impact and toxic chemicals are generally known to undergo a characteristic series of changes, and this process, named “apoptosis or programmed cell death”, is one of the ways to lead the damaged cells to death. In a multicellular organism, the cells were uniquely and highly organized, and the number of cells is tightly regulated not simply by controlling the rate of cell division but also by controlling the rate of cell death. Therefore, apoptotic cell death is considered to contribute to the maintenance of a healthy biofunction in developing and adult mammalian tissues. In a practical aspect, because the aberrant growth of tumor cells may be the result of avoiding the apoptotic cell death, a large number of apoptosis-inducible substances are speculated to bring about the prevention or suppression of tumor growth, thereby anticipating their availabilities for clinical use as anti-cancer agents. As an illustration of such substances, γ-tocotrienol, a kind of vitamin E, has been shown to inhibit the growth of gastric adenocarcinoma cells through the interruption of the cell cycle, resulting in the apoptotic cell death [22]. Cycloartenyl ferulate has also been reported to induce apoptosis of colon cancer cells through TRAIL-activated pathway [23]. Because these substances are known as the representative components of rice bran, it seems obviously reasonable to predict that FBRA may also have a potential intrinsic ability to induce the apoptotic death of cancer cells. Then, the aqueous extract was prepared from FBRA, and the cytotoxic effect of this extract was examined using cultured human acute lymphoblastic leukemia Jurkat cells.
Materials and Methods
Materials
Human acute lymphoblastic leukemia cells (Jurkat cells, RCB3052) were purchased from The RIKEN BRC Cell Bank (Tsukuba, Japan). FBRA powder was produced by fermenting the mixture of brown rice and rice bran with A. oryzae, and provided by Genmaikoso Co., Ltd. (Sapporo, Japan). Inhibitors of pan-caspase, caspase-8, and caspase-9 were obtained from R&D Systems, Inc. (Minneapolis, MN, USA). Antibodies against Bax, Bid, caspase-9, DR5, and Fas were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies against Bcl-2, caspase-3, caspase-8, and α-tubulin were from Bio Legend Inc. (San Diego, CA, USA). RPMI 1640 medium was from Sigma Chemical Co. (St Louis, MO, USA). Fetal bovine serum (FBS) was from Equitech Bio, Inc. (Kerrville, TX, USA). Other chemicals used were commercially available reagent grade or ultrapure grade.
Preparation of FBRA Extract
FBRA powder was suspended in distilled water at a ratio of 20 g to 100 ml, and sonicated at 40 kHz for 30 min on ice, and the mixture was then filtered through a Whatman no. 1 filter paper. The filtrate was centrifuged at 20,000×g for 2 h to remove insoluble materials, and the supernatant was sterilized by filtering through a 0.20-μm disk filter. The obtained extract was aliquoted and stored at −80 °C until use.
Cell Culture
Cells were grown in RPMI 1640 medium containing 10 % FBS, 100 units/ml of penicillin and 100 μg/ml of streptomycin at 37 °C in a humidified incubator containing 95 % air-5 % CO2 atmosphere.
Cell Viability Determination
Cell viability was assessed using a Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan). Briefly, the cells were plated on a 96-well microplate at a density of 2 × 104 cells/90 μl/well. FBRA extract was added to the cultures at the different concentrations in total volume of 100 μl, and incubated at 37 °C for 24, 48, and 72 h in a humidified incubator. CCK-8 solution (10 μl) was added to each well at the end of the exposure periods, and then incubated for additional 3 h. The absorbance at 450 nm was measured using an automated microplate reader (TECAN Japan, Kawasaki, Japan). In case of the application of caspase inhibitors, the inhibitors were added to the cell cultures at 4 h prior to the exposure to FBRA extract at the concentration of 100 μM pan-caspase inhibitor, 100 μM caspase-8 inhibitors and 25 μM caspase-9 inhibitor. In preliminary experiment, the cytotoxic effects of caspase inhibitors were examined, and the concentrations of the inhibitors used in this study were chosen as causing little or no toxic influence on the cell viability.
DNA Fragmentation Analysis
The cells were plated on a 60-mm culture dish at a density of 1.5 × 106 cells/dish, and exposed to the extract (100 μl/ml) for 24 h. The cells were collected by centrifuging at 600×g for 5 min at 4 °C, and washed with ice-cold PBS. The DNA fragmentation was determined according to the method of Zheng Dong, et al. [24] with slight modifications. Briefly, the cells were lysed in 400 μl of the hypotonic buffer [0.5 % Triton X-100, 10 mM Tris-HCl (pH 7.5), 10 mM EDTA]. Cell lysate was then centrifuged at 14,000×g for 20 min. The resultant supernatant was treated with proteinase K at 37 °C for 5 h followed by RNase A at 37 °C for 1 h. Proteins were removed with phenol/chloroform/isoamyl alcohol (25:24:1). DNA fragments were precipitated with 70 % ethanol at −20 °C overnight. The precipitated DNA was rinsed with 70 % ethanol, and then resuspended TE buffer [10 mM Tris-HCl, pH 7.5, 1 mM EDTA]. The DNA was separated on 2.0 % agarose gel in Tris-borate-EDTA buffer [90 mM Tris-borate, 2 mM EDTA]. Electrophoresis was carried out in the room temperature at 50 V for 2 h.
Western Blot Analysis
The cells were plated on a 60-mm culture dish at a density of 6 × 105 cells/dish, and exposed to the extract (0, 25, 50, and 100 μl/ml) for 24 h. The cells were collected by centrifugation at 2000×g for 5 min, washed with ice-cold PBS, resuspended in 50 μl lysis buffer [20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10 % glycerol, 1 % Nonidet P-40, 5 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin], and kept them for 30 min on ice. The lysate was centrifuged at 18,700×g at 4 °C for 15 min to remove the cell debris. The amount of proteins in supernatant was determined with BCA protein assay kit (Thermo Fisher Scientific Inc., USA). The samples (10 ∼ 30 μg of proteins) were boiled for 5 min in a sample buffer [62.5 mM Tris-HCl (pH 6.8), 2 % SDS (w/v), 50 mM DTT, 10 % glycerol (v/v), 0.01 % Bromophenol blue (w/v)], and then loaded on a 15 or 10 % SDS-polyacrylamide gel. The proteins were separated on the gel at 30 mA for 1.5 h, and electro-blotted onto a PVDF transfer membrane in a blotting buffer [5 % methanol, 100 mM Tris, 192 mM glycine] at 175 mA for 1.5 h. The membranes were soaked in Block Ace (DS Pharma Biomedical Co., Ltd. Suita, Japan) for 1 h at room temperature, and washed with 0.1 % TBST, and then incubated with competent primary antibodies. Anti-caspase-3, caspase-8, Bcl-2, and α-tubulin were diluted by 1:500. Anti-caspase-9, Bax, Bid, Fas, and DR5 were diluted by 1:1000. The membranes incubated overnight at 4 °C, and washed with 0.1 % TBST and further incubated with a peroxidase-conjugated secondary antibodies diluted by 1:2000 with Can Get Signal® (TOYOBO Co., Ltd. Osaka, Japan) for 1 h at room temperature. The membranes were washed, and the immunoreactive complexes were detected using the ECL™ prime Western Blotting Detection Reagent (GE Healthcare, Hino, Japan) following the manufacturer’s instructions. Together with the target proteins, α-tubulin was also determined as an internal standard.
Data Analysis
Results were presented as the mean ± standard deviation (SD). The data were analyzed by a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The differences between two groups at P < 0.05 were regarded statistically significant.
Results
Effect of FBRA Extract on the Cell Viability
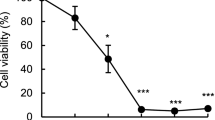
Jurkat cells were incubated with FBRA extract at the concentrations from 12.5 to 100 μl/ml for 24, 48, and 72 h, and the cell viabilities were then determined by a WST-8 assay. As shown in Fig. 1, the cell viability was reduced to approximately 62, 32, and 10 % of the control by the exposure to FBRA extract at the concentration of 25, 50, and 100 μl/ml for 24 h (Fig. 1a). Also, the cell viability was reduced to approximately 63 and 45 % of the control by the exposure to a low concentration of FBRA extract (12.5 μl/ml) for 48 and 72 h (Fig. 1b). Thus, FBRA extract was clearly shown to cause its toxic effect on Jurkat cells under the conditions used in the present study.
Direct effect of FBRA extract on viability of Jurkat cells. a Cells were exposed to various concentrations of FBRA extract for 24 h. b Cells were exposed to 12.5 μl/ml of FBRA extract for different periods. The cell viability was then determined as described in the text. Results were expressed as a percent of the control. Values are the mean ± SD (*p < 0.01 vs without FBRA extract, n = 3)
DNA Fragmentation
To address the question of whether the cytotoxic effect of FBRA extract observed here might be apoptotic or not, the genomic DNA was prepared from Jurkat cells treated with 100 μl/ml of FBRA extract for 24 h or 4 μM of camptothecin for 13 h as a positive control, and then analyzed using an agarose-gel electrophoresis. As shown in Fig. 2, the exposure to FBRA extract caused the fragmentation of genomic DNA, similar to the positive control. Therefore, FBRA extract was speculated to cause the damage to the genomic DNA in the nucleus without disrupting the plasma membrane, resulting in the cell death through the activation of apoptotic process.
Induction of genomic DNA fragmentation by FBRA extract in Jurkat cells. Cells were exposed to 100 μl/ml of FBRA extract for 24 h or 4 μΜ of camptothecin for 13 h. The genomic DNA was prepared from the cells and then separated by 2.0 % agarose-gel electrophoresis. The DNA fragments were visualized by the exposure to blue light (Safe Imager™ Transilluminator, Invitrogen), and captured by CCD camera using an image-capturing system
Recovery of the Cell Viability by Pan-caspase Inhibitor
To further verify that FBRA extract might cause the apoptotic damage, the cytotoxic effect of FBRA extract was examined again in the cells pretreated with pan-caspase inhibitor. As shown in Fig. 3, the cell viability was significantly reduced by exposure to FBRA extract for 24 h, and this reduction of the cell viability was significantly but not completely restored by pretreatment with a pan-caspase inhibitor. Therefore, it seemed possible to speculate that caspases might play an important role in the process connected with the cytotoxic effect of FBRA extract observed here.
Influence of pan-caspase inhibitor pretreatment on cytotoxic effect of FBRA extract on Jurkat cells. Cells were preincubated with 100 μΜ of pan-caspase inhibitor (Z-AVD-FMK) for 4 h, and then exposed to various concentrations of FBRA extract for 24 h. The cell viability was determined as described in the text. Results were expressed as a percent of the control. Values are the mean ± SD (*p < 0.01, vs without caspase inhibitor, n = 4)
FBRA Extract Altered the Expression of Caspases and Bcl-2 Family
Further studies on changes in apoptosis-associated proteins were carried. The cells were treated with a various concentration of FBRA extract for 24 h, and the cleavage of caspases was determined. Consequently, the amounts of cleaved caspase-3, caspase-8, and caspase-9 were increased in the cells exposed to FBRA extract (Fig. 4a). Furthermore, truncated Bid (tBid), which is active form of Bid, was increased and Bcl-2 was decreased by FBRA treatment, though Bax was not affected (Fig. 4b). Thus, FBRA extract was further confirmed to cause the cell death probably through the activation of apoptotic pathway.
Effect of FBRA extract on apoptosis-related protein expression in Jurkat cells. Cells were exposed to various concentrations of FBRA extract for 24 h, and the cell lysates were prepared for the protein analysis, and then Western blotting was carried out as described in the text. a active caspases, b Bcl-2 families, c α-tubulin
Effect of Initial Caspase Inhibitors on the Cell Viability
To clarify which apoptotic pathway might be involved, the effects of specific inhibitors for caspase-8 and -9 on the cytotoxic effect of FBRA extract were more precisely investigated. The cells were preincubated with caspase-8 inhibitor (Z-IETD-FMK) or caspase-9 inhibitor (Z-LEHD-FMK) for 4 h prior to the exposure to FBRA extract. Caspase-8 inhibitor restored the reduction of the cell viability induced by FBRA extract, but caspase-9 inhibitor failed to rescue the cells from the fatal damage induced by the extract (Fig. 5a, b). These results suggested that caspase-8 might be involved in the cellular mechanism underlying FBRA extract-induced cell death, proposing a possible role of non-mitochondrial apoptotic pathway in the cytotoxic effect of FBRA extract on Jurkat cells.
Effects of initial caspase inhibitors on cytotoxic effect of FBRA extract on Jurkat cells. Cells were preincubated with a 100 μM of caspase-8 inhibitor (Z-IETD-FMK) or b 25 μM of caspase-9 inhibitor (Z-LEHD-FMK) for 4 h, and exposed to various concentrations of FBRA extract for 24 h. The cell viability was then determined as described in the text. Results were expressed as a percent of the control. Values are the mean ± SD (**p < 0.01, *p < 0.05 vs without caspase inhibitor, n = 4)
Effect of FBRA Extract on the Death Receptor-related Protein Expression
To confirm a possible involvement of the death receptor-mediated pathway, the effect of FBRA extract on the expression of death receptors was examined, and the extract increased both DR5 and Fas protein levels in the cells (Fig. 6). Therefore, it seemed reasonable to consider that the cytotoxic effect of FBRA extract might be induced as a consequence of the activation of the death receptor-mediated pathway.
Discussion
In previous studies, FBRA has been reported to have the ability to suppress the growth of chemically-inducible carcinoma [14–20] and inflammation-related carcinoma [21] in rodents, but the mechanism underlying the suppressive effect of FBRA on tumor growth has not yet been fully understood, and therefore it seemed necessary to investigate the direct effect of FBRA on the growth of cancer cells in culture. Then, we first examined the effect of FBRA extract on the growth of human lymphoblastic leukemia Jurkat cells, and showed that FBRA extract markedly reduced the viability of Jurkat cells in a concentration- and time-dependent manner (Fig. 1a, b). The reduction of the cell viability was speculated to suggest that FBRA extract might be able to result in the suppression of cancer growth probably through the induction of tumor cell death, and therefore we next focused on the ability of FBRA extract to induce apoptosis, which is generally appreciated as one of the typical ways to prevent cancer growth [25].
Apoptosis is known to be mediated by various intracellular signal transduction cascades, and characterized by specific events, such as caspase activation, cell shrinkage, chromatin condensation, apoptotic bodies formation and so on [26]. In contrast, another mechanism of the cell death, called necrosis, is not accompanied by these specific events, and considered to be primarily initiated by the disruption of the plasma membrane function. Then, to determine whether the cytotoxity of FBRA extract was based on the induction of apoptosis or necrosis, the cells were exposed to FBRA extract, and the induction of DNA fragmentation and the activation of caspases in these cells were analyzed. The treatment of the cells with 100 μl/ml of FBRA extract was shown to cause the genomic DNA fragmentation, similar to that caused by a reference drug camptothecin (Fig. 2), and the pretreatment with a pan-caspase inhibitor (Z-VAD-FMK) was shown to restore the reduction of the cell viability induced by the extract (Fig. 3). These findings suggest that FBRA extract may be able to cause the toxic damage to Jurkat cells, thus resulting in their apoptotic death under the in vitro experimental conditions used in the present study. Moreover, it seemed worthy of attention that the cell viability reduced by FBRA extract could be partially but not fully restored by a pan-caspase inhibitor (Z-VAD-FMK) (Fig. 3). Therefore, it seems possible to speculate that FBRA extract may be able to cause the cell death probably through two separate pathways, one of which may be dependent on caspase activation and the other may not.
Next, we discuss the pathway toward activation of caspase-3. In addition to the mitochondrial apoptotic pathway, the non-mitochondrial death receptor-mediated pathway is appreciated as another process involved in the apoptotic cell death, and this process can be summarized as follows: The death receptor ligands, such as TNF-α and Fas ligand, bind to their cognate death receptors on the plasma membranes, leading to the activation of caspase-8, and it activate caspase-3 subsequently, thus resulting in the apoptotic cell death [27, 28]. On the other hand, the mitochondria apoptotic pathway is initiated by a variety of stress signals, which can induce the release of cytochrome c from mitochondria through modulating the expression of Bcl-2 family, resulting in the activation of caspase-9, and it also activate caspase-3, thus promoting the downstream signaling pathway to the apoptotic cell death [29–32]. Based on these findings, the effect of FBRA extract on individual caspases was examined, and the extract was shown to cause the cleavage of caspase-8, -9, and -3, thus resulting in the activation of these enzymes in the cells (Fig. 4a). Further studies also showed that FBRA extract caused a decrease in the anti-apoptotic protein Bcl-2 without altering the pro-apoptotic protein Bax (Fig. 4b). Since the increased Bax/Bcl-2 ratio is known to be connected with the release of cytochrome c from mitochondria, thereby resulting in the activation of apoptotic pathway [33, 34], it also seems possible to consider that FBRA extract may be able to stimulate the mitochondrial pathway as well as the death receptor-mediated pathway in the cells. Consistent with this interpretation, FBRA extract was practically shown to cause an increase in tBid by activating caspase-8 [35] as shown in Fig 4b, thus promoting the efflux of cytochrome c from mitochondria to cell cytoplasm [36, 37].
Furthermore, to confirm the apoptotic pathway involved in the cell death induced by FBRA extract, the additional studies were carried out using specific caspase inhibitors. The pretreatment with caspase-8 inhibitor was shown to rescue the cells from the cytotoxic effect of FBRA extract, but caspase-9 inhibitor failed to rescue the cells under the same conditions (Fig. 5a, b). These findings seem to propose the possibility that FBRA extract may trigger the apoptotic death of Jurkat cells as a consequence of activating the death receptor-mediated pathway, and that tBid-mediated mitochondrial pathway followed by caspase-9 activation may not be responsible for FBRA induced apoptotic cell death, because caspase-9 inhibitor was ineffective for the recovery of cell viability. Moreover, the expression of death receptor is considered to be involved in the input of apoptosis-inducing signal, and the upregulation of DR5 has recently been shown to enhance TRAIL-induced apoptotic cell death [38]. Then, the effect of FBRA extract on the death receptor expression was examined in Jurkat cells, and the exposure to FBRA extract was shown to cause the enhancement of DR5 and Fas protein expression in the cells (Fig. 6). Therefore, it seems possible to speculate that FBRA extract may enhance the expression of the death receptor, and make it more sensitive to the death receptor ligands, thus resulting in the induction of apoptotic death of Jurkat cells under the experimental conditions used in the present study.
Recently, we have reported that FBRA extract has caused the apoptotic death of human colorectal tumor HCT116 cells by activating mitochondrial pathway [39]. However, FBRA extract-induced apoptosis on Jurkat cells mainly via non-mitochondrial pathway in this study. It is interesting that the mechanism by which FBRA or other agents induce apoptotic death appears to depend on the cell type used. Desipramine, which is a tricyclic antidepressant, induced apoptotic cell death through non-mitochondrial and mitochondrial pathways in different types of human colon carcinoma cells [40]. TWEAK, which is a TNF family member and is produced by IFN-γ-stimulated monocytes, could induce multiple pathways of cell death, including both caspase-dependent apoptosis and also cathepsin B-dependent necrosis [41]. It seems possible to speculate that these multiple mechanisms of FBRA for apoptosis induction based on cell types would be a clue to recognize that FBRA has preventive effects on various chemical carcinogenesis in rodents.
In conclusion, we showed that FBRA extract-induced apoptosis of Jurkat cells probably through the death receptor-mediated pathway and supplementarily through the tBid-mediated mitochondrial pathway. Therefore, FBRA would be speculated as one of the functional foods effective for the suppression of cancer growth. However, the active components of FBRA are still unidentified, and therefore still remains to be further investigated.
References
Behall, K. M., Scholfield, D. J., & Hallfrisch, J. (2006). Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women. Journal of the American Dietetic Association, 106(9), 1445–1449.
Panlasigui, L. N., & Thompson, L. U. (2006). Blood glucose lowering effects of brown rice in normal and diabetic subjects. International Journal of Food Science and Nutrition, 57(3–4), 151–158.
Seki, T., Nagase, R., Torimitsu, M., Yanagi, M., Ito, Y., Kise, M., Mizukuchi, A., Fujimura, N., Hayamizu, K., & Ariga, T. (2005). Insoluble fiber is a major constituent responsible for lowering the post-prandial blood glucose concentration in the pre-germinated brown rice. Biological and Pharmaceutical Bulletin, 28(8), 1539–1541.
Das, U., Manna, K., Sinha, M., Datta, S., Das, D. K., Chakraborty, A., Ghosh, M., Saha, K. D., & Dey, S. (2014). Role of ferulic acid in the amelioration of ionizing radiation induced inflammation: a murine model. PLoS One, 9(5), e97599.
Wilson, T. A., Nicolosi, R. J., Woolfrey, B., & Kritchevsky, D. (2007). Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. Journal of Nutritional Biochemistry, 18(2), 105–112.
Canan, C., Delaroza, F., Casagrande, R., Baracat, M. M., Shimokomaki, M., & Ida, E. I. (2012). Antioxidant capacity of phytic acid purified from rice bran. Acta Scientiarum Technology, 34(4), 457–463.
Kim, A. J., Choi, J. N., Kim, J., Kim, H. Y., Park, S. B., Yeo, S. H., Choi, J. H., Liu, K. H., & Lee, C. H. (2012). Metabolite profiling and bioactivity of rice koji fermented by Aspergillus strains. Journal of Microbiology and Biotechnology, 22(1), 100–106.
Esaki, H., Kawakishi, S., Morimitsu, Y., & Osawa, T. (1999). New potent antioxidative o-dihydroxyisoflavones in fermented Japanese soybean products. Bioscience Biotechnology and Biochemistry, 63(9), 1637–1639.
Hirota, A., Taki, S., Kawaii, S., Yano, M., & Abe, N. (2000). 1,1-Diphenyl-2-picrylhydrazyl radical-scavenging compounds from soybean miso and antiproliferative activity of isoflavones from soybean miso toward the cancer cell lines. Bioscience Biotechnology and Biochemistry, 64(5), 1038–1040.
Justo, M. L., Rodriguez-Rodriguez, R., Claro, C. M., Alvarez de Sotomayor, M., Parrado, J., & Herrera, M. D. (2013). Water-soluble rice bran enzymatic extract attenuates dyslipidemia, hypertension and insulin resistance in obese Zucker rats. European Journal of Nutrition, 52(2), 789–797.
Kataoka, K., Kibe, R., Kuwahara, T., Hagiwara, M., Arimochi, H., Iwasaki, T., Benno, Y., & Ohnishi, Y. (2007). Modifying effects of fermented brown rice on fecal microbiota in rats. Anaerobe, 13(5–6), 220–227.
Kataoka, K., Ogasa, S., Kuwahara, T., Bando, Y., Hagiwara, M., Arimochi, H., Nakanishi, S., Iwasaki, T., & Ohnishi, Y. (2008). Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Digestive Diseases and Sciences, 53(6), 1601–1608.
Shibata, T., Nagayasu, H., Kitajo, H., Arisue, M., Yamashita, T., Hatakeyama, D., Iwasaki, T., & Kobayashi, H. (2006). Inhibitory effects of fermented brown rice and rice bran on the development of acute hepatitis in Long-Evans Cinnamon rats. Oncology Reports, 15(4), 869–874.
Katyama, M., Yoshimi, N., Yamada, Y., Sakata, K., Kuno, T., Yoshida, K., Qiao, Z., Vihn, P. Q., Iwasaki, T., Kobayashi, H., & Mori, H. (2002). Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncology Reports, 9(4), 817–822.
Katayama, M., Sugie, S., Yoshimi, N., Yamada, Y., Sakata, K., Qiao, Z., Iwasaki, T., Kobayashi, H., & Mori, H. (2003). Preventive effect of fermented brown rice and rice bran on diethylnitrosoamine and phenobarbital-induced hepatocarcinogenesis in male F344 rats. Oncology Reports, 10(4), 875–880.
Kuno, T., Hirose, Y., Hata, K., Kato, K., Qiang, S. H., Kitaori, N., Hara, A., Iwasaki, T., Yoshimura, T., Wada, K., Kobayashi, H., & Mori, H. (2004). Preventive effect of fermented brown rice and rice bran on N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats. International Journal of Oncology, 25(6), 1809–1815.
Kuno, T., Hirose, Y., Yamada, Y., Hata, K., Qiang, S. H., Asano, N., Oyama, T., Zhi, H., Iwasaki, T., Kobayashi, H., & Mori, H. (2006). Chemoprevention of mouse urinary bladder carcinogenesis by fermented brown rice and rice bran. Oncology Reports, 15(3), 533–538.
Long, N. K., Makita, H., Yamashita, T., Toida, M., Kato, K., Hatakeyama, D., & Shibata, T. (2007). Chemopreventive effect of fermented brown rice and rice bran on 4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats. Oncology Reports, 17(4), 879–885.
Tomita, H., Kuno, T., Yamada, Y., Oyama, T., Asano, N., Miyazaki, Y., Baba, S., Taguchi, A., Hara, A., Iwasaki, T., Kobayashi, H., & Mori, H. (2008). Preventive effect of fermented brown rice and rice bran on N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Oncology Reports, 19(1), 11–15.
Phutthaphadoong, S., Yamada, Y., Hirata, A., Tomita, H., Taguchi, A., Hara, A., Limtrakul, P. N., Iwasaki, T., Kobayashi, H., & Mori, H. (2009). Chemopreventive effects of fermented brown rice and rice bran against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in female A/J mice. Oncology Reports, 21(2), 321–327.
Phutthaphadoong, S., Yamada, Y., Hirata, A., Tomita, H., Hara, A., Limtrakul, P., Iwasaki, T., Kobayashi, H., & Mori, H. (2010). Chemopreventive effect of fermented brown rice and rice bran (FBRA) on the inflammation-related colorectal carcinogenesis in ApcMin/+ mice. Oncology Reports, 23(1), 53–59.
Sun, W., Xu, W., Liu, H., Liu, J., Wang, Q., Zhou, J., Dong, F., & Chen, B. (2009). gamma-Tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. Journal of Nutritional Biochemistry, 20(4), 276–284.
Kong, C. K., Lam, W. S., Chiu, L. C., Ooi, V. E., Sun, S. S., & Wong, Y. S. (2009). A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochemical Pharmacology, 77(9), 1487–1496.
Dong, Z., Saikumar, P., Weinberg, J. M., & Venkatachalam, M. A. (1997). Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death. Involvement of serine but not cysteine proteases. The American Journal of Pathology, 151(5), 1205–1213.
Fisher, D. E. (1994). Apoptosis in cancer therapy: crossing the threshold. Cell, 78(4), 539–542.
Elmore, S. (2007). Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35(4), 495–516.
Kruidering, M., & Evan, G. I. (2000). Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life, 50(2), 85–90.
Hirata, H., Takahashi, A., Kobayashi, S., Yonehara, S., Sawai, H., Okazaki, T., Yamamoto, K., & Sasada, M. (1998). Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. The Journal of Experimental Medicine, 187(4), 587–600.
Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S. M., Ahmad, M., Alnemri, E. S., & Wang, X. (1997). Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell, 91(4), 479–489.
Hengartner, M. O. (2000). The biochemistry of apoptosis. Nature, 407(6805), 770–776.
Zimmermann, K. C., & Green, D. R. (2001). How cells die: apoptosis pathways. Journal of Allergy and Clinical Immunology, 108(4 suppl), s99–s103.
Salvesen, G. S. (2002). Caspases: opening the boxes and interpreting the arrows. Cell Death and Differentiation, 9(1), 3–5.
Bhujade, A., Gupta, G., Talmale, S., Das, S. K., & Patil, M. B. (2013). Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food & Function, 4(2), 338–346.
Middleton, G., Cox, S. W., Korsmeyer, S., & Davies, A. M. (2000). Differences in bcl-2- and bax-independent function in regulating apoptosis in sensory neuron populations. The European Journal of Neuroscience, 12(3), 819–827.
Li, H., Zhu, H., Xu, C. J., & Yuan, J. (1998). Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell, 94(4), 491–501.
Eskes, R., Desagher, S., Antonsson, B., & Martinou, J. C. (2000). Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Molecular and Cellular Biology, 20(3), 929–935.
Kim, T. H., Zhao, Y., Barber, M. J., Kuharsky, D. K., & Yin, X. M. (2000). Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. The Journal of Biological Chemistry, 275(50), 39474–39481.
Todo, M., Horinaka, M., Tomosugi, M., Tanaka, R., Ikawa, H., Sowa, Y., Ishikawa, H., Fujiwara, H., Otsuji, E., & Sakai, T. (2013). Ibuprofen enhances TRAIL-induced apoptosis through DR5 upregulation. Oncology Reports, 30(5), 2379–2384.
Itoh, M., Nishibori, N., Sagara, T., Horie, Y., Motojima, A., & Morita, K. (2012). Extract of fermented brown rice induces apoptosis of human colorectal tumor cells by activating mitochondrial pathway. Phytotherapy Research, 26(11), 1661–1666.
Arimochi, H., & Morita, K. (2008). Desipramine induces apoptotic cell death through nonmitochondrial and mitochondrial pathways in different types of human colon carcinoma cells. Pharmacology, 81(2), 164–172.
Nakayama, M., Ishidoh, K., Kayagaki, N., Kojima, Y., Yamaguchi, N., Nakano, H., Kominami, E., Okumura, K., & Yagita, H. (2002). Multiple pathways of TWEAK-induced cell death. Journal of Immunology, 168(2), 734–743.
Acknowledgments
We thank Dr. Alan F. Hofmann (University of California, San Diego, CA) and Dr. Shigeo Ikegawa (Genmaikoso Co., Ltd) for their help in the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horie, Y., Nemoto, H., Itoh, M. et al. Fermented Brown Rice Extract Causes Apoptotic Death of Human Acute Lymphoblastic Leukemia Cells via Death Receptor Pathway. Appl Biochem Biotechnol 178, 1599–1611 (2016). https://doi.org/10.1007/s12010-015-1970-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1970-y