Abstract
The platform chemical 2,3-butanediol (2,3-BDO) is a valuable product that can be converted into several petroleum-based chemicals via simple chemical reactions. Here, we produced 2,3-BDO with the non-pathogenic and rapidly growing Corynebacterium glutamicum. To enhance the 2,3-BDO production capacity of C. glutamicum, we introduced budA encoding acetolactate decarboxylase from Klebsiella pneumoniae, a powerful 2,3-BDO producer. Additionally, budB (encoding α-acetolactate synthase) and budC (encoding acetoin reductase) were introduced from K. pneumoniae to reinforce the carbon flux in the 2,3-BDO production. Because budC had a negative effect on 2,3-BDO production in C. glutamicum, the budB and budA introduced strain, SGSC102, was selected for 2,3-BDO production, and batch culture was performed at 30 °C, 250 rpm and pH 6.86 with pure glucose, molasses, and cassava powder as carbon substrates. After batch culture, significant amount of 2,3-BDO (18.9 and 12.0 g/L, respectively) was produced from 80 g/L of pure glucose and cassava powder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the current instability in fossil fuel supplies and petroleum prices, microbial production of petroleum-based compounds is receiving significant attention globally. One chemical, 2,3-butanediol (2,3-BDO), is favorable for microbial production and is a platform chemical that can be converted into several petroleum-based chemicals via simple chemical reactions [1]. For example, 1,3-butadiene, which is an organic intermediate for synthetic rubber production, is a major derivative of 2,3-BDO. Methyl ethyl ketone is an effective fuel additive having a higher heat of combustion than ethanol [2], and diacetyl is a bacteriostatic food additive since it inhibits growth of some microorganisms and all of the above can be obtained from 2,3-BDO via simple chemical conversion [3]. Additionally, due to its low freezing point of −60 °C, 2,3-BDO can be used as an antifreeze agent substituting for ethylene glycol. For these reasons, production of 2,3-BDO from the microbial organisms has been intensively studied in recent years. A number of species in the genera Klebsiella, Bacillus, Pseudomonas, Enterobacter, and Serratia can majorly produce 2,3-BDO from pyruvate, which is the final product of the glycolysis pathway [1]. For example, significant amounts of 2,3-BDO was produced by engineered Enterobacter aerogenes and Serratia marcescens (118.05 and 139.92 g/L of 2,3-BDO produced from fed-batch fermentation, respectively) [4–5]. Among these species, Klebsiella pneumoniae, a gram-negative bacterium in the family Enterobacteriaceae, is one of the most useful industrial 2,3-BDO producers because of its high efficiency and ease of culture [6]. Fundamental 2,3-BDO production of K. pneumoniae is very powerful. For example, ldhA, which is involved in biosynthesis of lactate (a byproduct of 2,3-BDO production), was disrupted and budB and budA involved in 2,3-BDO biosynthesis were overexpressed, resulting in 111.3 g/L of 2,3-BDO produced with a productivity of 2.71 g/L h in fed-batch fermentation [7]. However, natural 2,3-BDO producers including K. pneumoniae are pathogenic, so attenuation of the pathogenicity of these species has to be carried out prior to industrial production. To avoid this limitation, 2,3-BDO production from non-pathogenic species has also been studied actively. For example, Escherichia coli, one of the well-known microbial producers of industrial chemicals, produced 73.8 g/L [8] and Saccharomyces cerevisiae, a prominent ethanol producer, produced 96.2 g/L of 2,3-BDO [9] from the fed-batch fermentation using glucose as a carbon source. Now, a new non-pathogenic industrial 2,3-BDO producer, Corynebacterium glutamicum is suggested. C. glutamicum is a non-pathogenic, non-sporulating, rapidly growing gram-positive soil bacterium that has been a producer of industrial amino acids. This species was found to produce glutamate efficiently, and to promote amino acid production by fermentation. Other amino acids, such as lysine, valine, and tryptophan, and organic acids, such as succinate and lactate, have subsequently been produced by the industrial fermentation of C. glutamicum [10]. C. glutamicum can also produce 2,3-BDO via its own carbohydrate metabolism naturally, but it does not have the key enzyme called acetolactate decarboxylase (encoded by budA in K. pneumoniae) that plays a key role in anaerobic 2,3-BDO synthesis; therefore, it cannot produce 2,3-BDO in significant quantities.
In this study, we produced 2,3-BDO by a minimal genetic engineered C. glutamicum harbors core genes participating in 2,3-BDO biosynthesis from the powerful 2,3-BDO producer, K. pneumoniae. In addition, we demonstrated the industrial advantage of this C. glutamicum that can utilize carbon sources such as cassava powder and molasses, and rapidly grow and produce 2,3-BDO in minimal media comprised by cheap inorganic compounds. Finally, we verified the potential for low cost industrial 2,3-BDO production by C. glutamicum.
Materials and Methods
Development of Strains and Plasmids
The strains, plasmids, and primers used in this study are listed in Table 1 [11]. budA, budB, and budC were derived from K. pneumoniae KCTC2242 (Korean Collection for Type Culture). Escherichia coli DH5α [F-(80d lacZ M15) (lacZYA -rgF)U1691 hsdR17(m+) recA1 endA1 relA1 deoR; Dong In Biotech Co., Korea] was used for the manipulation of plasmids and for gene cloning.
Enzymes and Chemicals
A DNeasy Tissue Kit (Qiagen, Netherlands) was used to isolate genomic DNA from K. pneumoniae KCTC2242. An AxyPrep Plasmid Miniprep Kit (Axygen, USA) was used to isolate bacterial plasmid DNA. Restriction enzymes and T4 DNA ligase were purchased from Takara Shuzo. A gel extraction kit (Takara, Japan, A550) was used for large-scale isolation of plasmid DNA from gels.
Strain Development
Sequence data for budA, budB, and budC in K. pneumoniae KCTC2242 were provided by the National Center for Biotechnology Information (NCBI) GenBank (CP002910.1) [12].
budA, budB, and budC and the whole budABC operon were amplified by polymerase chain reaction (PCR) using K. pneumoniae genomic DNA as a template and appropriate primers (Table 1.). The PCR conditions were as follows: 95 °C for 5 min, followed by 25 cycles at 95 °C for 30 s, the specific annealing temperature (budA 60 °C, budB 61 °C, budC 58 °C, budABC 59 °C) for 55 s, and 72 °C for 60 s. The PCR products were ligated into the pGEM-T Easy Vector (Promega, USA) with the four gene combinations. Subsequently, the target genes were double restriction enzyme digested and ligated into the pEKEx2 shuttle expression vector, and four sets of recombinant pEKEx2 plasmids were constructed. The constructed vectors (Table 1.) were transformed into C. glutamicum using the electroporation shock and heat shock methods [13–14].
Culture Conditions
Seed cultivation of all strains was performed in brain heart infusion (BHI) medium (Difco, Detroit, MI, USA), comprised of 5 g/L of beef heart, 12.5 g/L of calf brains, 2.5 g/L of disodium hydrogen phosphate, 2 g/L of glucose, 10 g/L of peptone, and 5 g/L of sodium chloride at 30 °C and 200 rpm for 12 h. In the cultivation of recombinant strains, 25 mg/L of kanamycin was added as a selection marker, and IPTG induction was conducted at a cell optical density (OD) of 0.6~0.8. Flask cultivation was performed in CGXII medium, comprised of 40 g/L of glucose, 20 g/L of ammonium sulfate, 0.25 g/L of magnesium sulfate heptahydrate, 10 mg/L of iron(II) sulfate heptahydrate, 10 mg/L of manganese(II) sulfate monohydrate, 10 mg/L of calcium chloride dehydrate, 1 mg/L of zinc sulfate heptahydrate, 0.2 mg/L of copper(II) sulfate pentahydrate, and 20 μg/L of nickel(II) chloride hexahydrate with 0.2 M potassium phosphate buffer at 30 °C and 200 rpm for 30 h in a 250-mL Erlenmeyer flask and 50-mL operating volume. In batch cultivation, all carbon substrates (pure glucose, cassava powder, and molasses) were adjusted to 80 g/L, and other medium compositions were the same as the CGXII medium with 0.2 M potassium phosphate buffer. Cassava powder was provided Changhae Ethanol (Korea) and pre-treated by liquefaction and saccharification to convert starch to glucose. The stock culture was inoculated into a 2.5-L bioreactor (Kobiotech, South Korea) adjusted to 0.25 of the initial cell OD and 1-L operating volume. The temperature of bioreactor was maintained at 30 °C. Dissolved oxygen was provided by injection of filtered air at a flow rate of 1.5 vvm, and the agitation speed was maintained at 250 rpm. The pH was maintained at 6.86 through automatic addition of 5 N NaOH and 5 N H3PO4 solutions. All batch cultures were repeated three times.
Analytical Methods
The cell OD of the cultures was assayed with a spectrophotometer (Jenway, UK) at 600 nm and an appropriate dilution. Samples were withdrawn periodically and then centrifuged at 12,470×g for 10 min. The amount of the carbon source, 2,3-BDO, and organic acids in the supernatants were measured on a high-performance liquid chromatography (HPLC) system using an RI2414 detector (Waters Co., USA) and an Aminex HPX-87H organic acid column (300 × 7.8 mm; Bio-Rad) [15]. Sulfuric acid solution (0.01 M) was used as the mobile phase. The temperature was 80 °C, and the flow rate was 0.6 mL/min. All solutions were filtered through a 0.2-μm PVDF membrane before use.
Results and Discussion
Development of 2,3-BDO Producing C. glutamicum Strains
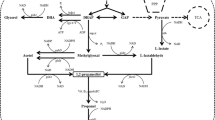
In general, 2,3-BDO is synthesized via three chemical reactions facilitated by the enzymes α-acetolactate synthase, α-acetolactate decarboxylase, and acetoin reductase in natural 2,3-BDO-producing strains. C. glutamicum is known as natural 2,3-BDO producer [3], but lacks the α-acetolactate decarboxylase that plays a key role in efficient 2,3-BDO production, according to pathway information from online databases such as the Kyoto Encyclopedia of Genes and Genomes (KEGG). Wild-type C. glutamicum produces 2,3-BDO via a diacetyl intermediate synthesized by a spontaneous reaction from α-acetolactate, but the driving force of this spontaneous reaction is weak when compared with the strong reaction catalyzed by α-acetolactate decarboxylase [16]. Therefore, to enhance the 2,3-BDO-producing ability of C. glutamicum, we introduced budA encoding the acetolactate decarboxylase from K. pneumoniae, a powerful 2,3-BDO producer. Also, budB (encoding α-acetolactate synthase) and budC (encoding acetoin reductase) were introduced from K. pneumoniae to reinforce the carbon flux in the 2,3-BDO production process, despite C. glutamicum naturally possessing ilvB and butA, with similar respective functions. As a result, four strains harboring K. pneumoniae genes participating in 2,3-BDO synthesis were developed and central anaerobic carbon metabolism including constructed 2,3-BDO pathway in C. glutamicum represented in Fig. 1.
Flask Cultures of Developed Strains in CGXII Minimal Medium with Potassium Phosphate Buffer
After strain development, we selected the best 2,3-BDO-producing strain among the four strains developed. The capacity for 2,3-BDO production was measured at 30 h in the 50-mL flask culture (Fig. 2). In the 30-h flask culture, the wild-type strain produces an undetectable amount of 2,3-BDO. In contrast, the engineered SGSC101 and SGSC102 strains produced significant amounts of 2,3-BDO (1.76 and 3.19 g/L, respectively) in the 30-h flask culture. In the cases of SGSC103 and SGSC104 strain, despite the capability of producing 2,3-BDO, they could not produce significant amounts of 2,3-BDO (0.426 and 0.0230 g/L, respectively). As a result, the SGSC102 strain, with budB and budA overexpressed, had the highest capacity for 2,3-BDO production and was selected as the main strain for subsequent experiments.
Comparison of cell growth and metabolite production in five developed C. glutamicum strains at 30 h of flask culture. a Cell optical density (black), glucose consumption (white). b Succinate concentration (dashed gray), lactate concentration (dashed light gray), acetate concentration (dashed white). Black bars represent standard deviation
Most importantly, the insertion of budA that plays a major role in 2,3-BDO biosynthesis provided C. glutamicum with this 2,3-BDO production capacity, and blocked the production of other organic acids, including succinate, lactate, and acetate. This result can explain the radical metabolic change that occurred with the introduction of only one gene: this budA has powerful central carbon metabolism. Overexpression of other genes involved in 2,3-BDO biosynthesis, budB and budC, also significantly influenced the 2,3-BDO production capacity of C. glutamicum. A significant decrease in 2,3-BDO production by SGSC103 and SGSC104 can be explained by budC overexpression, which has a negative effect to 2,3-BDO biosynthesis in C. glutamicum. The acetoin reductase encoded by budC in K. pneumoniae also participates in a reverse reaction that converts 2,3-BDO to acetoin, as has been demonstrated in previous studies [17]. Therefore, we suppose that the introduction of acetoin reductase from K. pneumoniae disturbed the 2,3-BDO synthesis by facilitating the reverse reaction at the physiological conditions of C. glutamicum. On the other hand, the highest capacity for 2,3-BDO production observed in strain SGSC102 can be explained by the knowledge that budB overexpression can reinforce the carbon flux from pyruvate to 2,3-BDO production in C. glutamicum. First, α-acetolactate synthase encoded by budB in K. pneumoniae has stronger activity and substrate affinity than other isozymes involved in branched amino acid biosynthesis (including ilvB of C. glutamicum) [8], and this powerful catalytic activity can facilitate the efficient conversion of pyruvate to α-acetolactate and 2,3-BDO production. Second, from the enhanced carbon source uptake of SGSC102 (Fig. 2a), we found that this reinforcement also promoted the carbon source uptake in C. glutamicum.
Validation of 2,3-BDO Production in the SGSC102 Strain
To validate the 2,3-BDO production capacity of strain SGSC102, a batch culture was performed. The batch culture profiles of wild-type and SGSC102 strain are shown in Fig. 3. Because CGXII has abundant nitrogen sources (5 g/L of urea and 20 g/L of ammonium sulfate), we increased the initial carbon substrate, glucose, from 40 to 80 g/L. An increase in the carbon substrate enabled us to identify the potential for mass, continuous 2,3-BDO production. Both strains grew similarly and consumed 80 g/L of glucose within a 76-h culture time. Commonly, until 12 h of culture time, cells grew by aerobic carbon metabolism (TCA cycle) and a very slight amount of glucose was consumed. In contrast, after 12 h of culture time, oxygen depletion due to high cell density changed the aerobic metabolism to anaerobic metabolism, and this anaerobic metabolism was radically altered in the SGSC102 strain. The wild-type strain produced primarily lactate (27.4 g/L), with succinate and acetate produced concomitantly (8.54 and 10.9 g/L, respectively). The SGSC102 strain, however, produced 18.9 g/L of 2,3-BDO and 2.49 g/L of acetoin, with a reduced amount of other organic acids (lactate 8.43 g/L, succinate 2.16 g/L, and acetate 2.36 g/L). In addition, a slight amount (1.93 g/L) of glycerol was produced only in the SGSC102 strain. The introduction of budB and budA from K. pneumoniae adjusted the carbon flux from pyruvate to other fermentative metabolites and promoted the expression of butA encoding acetoin reductase in C. glutamicum. This promotion of butA expression can be identified by the glycerol production in the SGSC102 strain, because acetoin reductase facilitates another reaction converting glyceraldehyde (an intermediate of glycolysis) to glycerol [18]. In addition, 2,3-BDO is neutral, and the non-toxic metabolites are physiologically favorable to C. glutamicum in comparison with the organic acids. From a high initial carbon substrate batch culture, the capacity and durability of 2,3-BDO production in SGSC102 was validated.
Batch culture profiles of wild-type strain and SGCG102 strain. Closed shapes represent wild-type strain, and open shapes represent SGCG102. a Cell optical density (diamonds), glucose concentration (squares). b Succinate concentration (squares), lactate concentration (circles), acetate concentration (triangles). c Glycerol concentration (inverted triangles), acetoin concentration (stars), 2,3-BDO concentration (diamonds). Black bars represent standard deviation
2,3-BDO Production Using an Industrial Carbon Substrate for the SGSC102 Strain
We took note of the carbon substrate as a key for industrial 2,3-BDO production. Because CGXII media is comprised of inorganic nitrogen sources and other trace elements, the carbon substrate, the main material of 2,3-BDO production, will determine the cost of 2,3-BDO production. Pure glucose is one of the best available carbon substrates, but the price of pure glucose is much more expensive than other industrial carbon sources. Therefore, microbial productions using pure glucose are losing competitiveness in industry. Recognizing these trends, we tested two widely available and cheaper carbon sources, molasses and cassava powder, that can be utilized by C. glutamicum in 2,3-BDO production. Because it is known that C. glutamicum can utilize only hexose sugars, industrial carbon substrates containing only hexose should be chosen. Sugar cane molasses, a viscous byproduct of the process of refining sugarcane into sugar, is comprised of three hexoses, mainly sucrose and an equal amount of fructose and glucose, that can be utilized by C. glutamicum. Because C. glutamicum can take up and utilize various sugars simultaneously [19], molasses is only one of the promising carbon substrates for 2,3-BDO production. Cassava is the third largest source of food carbohydrates in the tropics, after rice and maize, and gives the third highest yield of carbohydrates per cultivated area among crop plants, after sugarcane and sugar beets. The price of cassava is much lower than pure glucose, and dried cassava powder is comprised of predominantly sugars (80 % of dry weight, approximately), especially glucose. However, because glucose in cassava powder exists as a starch, a long polysaccharide chain consisting of a large number of glucose units, cassava powder must be pre-treated by liquefaction and saccharification in proper conditions. In molasses, 52.3 % of the initial polysaccharide content was released as glucose, fructose, and sucrose (9.12 g/L of glucose, 9.91 g/L of fructose, and 22.8 g/L of sucrose in 80 g/L of molasses), and in cassava powder, 74.9 % of the initial polysaccharide content was released as glucose and fructose (56.7 g/L of glucose and 2.17 g/L of fructose in 80 g/L of cassava powder). After batch culture using 80 g/L of cassava powder, 12.0 g/L of 2,3-BDO was produced within 60 h of culture time using the cassava powder (Fig. 4). In the case of molasses, a strong growth inhibition was observed during the initial culture time, and only a small amount of 2,3-BDO was produced. This inefficiency originated from the high viscosity of molasses, and high viscosity obstructed oxygen transfer and inhibiting the initial growth. In addition, co-consumption of glucose, fructose, and sucrose is expected to be less efficient than consumption of pure glucose. In the case of cassava powder, because the viscosity is less than molasses and the sugar composition is almost pure glucose, the production of 2,3-BDO was almost as efficient as culture using pure glucose.
Batch culture profiles of SGCG102 strain with the utilization of each industrial carbon substrate. Closed shapes represent molasses culture, and open shapes represent cassava powder culture. a Cell optical density (diamonds), glucose concentration (squares), fructose concentration (circles), sucrose concentration (triangles). b Succinate concentration (squares), lactate concentration (circles), acetate concentration (triangles). c Glycerol concentration (inverted triangles), acetoin concentration (stars), 2,3-BDO concentration (diamonds). Black bars represent standard deviation
Conclusion
In this paper, we identified the potential for 2,3-BDO production using non-pathogenic C. glutamicum and cassava powder as a cheap carbon source. Because of its non-pathogenicity and rapid growth in minimal media composed of only inorganic nitrogen sources, C. glutamicum has an advantage to the industrial production of 2,3-BDO. In addition, using cassava powder that is cheaper than pure glucose as a carbon substrate will reduce the raw material cost of industrial 2,3-BDO production. With further study, C. glutamicum has the possibility of becoming a non-pathogenic industrial 2,3-BDO producer, along with the current producers E. coli and S. cerevisiae.
References
Ji, X. J., Huang, H., & Ouyang, P. K. (2011). Microbial 2,3-butanediol production: a state-of-the-art review. Biotechnology Advances, 29, 351–364.
Syu, M. J. (2001). Biological production of 2,3-butanediol. Applied Microbiology and Biotechnology, 55, 10–18.
Celińska, E., & Grajek, W. (2009). Biotechnological production of 2,3-butanediol—current state and prospects. Biotechnology Advances, 27, 715–725.
Jung, M. Y., Ng, C. Y., Song, H. H., Lee, J. W., & Oh, M. K. (2012). Deletion of lactate dehydrogenase in Enterobacter aerogenes to enhance 2,3-butanediol production. Applied Microbiology and Biotechnology, 95, 461–469.
Zhang, L., Yang, Y., Sun, J., Shen, Y., Wei, D., Zhu, J., & Chu, J. (2010). Microbial production of 2,3-butanediol by a mutagenized strain of Serratia marcescens H30. Bioresource Technology, 101, 1961–1967.
Guo, X. W., Cao, C. H., Wang, Y. Z., Li, C. Q., Wu, M. Y., Chen, Y. F., Zhang, C. Y., Pei, H. D., & Xiao, D. G. (2014). Effect of the inactivation of lactate dehydrogenase, ethanol dehydrogenase, and phosphotransacetylase on 2,3-butanediol production in Klebsiella pneumoniae strain. Biotechnology for Biofuels, 7, 44.
Kim, B. R., Lee, S. J., Jeong, D. U., Yang, J. M., Oh, M. K., & Lee, J. W. (2014). Redistribution of carbon flux toward 2,3-butanediol production in Klebsiella pneumoniae by metabolic engineering. PLoS One, 9(10), e105322.
Xu, Y., Chu, H., Gao, C., Tao, F., Zhou, Z., Li, K., Li, L., Ma, C., & Xu, P. (2014). Systematic metabolic engineering of Escherichia coli for high-yield production of fuel bio-chemical 2,3-butanediol. Metabolic Engineering, 23, 22–33.
Kim, S. J., Heo, S. O., Jin, Y. S., & Seo, J. H. (2013). Production of 2,3-butanediol by engineered Saccharomyces cerevisiae. Bioresource Technology, 146, 274–281.
Smith, K. M., Cho, K. M., & Liao, J. C. (2010). Engineering Corynebacterium glutamicum for isobutanol production. Applied Microbiology and Biotechnology, 87, 1045–1055.
Patek, M., Krumbach, K., Eggeling, L., & Sahm, H. (1994). Leucine synthesis in Corynebacterium glutamicum: enzyme activities, structure of leuA, and effect of leuA inactivation on lysine synthesis. Applied and Environmental Microbiology, 60, 133–140.
Shin, S. H., Kim, S. H., Kim, J. Y., Lee, S. J., Um, Y. S., Oh, M. K., Kim, Y. R., Lee, J. W., & Yang, K. S. (2012). Complete genome sequence of the 2,3-butanediol-producing Klebsiella pneumoniae strain KCTC 2242. Journal of Bacteriology, 194, 2736–2737.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning: A laboratory manual. New York:Cold Spring Harbor Laboratory.
Tauch, A. L., Kirchner, O., Löffler, B., Götker, S., Pühler, A., & Kalinowski, J. (2002). Efficient electrotransformation of Corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Current Microbiology, 45(5), 362–367.
Kim, B. R., Lee, S. J., Yang, J. M., Jeong, D. U., Shin, S. H., Kook, J. H., Yang, K. S., & Lee, J. W. (2015). The influence of budA deletion on glucose metabolism related in 2,3-butanediol production by Klebsiella pneumoniae. Enzyme and Microbial Technol. doi:10.1016/j.enzmictec.2015.03.002.
Zhang, L., Zhang, Y., Liu, Q., Meng, L., Hu, M., Lv, M., Li, K., Gao, C., Xu, P., & Ma, C. (2015). Production of diacetyl by metabolically engineered Enterobacter cloacae. Scientific Reports, 5, 9033.
Kim, B. R., Lee, S. J., Park, J. H., Lu, M. S., Oh, M. K., Kim, Y. R., & Lee, J. W. (2012). Enhanced 2,3-butanediol production in recombinant Klebsiella pneumoniae via overexpression of synthesis-related genes. Journal of Microbiology and Biotechnology, 22, 1258–1263.
Jojima, T., Igari, T., & Moteki, Y. (2015). Promiscuous activity of (S,S)-butanediol dehydrogenase is responsible for glycerol production from 1,3-dihydroxyacetone in Corynebacterium glutamicum under oxygen-deprived conditions. Applied Microbiology and Biotechnology, 99, 1427–1433.
Eggeling, L., & Boot, M. (2005). Handbook of Corynebacterium glutamicum. New York:Taylor & Francis Group.
Acknowledgments
This work was supported by the R&D Program of MOTIE/KEIT (No. 10035578, Development of 2,3-Butanediol and Derivative Production Technology for C-Zero Bio-Platform Industry). This work was also supported by the Graduate School of Specialization for Biotechnology Program of the Ministry of Knowledge Economy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Kim, B., Kim, H. et al. Industrial Production of 2,3-Butanediol from the Engineered Corynebacterium glutamicum . Appl Biochem Biotechnol 176, 2303–2313 (2015). https://doi.org/10.1007/s12010-015-1719-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1719-7