Abstract
To reduce environmental problems caused by glycerine accumulation and to make the production of biodiesel more profitable, crude glycerin without treatment was used as substrate for obtaining higher value-added bioproducts. Monascus ruber is a filamentous fungus that produces pigments, particularly red ones, which are used for coloring foods (rice wine and meat products). The interest in developing pigments from natural sources is increasing due to the restriction of using synthetic dyes. The effects of temperature, pH, microorganism morphology, aeration, nitrogen source, and substrates have been studied in the cultivation of M. ruber. In this work, it was observed that light intensity is also an important factor that should be considered for understanding the metabolism of the fungus. In M. ruber cultivation, inhibition of growth and pigment production was observed in Petri dishes and blaffed flasks exposed to direct illumination. Growth and pigment production were higher in Petri dishes and flasks exposed to red light and in the absence of light. Radial growth rate of M. ruber in plates in darkness was 1.50 mm day−1 and in plates exposed to direct illumination was 0.59 mm day−1. Maximum production of red pigments (8.32 UA) and biomass (8.82 g L−1) were obtained in baffled flasks covered with red film and 7.17 UA of red pigments, and 7.40 g L−1 of biomass was obtained in flasks incubated in darkness. Under conditions of 1248 lux of luminance, the maximum pigment production was 4.48 UA, with production of 6.94 g L−1 of biomass, indicating that the fungus has photoreceptors which influence the physiological responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Monascus ruber van Thiehen is a fungus that metabolizes cellulose, maltose, fructose, and glucose producing pigments known as Monascus pigments. Easily found in many ecosystems, fungi of the genus Monascus are traditionally used in oriental countries, originally in China and Thailand. The pigments are commonly used in the preparation of red color rice but also find several other applications, from giving color to wine, cheese and meat, and in medical uses [1].
Monascus pigments are traditionally produced by biotechnological processes in solid state (bread or rice). The use of submerged fermentation has been studied to minimize the problems of space, scale, and process control. The solid-state fermentation requires complex control systems, whereas in submerged fermentation, control is simpler, and the cultivation time may be reduced. The use of submerged fermentation can benefit the production of secondary metabolites and reduce the production cost [2].
The factors that influence the growth and pigment production are as follows: culture medium, temperature, presence of oxygen and aeration [3, 4], pH [5–7], nitrogen source [3, 7, 8], culture medium rheology [9, 10], and morphology [11].
Monascus also responds to light (blue, red, dark) regulating the secondary metabolite production and cellular development. This light sensivity system may have an important commercial application [12]. The responses are mediated by light photoreceptors capable of initiating the signal transmission that result in changes in the gene expression encoding enzymes responsible for mycelial growth and secondary metabolite productions in fungi [13, 14].
Various carbon sources have been used as substrate for Monascus spp. growth, being the most common glucose, sucrose, and starch. Best growth is generally observed in glucose [15]. Some alternative substrates for pigment production by Monascus spp. are as follows: pear juice [4], jackfruit seeds [6], ethanol [16], fructose, maltose, sucrose, and lactose [17], corn syrup [18], grape waste [19], corn flour [20], cassava starch [21], and glyverol [22]. In addition, actually, there has been a demand for alternative energy sources due to the depletion of oil reserves and the increasing concern on environmental issues. Biodiesel is a renewable fuel recently considered the best substitute for petroleum-based diesel because of the possibility of use in many ignition engines. Chemically, biodiesel is a mixture of methyl or ethyl long chain esters and is obtained from nontoxic biological sources: vegetable oils, animal fats, and cooking oils [16]. The biodiesel coproduct is formed approximately by 50 % of glycerine, water, salts, and unreacted alcohol and catalyst. Generally, water and alcohol are removed to produce 80–88 % pure glycerine that can be traded as crude glycerine. Glycerine can also be distilled for obtaining glycerol with a purity of 99 % or greater and commercialized for several markets [17].
A concern is the management of the biodiesel coproducts due to the implementation of this fuel in the Brazilian energy matrix. In general, 10 kg of glycerine is generated as by-product from 100 kg of biodiesel. About 2.4 billion liters of biodiesel was produced to reach the legal requirement established (addition of 5 % of biodiesel to fossil diesel), which generated approximately 240 million liters of glycerine [18].
Based in these aspects, the aim of this study was to investigate the influence of light intensity on growth and pigment production in cultives of M. ruber in Petri dishes and baffled flasks using crude glycerine as substrate.
Materials and Methods
Microorganism
The microorganism used was the filamentous fungus M. ruber CCT3802, obtained from the Tropical Culture Collection André Tosello Foundation (Campinas-SP, Brazil). The stock culture was maintained on potato dextrose agar (PDA) in tubes. The tubes were inoculated, incubated at 30 °C for 7 days, and stored at 4 °C.
Culture Medium
The culture medium contained per liter: 20 g glycerine, 5 g glycine, 5 g K2HPO4, 0.1 g CaCl2, 0.5 g MgSO4·7H2O, 0.01 g FeSO4·7H2O, 0.01 g ZnSO4·7H2O, and 0.03 g MnSO4·H2O [3, 19].
The glycerine used in this study was obtained from a biodiesel plant located in Brazil. Crude glycerine is a coproduct of biodiesel production and was used without purification or pretreatment.
Experiments
The assays were performed in Petri dishes and baffled Erlenmeyer flasks exposed to continuous lighting. The dishes and flasks were covered with colored film (blue, yellow, red, green, and black). The colorful translucent film allows only the passage of particular color of light—the other colors of the spectrum are filtered [20, 14].
A fluorescent lamp of 15 W (ECONOLIFE TASCHIBRA, Brazil) was used as light source. A digital luximeter (LUTRON LX 81, Brazil) was used to determine the luminance (lux). The calibrated luximeter was placed in the same distance from the lamp to the culture medium, and the average luminance was determined with 10 measures of luminance at different points. To ensure that each dish/flask received same intensity of light, dishes and/or flasks were handled, after each measurement of the radius and sample removal, for different positions (distance from the source of light). It was carried out 10 measurements of luminance at different points for determination of mean value of luminance (Em) and the respective standard deviation.
Growth in Petri Dishes and Determination of Radial Growth Rate
The plates covered with colored film were incubated in a closed box and exposed to light. The spore suspension (2.0 mL) obtained by adding sterile water in tubes containg M. ruber were transferred to the surface of solid medium in Petri dishes containing bacteriological agar (20 g L−1) and the culture medium described in item 2.2.
To determine the radial growth rate, the M. ruber was inoculated in Petri dishes containg PDA. The inoculum was prepared transferring the microorganism to eppendorf tubes containing 2.0 mL of 0.2 % agar. The spore suspension was inoculated in the center of each plate with a 1.0-mm diameter sterile pipette. Assays were performed in duplicate, and the dishes were incubated at 30 °C. Tree lines were drawn at the plates to measure the diameter of each colony to calculate the average value [21].
The radial growth rate was obtained by linear regression of the mean radius as a function of time (Eq. 1). The value 0.5 mm was considered the linear coefficient of the equation, since this is the initial radius of the inoculum.
where V R is the radial growth rate (mm h−1), t is the time (h), and r is the radius (mm).
Cell Growth and Pigment Production in Flasks
The experiments were performed in baffled Erlenmeyer flasks (1 L) with 360 mL of culture medium added of 400 mL of M. ruber inoculum. The flasks were incubated at 30 °C on rotary shaker (TECNAL, Brazil) at 140 min−1. The initial pH of the medium was adjusted to 6.5 with NaOH or H3PO4 solutions. The end of the fermentation was defined when there was a decrease in pigment production and biomass concentration kept constant.
The inoculum was obtained by germination of M. ruber spores suspended in 1-L baffled flasks, containing 0.4 L of culture medium incubated at 30 °C in an orbital shaker for 60 h [10].
The biomass was quantified gravimetrically. An amount of 5.0 mL of the culture medium was filtered under vacuum through previously weighed quantitative filter paper (UNIFIL), and the retained material was submitted to a drying process in a microwave oven (CONSUL FACILITATE MIDI, Brazil) for 15 min under power of 180 W [22]. Paper and biomass were cooled for 15 min in a desiccator and then weighed on an analytical balance (BEL ENGINEERING, Italy, accuracy = 0.1 mg) for determining the dry weight.
The filtrate was used for the quantification of pigments and pH. A digital pH meter (LUTRON–Model pH 221, Brazil) was used to determine the pH in the culture medium. The pigments were quantified using a spectrophotometer (SP-1100 Series Model SP-105, TECNAL, Brazil). The concentration of extracellular pigment was estimated by measuring the absorbance of filtrates at 510 nm (red pigments) [23, 24], and the pigment production was expressed in absorbance units (UA).
The maximum specific growth velocity was calculated from the slope of the linearized curve of logarithm residual biomass over time, according to Eq. 2.
where X is the biomass in the exponential phase (g L−1); X 0 is the biomass in the initial exponential phase (g L−1); μ máx is the specific growth rate (h−1); and t is the time (h).
The maximum productivity of cells was calculated by the difference between the highest amount of biomass at a time t and the initial amount of biomass divided by the corresponding period of time, as seen in Eq. 3:
where P cells (g h−1) is the maximum productivity of biomass at the period of time (t–t 0); X MÁXt is the maximum amount of biomass at time t; X to is the amount of biomass at time t 0; t is the time to achieve the maximum value of biomass; and t 0 is the initial time of cultivation.
The maximum productivity of pigments was calculated by the difference between the highest amount of pigment (UA510) at a time t and the initial amount of pigments divided by the corresponding period of time, as seen in Eq. 4:
where P M (UA h−1) is the maximum productivity of pigments at the period of time (t–t 0); UA MÁXt is the maximum amount of pigment at time t; UA t0 is the amount of pigment at time t 0; t is the time to achieve the maximum value of UA; and t 0 is the initial time of cultivation.
Stability of Pigments to Light
To evaluate the stability of pigments to light, the culture medium containing Monascus pigments produced in submerged fermentation (baffled flasks) was filtered. Solution of red pigments (7.5 UA) was added to Erlenmeyer flasks covered with colored film, black film, and without film.
The flasks with 400 mL of cultive medium were incubated at 30 °C for 192 h in an orbital skaker (140 min−1) and exposed to continuous ilumination (fluorescent lamp of 15 W). The luminance (Em) was determined inside the flasks using a digital luximeter. A sample of 2.0 mL was taken from each flask at intervals of 12 h for quantification of pigments (absorbance).
Results and Discussion
Crude Glycerine Characterization
Composition of crude glycerine varies depending on the original raw material and biodiesel production process. It is necessary to characterize the physical, chemical, and nutritional properties of crude glycerine before its use for obtaining substances with higher added value [25]. The composition of industrial by-products like glycerine obtained from biodiesel production can influence the microorganism metabolism. The physicochemical properties of crude glycerine used in this work are presented in Table 1. The results presented in this table are similar to those found in the literature. These properties depend on the process and fat or oil used for biodiesel production. In general, glycerine is composed of glycerol (40–80 %), water, methanol, and dissolved salts.
Radial Growth Rate of M. ruber in Petri Dishes Covered with Colored Film and Exposed to Light
Petri dishes with PDA and covered with yellow, red, blue, green, and black films were inoculated with M. ruber and exposed to the fluorescent lamp. The incubation temperature was 31.3 °C and luminance of 4256 lux.
Petri dishes in duplicate were exposed to light for 264 h, and the diameter of the colonies was measured in intervals of 24 or 48 h. The results are shown in Table 2. From this table, one can observe lower radial growth rate and lower pigmentation in dishes exposed to direct light (no film) and dishes covered with yellow film, indicating inhibition of growth and pigment production by light. The highest radial growth rates were observed in dishes covered with red and black films (darkness).
Colonies with more pigmentation were observed in Petri dishes covered with red and black films. Pigment production in the medium and pigmented colonies was not observed in dishes covered with yellow and without film. Darkness and red film favored growth and pigmentation of M. ruber on PDA. Colony growth was not observed in dishes initially exposed to light. However, after incubation in the dark, the colonies developed with growth and pigmentation dissolved in the medium.
Light has a stagnation effect, but not able to inactivate the spores and aerial mycelium. If a colony that did not grow when exposed to light is subjected to absence of light (darkness), it usually grows [26]. The average radial growth rate of four strains of Monascus purpureus on PDA at 30 °C was determined by other research [26]. For these strains, they found rates between 2.3 and 3.1 mm day−1.
Influence of Light Intensity on Growth and Pigment Production on Petri Dishes Covered with Colored Film
Petri dishes covered with colored film were incubated to evaluate the influence of the light wavelenght on the growth and pigmentation. The colors tested were red, blue, green, and yellow, and pigment production was compared to that observed on dishes covered with black film and dishes without film (exposed to direct light). The solidified medium was composed of crude gycerine, glycine, and salts.
The luminance corresponding to each color was determined with luximeter covered with colored film and exposed to the light at the same distance from the dishes to the lamp inside the box. The following values of luminance (lux) were found: 439 ± 68 for dishes covered with red film, 702 ± 71 for dishes covered with blue film, 898 ± 101 for dishes covered with green film, 3820 ± 632 for dishes covered with yellow film, and 4952 ± 749 for dishes without film.
There was growth with pigmentation in dishes covered with black film (darkness) and inhibition of growth and pigmentation in dishes exposed to direct light (without film). This behavior was also observed in dishes partially covered with black film.
M. ruber growth and pigment production were smaller in dishes covered with green, red, and blue films than those observed in dishes covered with black film. In dishes covered with red film, pigment production was higher than in dishes covered with other color films. This indicates that light at various wavelenghts and luminance interferes in growth and pigment production, a factor that must be observed in research using M. ruber.
Colonies with aerial mycelium were formed in dishes exposed to red light. A decrease in the size of the colonies was observed when blue light was used, and growth in green light showed no significant difference in the appearance of growth compared to blue light [27].
In luminance of 6000 lux, mycelium of the fungus Inonotus obliquos grew faster on dishes containing PDA exposed to darkness (no light) than in dishes covered with red and blue films. This indicates that light regulates the mycelium growth in fungus belonging to the basidiomycete class [13].
At higher luminance values, determined in dishes without film and covered with yellow film, there was inhibition of pigment production. Higher pigment production and cell growth were observed in dishes covered with black and red films, when lower values of luminance were determined.
Influence of Light on Growth and Pigment Production in Submerged Fermentation in Baffled Flasks
To evaluate the influence of light intensity on cell growth and pigment production in submerged fermentation, assays were performed in Erlenmeyer flasks incubated at 30 °C on orbital shaker. A sample of culture medium was taken from each flask every 24 h to determine pigment production, biomass, and pH. The luminance on the surface of the flasks was determined by luximeter. The luminance inside the flasks covered with red film was lower than in the other flasks (Table 3).
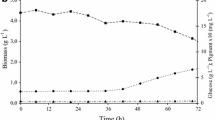
Biomass and production of pigments were observed in all flasks. Evolution of red pigment production is shown in Fig. 1. Higher pigment production was observed in flasks covered with red (8.36 UA) and black films (7.21 UA). The lowest pigment production was observed in flasks without film (4.52 UA). In flasks covered with green, yellow, and blue films, the pigment production was around 6.20 UA.
It was observed that direct light inhibits the formation of extracellular pigments by M. ruber. Inhibition in direct light and increased production in flasks exposed to red light and in the darkness were also observed in cultures of Monascus purpureus [27, 14].
The biomass production in the flasks is shown in Fig. 2. Maximum biomass concentration in flasks covered with green, blue, and red film was about 8.5 g L−1 and in darkness in direct light (without film) was 7.0 g L−1. The kinetic parameters obtained are shown in Table 4. The highest yield was observed in flasks with red film and the lowest in flasks without film (exposed to direct light).
Pigment production, biomass, and pH evolution for the flasks with red film are shown in Fig. 3. The pH remained around 6.5 indicating that the maximum pigment formation was observed at the end of the growth phase. There was an increase in the red pigment production with the increase in biomass. Maximum pigment production was observed in the stationary growth phase, indicating that pigment production is partially associated to growth.
In cultives of Monascus purpureus in 250-mL flasks containing glucose as substrate, other research obtained 11 UA of red pigments and 5 g L−1 of biomass [28]. In cultives of M. ruber in 1-L flasks containing 200 mL of cultive medium consisting of glucose (5 g L−1), monosodium glutamate (5 g.L−1) and salts, other authors reported 1.8 g L−1 of biomass and 8 UA of red pigments after 90 h [15].
For Babitha et al. (2008), the incubation of Monascus purpureus in 250-mL flasks, in darkness, resulted in an increase in pigment production (approximately 2 times) and incubation in direct and continuous illumination resulted in reduction of pigment productivity. Pigment production increased with exposition to red light, while green, and blue wavelenghts inhibited the pigment production. Similar behavior was observed in the assays performed in this work.
Velmurugan et al. [29] observed changes in the composition of pigments, which depends on the light condition in cultives of Monascus purpureus in flasks covered with colored film containg 100 mL of culture medium composed of glucose and salts. The authors concluded that the effect of light on the intracellular and extracellular pigments and biomass production by the fungus revealed that incubation in the absence of light was most effective, followed by red and blue lights and direct exposure. Yellow and green lights inhibit pigment production and cell growth.
Reduction of light intensity of 5000 to 2000 lux increased chlorophyll production in submerged cultivation of Spirulina platensis. However, cell growth was higher in light intensity of 5000 lux, indicating that light conditions influence the metabolism for pigment production [30].
In submerged cultivation of the fungus Phanerochaete chrysosporium, researchers studied the influence of various wavelenghts in the development and production of lignin peroxidase. The green light had significant positive effect on the enzyme activity and biomass production compared to cultives exposed to direct, yellow, blue, red light, and darkness [30].
Light is a factor that influences the production of secondary metabolites, like toxin by the fungus Alternaria alternata. The production of toxins can be reduced by blue light, and direct light can reduce the biosynthesis of polyketides [31, 32].
Further studies on a larger scale are necessary in cultives of M. ruber. However, it is now known that light is an important factor for the fungi, with influence on the different physiological responses, like pigmentation, sexual development, asexual conidiation, the circadian clock, and the secondary metabolite production. Analyses of the genomes of fungi identified several genes that encode proteins involved in light detection and activation of mechanisms for physiological ans morphological responses [33].
Stability of Pigments Exposed to Light
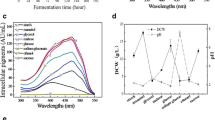
Degradation of pigments and changes in the solution color was observed at higher values of luminance (direct light and yellow film). Less degradation and less color change were observed in solutions of pigments in flasks covered with black film (darkness) and flasks covered with red film (lower value of luminance).
The variation of color versus time of the pigment solutions in different luminance values (Table 5) are shown in Fig. 4. A linear regression was calculated with the values (UA–UA0), ln (UA/UA0) and 1/(UA/UA0) versus time for zero order, first order, and second order reactions, where UA0 is the initial red pigment concentration. A nonlinear behavior was observed through analysis of the correlation coefficient (R 2). There was a variation in the degradation kinetics of the pigment solutions exposed to different conditions of luminance.
Carvalho et al. [34] also observed nonlinear behavior of the color of Monascus pigment solution at different temperatures and pH values.
The stability of Monascus pigments can be improved by addition of amino acids. Pigments are more stable at various conditions of pH, solvent, temperature, and initial concentration of pigments when different amino acids are added to the medium. For red pigments exposed to solar radiation, without addition of amino acids, degradation constant (k) was 3.2 ± 0.105 h−1 and half-life (t 1/2) was 0.22 ± 0.01 h. For red pigments added of glycine, degradation constant to sunlight was 0.137 ± 0.001 h−1 and half-life of 5.06 ± 0.02 h. The degradation constant of solutions exposed to UV lamp was 0.0002 ± 0.003 h−1 and half-life of 245.36 ± 17.72 h [35].
Anthocyanin extract from purple cherry protected from light remained more stable than the extract exposed to light (80 W fluorescent lamp). The degradation constants (k) obtained were 7.3 × 10−4 h−1 for samples in the presence of light and 5.7 × 10−4 h−1 for sample extracts in the absence of light. The 50 % loss of the original color (half-life) was achieved after 948.3 h (39 days) for the extract exposed to light and after 1205.7 h (50 days) for the extract protected from light, showing greater instability of anthocyanin extract to luminosity [36].
The degradation of pigments is photochemical and is not influenced by the presence of dissolved oxygen. Pigments have a continuous degradation in aqueous solutions, while are stable for weeks in butanol solution and protected from light [37].
Conclusions
The results obtained here permitted us to verify that incubation in complete darkness was more effective in inducing growth and production of pigments. There was a greater pigment production in submerged cultivation in flasks maintained in darkness and covered with red film. Inihibition in growth was observed in direct illumination. Radial growth rate of M. ruber in plates in darkness was 1.50 mm day−1 and in plates exposed to direct illumination was 0.59 mm day−1. Maximum production of red pigments (8.32 UA) and 8.82 g L−1 of biomass were obtained in baffled flasks covered with red film and 7.17 UA of red pigments and 7.40 g L−1 of biomass was obtained in flasks incubated in darkness. Under conditions of 1248 lux of luminance, the maximum pigment production was 4.48 UA, with production of 6.94 g L−1 of biomass, indicating that the fungus has photoreceptors which influence the physiological responses of luminance.
References
Wong, H. C., & Koehler, P. (1981). Production and isolation of an antibiotic from Monascus purpureus and its relationship to pigment production. Journal of Food Science, 46, 589–592.
Domínguez-espinosa, R. M., & Webb, C. (2003). Submerged fermentation in wheat substrates for production of Monascus pigments. World Journal of Microbiology and Biotechnology, 19, 329–336.
Pastrana, L., Blanc, P. J., Santerre, A. L., Loret, M. O., & Goma, G. (1995). Production of red pigments by Monascus ruber in synthetic media with a strictly controlled nitrogen source. Process Biochemistry, 30, 333–341.
Hamdi, M., Blanc, P. J., & Goma, G. (1996). Effect of aeration conditions on the production of red pigments by Monascus purpureus growth on prickly pear juice. Process Biochemistry, 31, 543–547.
Yongsmith, B., Tabloka, W., Yongmanitchai, W., & Bavavoda, R. (1993). Culture conditions for yellow pigment formation by Monascus sp. KB 10 grown on cassava medium. World Journal of Microbiology and Biotechnology, 9, 85–90.
Babitha, S., Soccol, C. R., & Pandey, A. (2006). Jackfruit seed – a novel substrate for the production of Monascus pigments throught solid-state fermentation. Food Technology and Biotechnology, 44, 465–471.
Mukherjee, G., & Singh, S. K. (2011). Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochemistry, 46, 188–192.
Jung, H., Kim, C., Kim, K., & Shin, C. S. (2003). Color characteristics of Monascus pigments derived by fermentation with various amino acids. Journal of Agricultural and Food Chemistry, 5, 1302–1306.
Ahn, J., Jung, J., Hyung, W., Haam, H., & Shin, C. (2006). Enhancement of Monascus pigment production by the culture of Monascus spp. J101 at low temperature. Biotechnology Progress, 22, 338–340.
Vendruscolo, F., Pitol, L. O., Carciofi, B. A. M., Moritz, D. E., Laurindo, J. B., Schmidell, W., & Ninow, J. L. (2010). Construction and application a vane system in a rotational rheometer for determination of the rheological properties of Monascus ruber CCT 3802. Journal of Biorheology, 24, 29–35.
Carels, M., & Shepherd, D. (1975). Sexual reproductive cycle of Monascus in submerged shaken culture. Journal of Bacteriology, 122, 288–294.
Miyake, T., Mori, A., Okuno, A. K. T., Usui, Y., Sammoto, F. S. H., & Kariyama, A. W. M. (2005). Light effects on cell development and secondary metabolism in Monascus. Journal of Industrial Microbiology and Biotechnology, 32, 103–108.
Zheng, W., Zhang, M., Zhao, Y., Miao, K., & Jiang, H. (2009). NMR-based metabonomic analysis on effect of light on production of antioxidant phenolic compounds in submerged cultures of Inonotus obliquus. Bioresource Technology, 100, 4481–4487.
Velmurugan, P., Lee, Y. H., Venil, C. K., Lakshmanaperumalsamy, P., Chae, J. C., & Oh, B. T. (2010). Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. Journal of Bioscience and Bioengineering, 109, 346–350.
Hajjaj, H., Blanc, P. J., Groussac, E., Uribelarrea, J. L., Goma, G., & Loubiere, L. (2000). Kinetics analysis of red pigment and citrinin production by Monascus ruber as a function of organic acid accumulation. Enzyme and Microbial Technology, 27, 619–625.
Hamdi, M., Blanc, P. J., Loret, M. O., & Goma, J. L. (1997). A new process for red pigment production by submerged culture of Monascus purpureus. Bioprocess Engineering, 17, 75–79.
Tseng, Y. Y., Chen, M. T., & Lin, C. F. (2000). Growth, pigment production and protease activity of Monascus purpureusas affected by salt, sodium nitrite, polyphosphate and various sugars. Journal of Applied Microbiology, 88, 31–37.
Hamano, P. S., & Kilikian, B. V. (2006). Production of red pigments by Monascus ruber in culture media containing corn steep liquor. Brazilian Journal of Chemical Engineering, 23, 443–449.
Silveira, S. T., Daroit, D. J., & Brandelli, A. (2008). Pigment production by Monascus purpureus in grape waste using factorial design. LWT - Food Science and Technology, 41, 170–174.
Xu, W. (2001). Study on the liquid fermentation to produce Monascus pigment with corn starch and antibacteria. Advanced Materials Research, 1336, 183–185.
Kongruang, S. (2011). Growth kinetics of biopigment production by Thai isolated Monascus purpureus in a stirred tank bioreactor. Journal of Industrial Microbiology and Biotechnology, 38, 93–99.
Meinicke, R. M., Vendruscolo, F., Moritz, D. E., Oliveira, D., Schmidell, W., Samohyl, R. W., & Ninow, J. L. (2012). Potential use of glycerol as substrate for the production of red pigments by Monascus ruber in submerged fermentation. Biocatalysis and Agricultural Biotechnology, 3, 238–242.
Gomes, M. C. S., Arroyo, P. A., & Pereira, N. C. P. (2011). Biodiesel production from degummed soybean oil and glycerol removal using ceramic membrane. Journal of Membrane Science, 378, 453–461.
Leung, D. Y. C., Wu, X., & Leung, M. K. H. (2010). A review on biodiesel production using catalyzed transesterification. Applied Energy, 87, 1083–1095.
Leoneti, A. B., Aragão-Leoneti, V., & Oliveira, S. V. W. B. (2012). Glycerol as a by-product of biodiesel production in Brazil: alternatives for the use of unrefined glycerol. Renewable Energy, 45, 138–145.
Hajjaj, H., Blanc, P. J., Groussac, E., Goma, G., Uribelarrea, J. L., & Loubiere, P. (1999). Improvement of red pigment/citrinin production ratio as a function of environmental conditions by Monascus ruber. Biotechnology and Bioengineering, 64, 497–501.
Babitha, S., Carvalho, J. C., Soccol, C. R., & Pandey, A. (2008). Effect of light on growth, pigment production and culture morphology of Monascus purpureus in solid-state fermentation. World Journal of Microbiology and Biotechnology, 24, 2671–2675.
Gabiatti Junior, C., Vendruscolo, F., Piaia, J. C. Z., Rodrigues, R. C., Durrant, L. R., & Costa, J. A. V. (2004). Radial growth rate as a tool for the selection of filamentous fungi for use in bioremediation. Brazilian Archives of Biology and Technology, 47, 225–232.
Hamano, P. S., Orozco, S. F. V. B., & Kilikian, B. V. (2005). Concentration determination of extracellular and intracellular red pigments produced by Monascus spp. Brazilian Archives of Biology and Technology, 48, 43–49.
Juzlova, P., Martinkova, L., & Kren, V. (1996). Secondary metabolities of the fungus Monascus: a review. Journal of Industrial Microbiology, 16, 163–170.
Kim, H. J., Kim, J. H., Oh, H. J., & Shin, C. S. (2002). Morphology control of Monascus cells and scale-up of pigment fermentation. Process Biochemistry, 38, 649–655.
Thomson, J., & He, B. (2006). Characterization of crude glycerol from biodiesel production from multiple feedstocks. Applied Engineering in Agriculture, 22, 261–265.
Schmidt-Heydt, M., Rüfer, C., Raupp, F., Bruchmann, A., Perrone, G., & Geisen, R. (2011). Influence of light on food relevant fungi with emphasis on ochratoxin producing species. International Journal of Food Microbiology, 145, 229–237.
Carvalho, J. C., Oishi, B. O., Pandey, A., & Soccol, C. R. (2005). Biopigments from Monascus: strain selection, citrinin production and color stability. Brazilian Archives of Biology and Technology, 48, 885–894.
Lee, B. K., Park, N. H., Piao, H. Y., & Chung, W. J. (2001). Production of red pigments by Monascus purpureus in submerged culture. Biotechnology and Bioprocess Engineering, 6, 341–346.
Danesi, E. D. G., Rangel-Yagui, C. O., Carvalho, J. C. M., & Sato, S. (2004). Effect of reducing the light intensity on the growth and production of chlorophyll by Spirulina platensis. Biomass and Bioenergy, 26, 329–335.
Ramirez, D. A., Munoz, S. V., Atehortua, L., & Michel, F. C., Jr. (2010). Effects of different wavelengths of light on lignin peroxidase production by the white-rot fungi Phanerochaete chrysosporium grown in submerged cultures. Bioresource Technology, 101, 9213–9220.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bühler, R.M.M., Müller, B.L., Moritz, D.E. et al. Influence of Light Intensity on Growth and Pigment Production by Monascus ruber in Submerged Fermentation. Appl Biochem Biotechnol 176, 1277–1289 (2015). https://doi.org/10.1007/s12010-015-1645-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1645-8