Abstract
Carbonic anhydrate is a zinc-containing metalloenzyme and involved in plant abiotic stress tolerance. In this study, we found that heat stress could induce rice mature carbonic anhydrate gene over-expression in rice plants. An Escherichia coli heterologous expression system was performed to identify the function of rice mature carbonic anhydrate in vitro. By sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), mature OsCA fusion protein was identified and proved to be soluble. The results of spot, survival rate, and growth curve assay demonstrated that the expression of the mature OsCA could enhance the thermo-tolerance of the induced mature OsCA recombinants in comparison with controls under heat stress. Meanwhile, compared with controls, the levels of reactive oxygen species in induced mature OsCA recombinants were apparently low under heat stress, and correspondingly, activities of the critical antioxidant enzymes including superoxide dismutase, catalase, and peroxidase in the induced mature OsCA recombinants were significantly increased. Additionally, relative to controls, the activity of the lactate dehydrogenase decreased in the induced mature OsCA recombinants under heat stress. Based on these results, we suggest that mature OsCA protein could confer the E. coli recombinants’ tolerance to heat stress by a synergistic fashion of increasing the antioxidant enzymes’ activities to reduce the oxidative damage and maintaining the lactate dehydrogenase (LDH) activity of E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Along with the development of industrialization course, carbon dioxide concentration is increasing, temperature is rising, and the resultant global warming is becoming a seriously ecological problem. The rise of temperature results in serious impact on global agricultural production [1]. In rice production, heat stress has been one of the major adverse environmental factors, which impairs crop plant growth, development, fertility, and quality [2–4]. In response to this challenge, identifying novel genes related to heat stress and developing thermo-tolerance-improved crop plants using various genetic approaches will be an alternative strategy.
Carbonic anhydrase (CA) is a zinc-containing metalloenzyme, catalyzes the reversible interconversion of CO2 and HCO3 − (CO2 + H2O ← →HCO3 − + H+) [5, 6]. Recently, some reports have suggested that the expression of plant carbonic anhydrase genes is linked with multiple stresses [7, 8]. In Pisum sativum, a carbonic anhydrase can retain 40 % activity under 60 °C for 15 min [9]. Cotton carbonic anhydrase can retain 60 % activity under 55 °C for 20 min [10]. Besides, the activity of carbonic anhydrase is increased under the stress of ABA and NaCl and drought in pea [11]. The activity of CA in Oryza sativa is increased under low-salt conditions [12]. The enzyme activity of recombinant β-carbonic anhydrase from Pennisetum glaucum is stable at 80 °C. Meanwhile, transcript levels of carbonic anhydrase from P. glaucum were both upregulated under the treatments of 150 mM NaCl and dehydration [13]. The carbonic anhydrase isolated from the Dunaliella salina could retain activity under 1.5 M NaCl [14]. However, until now, there is little information available on the relation between heat stress and CA genes in rice.
In this study, we isolated a gene-encoded mature β-type carbonic anhydrase (OsCA-M, GenBank Accession No. U08404.1) which contains 210 amino acid residues from rice. As a result of the poor information on the relation between heat stress and the OsCA-M gene, we focused on the thermo-tolerance of OsCA-M. We constructed the recombinant plasmids expressing the OsCA mature protein (OsCA-M) in Escherichia coli. The expressed polypeptide was proved to be soluble. The results of spot assay, the survival ratios, and the growth curves showed that the expression of OsCA-M in E. coli enhanced the tolerance of E. coli to heat stress (55 °C). Meanwhile, decreased intracellular reactive oxygen species (ROS) and increased activities of antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD)] were detected in the induced OsCA-M transformants under high temperature (55 °C). Furthermore, it was demonstrated that, compared with controls, the lactate dehydrogenase (LDH) activity was less in the induced OsCA-M transformants under high temperature (55 °C).
Materials and Methods
Materials
The indica rice variety Yuetai (O. sativa L.), which was supplied by our laboratory, was used in this research. Rice was planted in a growth chamber at 25 °C for 2 weeks in a 16/8-h light cycle. Fourteen-day-old seedlings were treated at 42 °C for 72 h. The leaves of seedlings were harvested every 12 h and stored at −80 °C for further analysis.
The E. coli strain BL21 was used as the expression host strain in our study. The vector pGEX-6P-1 (Invitrogen, USA) with glutathione S-transferase (GST) tag was used to construct expression cassette.
Expression of OsCA in Rice
Total RNA was isolated with TRIzol reagent (Invitrogen) and reversely transcribed to single-strand complementary DNA (cDNA) using the ReverTra Ace transcriptase kit (Toyobo, Japan) according to the manufacturer’s instructions. The primers for cDNA amplification were OsCA-M-F1 (5-AACTCATCGTGGTGATTGGC-3) and OsCA-M-R1 (5-CGCATTGGTCATCGAAAGG-3). The rice ubiquitin gene was used as an internal standard, and the gene-specific primers were as follows: sense, 5- CGCAAGAAGAAGTGTGGTCA-3, and antisense, 5- ACGATTGATTTAACCAGTCCATGA-3. The programs of real-time polymerase chain reaction (RT-PCR) were the following: 1 cycle at 94 °C for 5 min followed by 25 cycles for 10 s at 94 °C, 10 s at 54 °C, and 10 s at 72 °C and final extension for 5 min at 72 °C. The RT-PCR products (25 μl) were sized on a 2 % agarose gel. SYBR Green-based real-time quantitative PCR (qPCR) was performed on the iCycler iQ5 Real-Time PCR Detection System (Bio-Rad, USA). Reactions were consisted of 1 cycle at 94 °C for 5 min, followed by 35 cycles at 94 °C for 10 s, 54 °C for 10 s, and 72 °C for 10 s and final extension at 72 °C for 5 min. Each sample was run in triplicates, and data analysis was performed with the Bio-Rad iCycler software (version 3.06070).
Expression and Solubility of OsCA-M in E. coli
The complete coding region of the rice CA-M gene was amplified by PCR using rice cDNA from rice leaves as a template. The primers were 5′-CGGGATCCCGTCTTCGCCGCCCCCGT-3′ and 5′-CGGGATCCAACTGACGAACTGAACGGAC-3′ (underline indicates a BamHI site). The sequenced fragment was introduced into the pGEX-6P-1 vector (Invitrogen, USA) which had been treated with the same restriction enzymes. The recombinant plasmid was also confirmed by DNA sequencing and named pGEX:OsCA-M. Then, pGEX:OsCA-M vector was transformed into E. coli BL21 to create BL/OsCA-M. The empty vector of pGEX-6P-1 was introduced into E. coli to create BL/pGEX-6P-1 as the control.
BL/OsCA-M and BL/pGEX-6P-1 were cultured overnight in a lysogeny broth (LB) medium containing 50 μg/ml ampicillin and amplified at 37 °C with shaking at 220 rpm (THZ-C, China). When an optical density at 600 nm (OD600) reached 0.8, the bacterial cultures of BL/OsCA-M were induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and 400 μM ZnSO4 for an additional 8 h at 30 °C. Expressed proteins were detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the bands in gels were stained with Coomassie brilliant blue. In order to study the solubility of expressed heterogeneous polypeptides in recombinant E. coli, the induced bacteria were broken using a sonication apparatus (Sonics, USA) and then centrifuged at 14,000×g for 30 min at 4 °C. The supernatant and the pellet which contains inclusion bodies were kept in a hot bath at 100 °C for 10 min, placed on ice for 15 min, and finally separated on 15 % SDS-PAGE. The bacterial cultures of BL/pGEX-6P-1 were used as the control.
Western Blot Analysis
For Western blotting, an equal amount of proteins were loaded per lane, separated on a 15 % SDS-PAGE, and transferred onto a PVDF membrane (Amersham). Detection of the GST-OsCA-M fusion protein was done using an anti-GST monoclonal antibody followed by horseradish peroxidase-conjugated secondary antibody goat anti-rabbit IgG, and the signals were detected with a SuperSignal West Pico Assay Kit (Thermo, USA).
Spot Assay and Survival Ratio Determination of Recombinant E. coli Under Heat Stress
To assay the heat tolerance of E. coli recombinants, the non-induced and induced cultures of BL/OsCA-M and BL/pGEX-6P-1 were adjusted to OD600 = 0.8 in a spectrophotometer and exposed to 55 °C for 45 min. The cultures were diluted serially (1:10, 1:100, 1:1000, and 1:10,000). Five microliters of each sample and 100 μl of the 100-fold diluted samples were spotted onto LB plates, respectively, to incubate overnight at 37 °C for 1 day. The colony number on each plate was recorded to determine the survival ratio [15]. All of the experiments were repeated three times.
In addition, to test the growth of E. coli cells, the non-induced and induced cultures of BL/OsCA-M and BL/pGEX-6P-1 were adjusted to OD600 = 0.4 in a spectrophotometer and exposed to 55 °C for 45 min. Then, the cultures were grown at 37 °C with shaking at 220 rpm, and the OD600 was monitored at 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, and 5 h.
Measurement of Intracellular Oxidation Levels
The oxidant-sensitive probe, 6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) was utilized to measure the intracellular oxidation levels in E. coli. After being treated at 55 °C for 45 min, the ROS of the recombinant cells were measured with a GENMED bacterial reactive oxygen species fluorometric assay kit (GENMED Scientifics Inc., USA) according to the manufacturer’s instructions. Relative fluorescence units (RFU) reflect the content of ROS. Time courses of ROS in the E. coli cells were measured using the fluorescence intensity of CM-H2DCFDA excited at 490 nm and sent out at 530 nm.
Enzymatic Activity Assay
The non-induced and induced cultures of BL/OsCA-M and BL/pGEX-6P-1 were treated at 55 °C for 45 min and measured for SOD, CAT, POD, and lactate dehydrogenase activity. The activities of SOD, CAT, and POD were measured using an enzyme assay kit (Naniing Jiancheng Bioengineering Institute, China). The activity of LDH in E. coli was measured in vivo using a GENMED bacterial lactate dehydrogenase assay kit (GENMED Scientifics Inc., USA). All procedures were carried out according to the manufacturer’s instructions.
Statistical Analysis
All experimental data are represented as the mean over three independent replicates. The values shown in the tables and figures represent means ± SD of triplicate. Statistical significance is determined as P ≤ 0.05.
Results
Expression of OsCA-M Gene Is Induced by Heat Stress
The full-length cDNA of 1148 bp encodes a protein containing a chloroplast transit peptide of 63 amino acid residues and a mature protein of 210 amino acid residues (Electronic Supplementary Material Fig. S1). It is known that the chloroplast transit peptide is for protein localization, so we removed the transit peptide from the full-length OsCA to study the OsCA mature protein (OsCA-M). RT-PCR and real-time quantitative PCR were employed to analyze the expression patterns of OsCA-M under heat stress (Fig. 1). At 42 °C for 72 h, OsCA-M mRNA revealed different expression patterns under different times (Fig. 1a). The expression levels of OsCA-M were increased by 3.3-fold and 3.1-fold at the 24 and 36 h time points, respectively, but no any remarkable change occurred at other time points (Fig. 1b). These results implied that OsCA-M was an early-induced gene in response to heat stress.
Analysis of the expression patterns of OsCA-M gene in rice (Oryza sativa. L) seedlings by RT-PCR (a) and qRT-PCR (b) under high temperature. All values are expressed relative to the gene expression observed in wild-type plants (1 unit). Endogenous ubiquitin was used as a control. All experiments were performed in triplicate. ***P < 0.001
Expression and Solubility of OsCA-M Fusion Proteins in E. coli
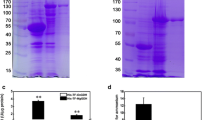
Cell pellets and soluble cell extracts of BL/OsCA-M were analyzed after induction. The predicted molecular mass of OsCA-M protein was 22.8 kDa. Coomassie blue staining revealed that a 48.8-kDa band (22.8 kDa of OsCA-M plus 26 kDa of GST) was observed in the cell pellets and soluble cell extracts from a GST-OsCA-M recombinant (Fig. 2a). The expression of GST-OsCA-M was further confirmed by Western blot (Fig. 2b). It was implied that the expressed GST-OsCA-M protein was soluble.
Expression of OsCA-M in E. coli was analyzed by SDS-PAGE (a) and Western blot (b). M molecular marker; line 1 total protein extracts of BL/pGEX-6P-1 without IPTG induction; line 2 total protein extracts of BL/pGEX-6P-1 with IPTG induction; line 3 total protein extracts of BL/OsCA-M without IPTG induction; line 4 inclusion bodies of BL/OsCA-M with IPTG induction; line 5 supernatant of BL/OsCA-M with IPTG induction. The antibody is a GST antibody
Expression of OsCA-M Gene in E. coli Improves the Cell Survival Ratio Under Heat Stress
To determine the function of OsCA-M gene in heat stress, we analyzed the growth of the OsCA-M transformants of E. coli at 55 °C. The spot and growth curve assay showed that the growth levels of non-induced and induced cultures of BL/pGEX-6P-1 and BL/OsCA-M recombinants were similar at 37 °C. When treated with 55 °C, the non-induced cultures of BL/pGEX-6P-1 and BL/OsCA-M grew slowly or almost had no growth. However, the induced cultures of BL/pGEX-6P-1 and BL/OsCA-M grew normally and the growth of the induced BL/OsCA-M transformants was better than that of the induced BL/pGEX-6P-1 transformants (Fig. 3). This result (Table 1) revealed that the cell survival ratio of the induced culture of BL/OsCA-M transformants was about 13.4-fold higher than that of the induced culture of BL/pGEX-6P-1 transformants at 55 °C for 45 min.
The OsCA-M gene increases E. coli resistance to heat shock. a Spot assay of the E. coli cells harboring either the BL/OsCA-M or BL/pGEX-6P-1 vector. The E. coli cultures were adjusted to OD600 = 0.8 and treated with high temperature at 55 °C for 45 min and then returned to normal growth conditions. Five microliters of the serially diluted (10-fold, 100-fold, 1000-fold, 10,000-fold) bacterial suspensions were spotted onto the LB plates. Spot assay at 37 °C was the control. b Growth curves of the E. coli cells harboring either the BL/OsCA-M or BL/pGEX-6P-1 vector were measured at 37 °C (left) or at 55 °C (right). The induced E. coli cultures were adjusted to OD600 = 0.4 and treated with high temperature at 55 °C for 45 min and then returned to normal growth conditions, and the OD600 was monitored; 37 °C was the normal temperature as the control. The data are the averages ± standard deviations of the three independent experiments
Expression of OsCA-M Reduces ROS Under Heat Stress
Heat stress could cause oxidative stress to release ROS; thereby, we detected the change tendency of ROS expressed in RFU. The change of RFU is consistent with ROS. Time courses of RFU showed that the ROS of BL/pGEX-6P-1 and BL/OsCA-M did not show an obvious difference between non-induced and induced cultures at 37 °C (Fig. 4). When the cells were subjected to 55 °C, the ROS was induced in cultures of BL/OsCA-M and BL/pGEX-6P-1 transformants. The induced BL/OsCA-M bacteria had the lowest ROS level, and the induced BL/pGEX-6P-1 bacteria had moderate ROS level, while the non-induced cultures of BL/OsCA-M and BL/pGEX-6P-1 had high ROS level (Fig. 4).
The dose of ROS of the E. coli cells harboring either the BL/OsCA-M or BL/pGEX-6P-1 vector was measured at 37 °C (a) or 55 °C (b). The induced E. coli cultures were adjusted to OD600 = 0.8 and treated with high temperature at 55 °C for 45 min, and the relative fluorescence units (RFU) were monitored; 37 °C was the normal temperature as the control. The data are the averages ± standard deviations of the three independent experiments
Enzymatic Activity of Antioxidative System in E. coli Under Heat Stress
It is well known that excessive ROS is harmful to bacteria, and antioxidant enzymes play an important role for preventing the ROS accumulation. SOD, CAT, and POD are the three main types of antioxidant enzymes. Thus, we measured the enzymatic activities of SOD, CAT, and POD. As shown in Table 2, in the non-induced and induced cultures of BL/pGEX-6P-1 and BL/OsCA-M recombinants, the activities of these three enzymes were almost similar at 37 °C. However, when the cells were subjected to 55 °C, the activities of SOD, CAT, and POD were increased both in non-induced and induced cultures of BL/OsCA-M and BL/pGEX-6P-1 transformants. Moreover, we also observed that relative to the non-induced cultures of BL/OsCA-M and BL/pGEX-6P-1 transformants, the activities of these three enzymes in the induced cultures were all increased at 55 °C. Meanwhile, in the induced cultures, the SOD, CAT, and POD activities of the BL/OsCA-M increased at 18.7, 109, and 3.0 % compared with that of the induced BL/pGEX-6P-1 transformants at 55 °C, respectively (Table 2).
Expression of OsCA-M Protects LDH Activity Under Heat Stress
Lactate dehydrogenase, which is an enzyme found in animals, plants, and prokaryotes, is known to be a marker of injuries and is important for organisms. It has been reported that the activity of this enzyme increases under the condition of oxidative stress, so the thermo-protective action of OsCA-M on LDH activity was measured. We found that there were no significant differences on LDH activities between non-induced and induced cultures of BL/pGEX-6P-1 and BL/OsCA-M at 37 °C (Table 3). However, when the cells were subjected to 55 °C, the LDH activities were both upregulated in non-induced and induced cultures of BL/OsCA-M and BL/pGEX-6P-1 transformants. The upregulation levels of LDH in the non-induced cultures of BL/OsCA-M and BL/pGEX-6P-1 transformants were both higher than those in the induced cultures of them. Furthermore, in the induced cultures, the LDH activity of the BL/OsCA-M decreased at 37.2 % compared with that of the induced BL/pGEX-6P-1 transformants (Table 3).
Discussion
Recently, many plant genes related to stresses were introduced into E. coli and these genes conferred stress tolerances of the host cells [2, 16, 17]. It is indicated that some protective mechanisms might be common in prokaryote and eukaryote under stress conditions [18].
It is reported that the expression of CA has been linked with stress resistance in plants [11–13]. However, it is still unknown whether OsCA could contribute to protect organisms from heat stress. In the present study, a mature β-type rice carbonic anhydrase gene (OsCA-M) was induced in rice when rice plants were treated with heat stress. It was implied that OsCA-M was possibly an early-induced gene in response to heat stress (Fig. 1). On the other side, the spot, survival rate, and growth curve assay (Fig. 3, Table 1) strongly suggested that the expression OsCA-M protein could not only promote the normal growth of host cells but also contribute to thermo-tolerance of the host cells. Thus, we consider that OsCA-M-expressing transformants have more rapid recovery compared with non-expressing control transformants and the improved growth performance of E. coli was due to the function of the OsCA-M protein.
As we known, heat stress has been found to increase oxidative damage in the microbial cell and affect the microbial cell survival [19, 20]. Here, we found that the treatment of 55 °C to E. coli cells for 45 min clearly induced ROS generation and oxidative stress compared with normal temperature (Fig. 4), and it was consistent with previous reports [21, 22]. However, the ROS of E. coli transformants harboring the OsCA gene was much lower than that of the control transformants at 55 °C (Fig. 4). It means that the expression of OsCA can reduce ROS content in E. coli to protect the microbial cell from oxidative damage under heat stress. Meanwhile, heat stress induced the activities of antioxidant enzymes (SOD, CAT, and POD) of E. coli cells in our study (Table 2) and the activities of the antioxidant enzymes of E. coli transformants harboring the OsCA-M gene were all higher than those of the control transformants (Table 2). The antioxidant enzymes may serve as the frontline of defense against oxidative stress by preventing the ROS accumulation. Antioxidant enzymes could lead to protection yeast and bacteria from lethal heat shock [23, 24]. The results of ROS and antioxidant enzymes strongly suggested that ectopic expression of OsCA-M could reduce ROS content by inducing and increasing antioxidant enzymes’ activities to protect the microbial cell from oxidative damage under heat stress. Besides, we also found that, at 55 °C, the LDH activity of E. coli transformants harboring the OsCA-M gene was lower than that of control transformants (Table 3). It was known that excessive ROS could cause damage to tissue and LDH was released during tissue damage; our results also showed that the change of LDH activity was consistent with the change of ROS (Table 3, Fig. 4). It was also implied that the OsCA-M protein could reduce ROS accumulation to protect LDH activity to some extent in vivo under high-temperature stress.
In conclusion, our results showed that the OsCA-M protein could provide protective functions for the host cells’ survival under heat stress by a synergistic fashion of increasing the antioxidant enzymes’ activities to reduce the oxidative damage and regulate the LDH activity of E. coli. Our results established a basis for further research on the rice carbonic anhydrase to help crop plant to have heat tolerance and provided a potential method to improve the thermo-tolerance of rice by over-expressing OsCA-M in rice.
References
Craufurd, P. Q., Jagadish, S. V. K., Wheeler, T. R., Bennett, J., & Lafitte, R. (2007). Heat tolerance at flowering. Abstract: An international workshop on “Cool rice for a warmer world”, Wuhan, China.
Liu, Y., Zheng, Y. Z., Zhang, Y. Q., Wang, W. M., & Li, R. H. (2010). Soybean PM2 protein (LEA3) confers the tolerance of Escherichia coli and stabilization of enzyme activity under diverse stresses. Current Microbiology, 60, 373–378.
Lobell, D. B., & Asner, G. P. (2003). Climate and management contributions to recent trends in U.S. agricultural yields. Science, 299, 1032.
Zhang, G. L., Chen, L. Y., Lei, D. Y., & Zhang, S. T. (2005). The research progress of heat resistance in rice. Hybrid Rice, 20, 1–5.
Supuran, C. T. (2008). Carbonic anhydrases—an overview. Current Pharmaceutical Design, 14, 603–614.
Tiwari, A., Kumar, P., Singh, S., & Ansari, S. A. (2005). Carbonic anhydrase in relation to higher plants. Photosynthetica, 43, 1–11.
Karlsson, J., Clarke, A. K., Chen, Z. Y., Hugghins, S. Y., Park, Y. I., Husic, H. D., Moroney, J. V., & Samuelsson, G. (1998). A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO Journal, 17, 1208–1216.
Moskvin, O. V., Ivanov, B. N., Ignatova, L. K., & Kollmeier, M. A. (2000). Light-induced stimulation of carbonic anhydrase activity in pea thylakoids. FEBS Letters, 470, 375–377.
Kisiel, W., & Graf, G. (1972). Purification and characterization of carbonic anhydrase from Pisum sativum. Phytochemistry, 11, 113–117.
Chang, C. W. (1975). Activation energy for thermal inactivation and Km of carbonic anhydrase from the cotton plant. Plant Science Letters, 4, 109–113.
Lazova, G. N., Kicheva, M. I., & Popova, L. P. (2000). The effect of abscisic acid and methyl jasmonate on carbonic anhydrase activity in pea. Photosynthetica, 36, 631–634.
Yu, S., Zhang, X. X., Guan, Q. J., Takano, T., & Liu, S. K. (2007). Expression of a carbonic anhydrase gene is induced by environmental stresses in rice (Oryza sativa L.). Biotechnology Letters, 29, 89–94.
Kaul, T., Reddy, P. S., Mahanty, S., Thirulogachandar, V., Reddy, R. A., Kumar, B., Sopory, S., & Reddy, M. K. (2011). Biochemical and molecular characterization of stress-induced β-carbonic anhydrase from a C4 plant, Pennisetum glaucum. Journal of Plant Physiology, 168, 601–610.
Bageshwar, U. K., Premkumar, L., Gokhman, I., Savchenko, T., Sussman, J., & Zamir, A. (2004). Natural protein engineering: a uniquely salt-tolerant, but not halophilic, α-type carbonic anhydrase from algae proliferating in low-to hyper-saline environments. Protein Engineering Design and Selection, 17, 191–200.
Liu, Y., & Zheng, Y. Z. (2005). PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochemical and Biophysical Research Communications, 331, 325–332.
Pan, J., Wang, J., Zhou, Z. F., Yan, Y. L., Zhang, W., Lu, W., Ping, S. Z., Dai, Q. L., Yuan, M. L., Feng, B., Hou, X. G., Zhang, Y., Ma, R. Q., Liu, T. T., Feng, L., Wang, L., Chen, M., & Lin, M. (2009). IrrE, a global regulator of extreme radiation resistance in Deinococcus radiodurans, enhances salt tolerance in Escherichia coli and Brassica napus. PLoS One, 4, e4422.
Tanaka, S., Ikeda, K., Ono, M., & Miyasaka, H. (2002). Isolation of several anti-stress genes from a mangrove plant Avicennia marina. World Journal of Microbiology and Biotechnology, 18, 801–804.
Garay-Arroyo, A., Colmenero-Flores, J. M., Garciarrubio, A., & Covarrubias, A. A. (2000). High hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. Journal of Biological Chemistry, 275, 5668–5674.
Bai, Z., Harvey, L. M., & McNeil, B. (2003). Elevated temperature effects on the oxidant/antioxidant balance in submerged batch cultures of the filamentous fungus Aspergillus niger B1-D. Biotechnology and Bioengineering, 83, 772–779.
Abrashev, R. I., Pashova, S. B., Stefanova, L. N., Vassilev, S. V., Dolashka-Angelova, P. A., & Angelova, M. B. (2008). Heat-shock-induced oxidative stress and antioxidant response in Aspergillus niger 26. Canadian Journal of Microbiology, 54, 977–983.
Kim, I. S., Moon, H. Y., Yun, H. S., & Jin, I. (2006). Heat shock causes oxidative stress and induces a variety of cell rescue proteins in Saccharomyces cerevisiae KNU5377. Journal of Microbiology, 44, 492–501.
Belozerskaya, T. A., & Gessler, N. N. (2007). Reactive oxygen species and the strategy of antioxidant defense in fungi: a review. Applied Biochemistry and Microbiology, 43, 506–515.
Begonia, G. B., & Salin, M. L. (1991). Elevation of superoxide dismutase in Halobacterium halobium by heat shock. Journal of Bacteriology, 173, 5582–5584.
Romandini, P., Bonotto, C., Bertoloni, G., Beltramini, M., & Salvato, B. (1994). Superoxide dismutase, catalase and cell dimorphism in Candida albicans cells exposed to methanol and different temperatures. Comparative Biochemistry and Physiology. Pharmacology, Toxicology and Endocrinology, 108, 53–57.
Acknowledgments
This research was partly supported by the National Transgenic Research and Development Program (2011ZX08001-004) and the 863 program (31071391) of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The amino acid sequences of the β-type carbonic anhydrate (OsCA). It contains a chloroplast transit peptide of 63 amino acid residues and a mature protein of 210 amino acid residues (GIF 133 kb)
Rights and permissions
About this article
Cite this article
Tianpei, X., Mao, Z., Zhu, Y. et al. Expression of Rice Mature Carbonic Anhydrase Gene Increase E. coli Tolerance to Heat Stress. Appl Biochem Biotechnol 176, 625–635 (2015). https://doi.org/10.1007/s12010-015-1600-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1600-8