Abstract
Dilution was employed as a pretreatment strategy to increase light transmittance and decrease ammonia toxicity in piggery effluent prior to the cultivation of microalgae. The dilution effect was quantitatively determined based on both the maximum specific nutrient consumption rate and the maximum growth coefficient to minimize the usage of diluent. The biomass productivity of microalgae was also evaluated to select the best species among the five different candidates examined. A 20-fold dilution of piggery wastewater resulted in decreased chromaticity (584 mg Pt-Co L−1) and total nitrogen (76 mg L−1), on which the microalgae cultivation was more effective for an algal growth compared to the other dilution factors. If the initial cell concentration of Scenedesmus quadricauda increased, the production of biomass tended to improve. Robust growth and harvesting of S. quadricauda were achieved, and the associated consistent removal of inorganic nutrients was accomplished during the semi-continuous cultivation of the best species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many countries, attention has been paid to strategies for a reliable treatment of piggery wastewater. Due to its high levels of organic matter, inorganic nutrients, and pathogens, piggery wastewater can cause a wide variety of latent issues in water environments. Recent political commitments indicate that serious actions must now be taken in order to reduce the adverse impacts of various contaminants including nutrients on receiving water bodies. Among the various treatment strategies for the remediation of piggery effluent, anaerobic digestion is credited as the most promising alternative [1–4]. The advantages of using anaerobic digestion for this purpose include pathogen stabilization, odor reduction, energy recovery, and cost-effectiveness. Nevertheless, the anaerobically digested effluent which still contains high concentrations of inorganic nutrients (e.g., nitrogen and phosphorus) contributes readily to these nutrient loads that are implicated in the eutrophication of natural water bodies. Post-treatment with aerobic microbes is commonly employed for the removal of inorganic nutrients that remain after the fermentative treatment processes. However, such processes generally require considerable air supply for the conversion of NH4-N to NO3-N and require a supplement of external carbon sources due to the low C/N ratio of anaerobic digestion effluent [5]. Therefore, strategies that are simpler and more cost-effective are needed for the robust removal of residual nutrients.
Microalgae use solar energy to combine water with CO2, resulting in the splitting of H2O and the resultant O2 evolution [6–8]. The photoautotrophic growth of microalgae can result in the conversion of both nitrogen and phosphorus into algal biomass through photosynthesis with CO2 used as an inorganic carbon source. Thereby, the cultivation of microalgae can be employed as a post-treatment strategy to remove nutrients from anaerobically digested piggery wastewater, where the biosolids have previously been removed using a common solid–liquid separation technique (e.g., membrane filtration). Furthermore, the microalgae-based wastewater treatment can also produce a potentially valuable algal biomass that can be utilized as feedstock for the production of biofuels [9, 10]. However, the production of microalgae using wastewater leads to the contamination of the biomass with undesirable compounds; thus, a biomass cultivated with wastewater may not be appropriate for feed, food, or other applications [11]. Increased attempts have been made to use biofuel production with algal biomass to explore alternate energy sources, to utilize domestic resources, and to reduce greenhouse gas emissions [12]. The main obstacle to the widespread use of algal biofuels is the high costs of microalgae cultivation [9, 13]. It is therefore crucial to utilize nutrient-rich wastewater as a good growth medium for microalgae instead of using commercialized chemicals for the culture medium preparation.
The goal of this study was to characterize the algal growth and the associated removal of inorganic nutrients from anaerobically digested piggery wastewater in which residual solids were completely removed through microfiltration prior to the microalgae cultivation. The research objectives were as follows: (1) minimize the adverse effects of free ammonia and/or color on the microalgae growth using the dilution of the pretreated wastewater with distilled water, (2) select the most promising microalgal species among five different candidates in terms of biomass productivity and the maximum specific nutrient consumption rate, and (3) optimize the initial concentration of algal biomass inoculated on the pretreated wastewater for the robust growth and harvesting of microalgae during the semi-continuous cultivation of the best species.
Materials and Methods
Microalgal Strains and Culture Conditions
Five different algal cultures were examined in this study, and they are Scenedesmus accuminatus AG 10316, Scenedesmus quadricauda AG 10003, Chlorella vulgaris AG 30007 from Korea Collection for Type Culture in Korea Research Institute of Bioscience and Biotechnology, Ankistrodesmus gracilis SAG 278-2 from the bank SAG Culture Collection of the University of Göttingen, and Botryococcus braunii UTEX LB 572 obtained from the University of Texas. These microalgal species were separately inoculated into 1,000-mL tubular photobioreactors containing 800 mL BG-11 medium [14]. The photobioreactors were incubated under white fluorescent light illumination at 75 μmol photon m−2 s−1 at 25 °C for 2 weeks while continuously supplementing 5 % CO2 (v/v) into the reactors.
Piggery Wastewater Pretreatment
The biologically treated piggery effluent was collected from commercial treatment facilities (ENR Solution Co., Ltd., South Korea) which use the upflow sludge blanket reactor process for the typical mesophilic anaerobic digestion of organic substrates. The effluent was then filtered to remove microbials and suspended solids in lab-scale submerged membrane reactors (SMR) with 0.2-μm polyethylene membranes (Yuasa Membrane Systems Co., Ltd., Japan) placed in a filtration testing module (Kubota Corporation, Japan), providing an effective filtration area of 0.1 m2. The membrane filtration was conducted at a constant flux of 0.25 L m−2 h−1 using a peristaltic pump for the permeate side of the SMR with a working volume of 6 L. The characteristics of SMR effluent are shown in Table 1.
Incubation of Algal Cells in Photobioreactors
The SMR effluent was diluted with distilled water to prepare five different growth media regarding the concentration of wastewater. Microalga S. accuminatus was employed to select the growth medium that required the smallest volume of diluent among the prepared media for the consistent growth of microalgae. The subsequent comparison in biomass productivity between different algal species was conducted using the dilution factor identified in the preliminary test [15–17]. Batch experiments were conducted to determine the growth rate of S. accuminatus when using a series of tubular borosilicate reactors (34-mm inner diameter and 520-mm height) as illustrated in Fig. 1. Algal biomass from a 2-week-old culture was harvested by centrifugation (3,000 rpm for 10 min at room temperature) and was inoculated to be 500 mg L−1 as dry cell weight concentration into each photobioreactor containing 400 mL SMR effluent with various dilution ratios. The photobioreactors were incubated under white fluorescent light illumination at a light intensity of 150 μmol photon m−2 s−1 at 25 °C for 94 h, while a 5 % CO2 (v/v) supplement was passed through an inlet port at the conical base of the reactor. A gas mixture of 5 % CO2 and 95 % ambient air was sterilized with 0.2-μm membrane filters installed at the bottom of the reactor. During the incubation, 10 mL mixed liquor was collected at 19, 43, and 94 h of cultivation from each photobioreactor for measurement of dry cell weight, T-N, NH4-N, and T-P. In some experiments, the incubation extended to 113 h and the identical volume of mixed liquor was collected at 15, 39, 63, and 113 h of cultivation for both non-diluted and fourfold diluted SMR effluent.
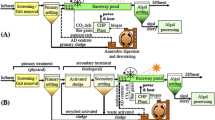
Experimental setup for the evaluation of algal growth and the associated removal of nutrients from pretreated piggery wastewater under continuous illumination: 1 air pump, 2 gaseous CO2 cylinder, 3 equilibration reactor for supplying a gas mixture of 5 % CO2 and 95 % ambient air, 4 tubular photobioreactors (ID 34 mm × H 520 mm) with a working volume of 400 mL, 5 white fluorescent light sources (150 μmol photon m−2 s−1), and 6 flow meters with membrane (0.2-μm pore size) gas sterilizers
Based on the results from the cultivation test, the optimal dilution factor was selected and used for further experiments. Separate cultivation experiments were conducted to select the best algal species among the five different candidates and to evaluate the impact of the initial culture media biomass density which varied from 0.5 to 2.0 g cell L−1 (as dry cell weight) on the performance of the algae-based wastewater remediation. The identical cultivation conditions (e.g., light intensity etc.) and apparatuses as described above were used in these cultivation tests. Finally, the sequential algal cultivation and harvesting on pretreated piggery wastewater were demonstrated. This was performed by conducting a fed-batch experiment using the most promising candidate among the examined algal species and under the optimal bioremediation conditions. The piggery wastewater temperature was equivalent to the room temperature (≈25 °C).
Analytical Methods
Salicylate, acid persulfate digestion, and dichromate methods were used to measure NH4-N, T-P, and COD in the water samples, equivalent to the Standard Method 4,500-NH3 G, 4,500 P. B. 5, and 5,220 C, respectively, for water and wastewater [18]. T-N was also determined (TOC TNM-1, Shimadzu, Japan) using whole samples. A DR 5000 spectrophotometer (Hach, USA) was used to measure the chromaticity. Light intensity was determined using LI-250A (LI-COR, USA). The pH was measured using a pH meter (Orion 3 STAR, USA). Metals were analyzed using an iCE 3500Z atomic absorption spectrometer (Thermo Scientific, USA). Each measurement was carried out in triplicate and average values were reported.
Kinetic Analysis
The cell growth was determined by measuring the dry cell weight concentration using the same procedure as that found in the Standard methods for the examination of water and wastewater [18]. The growth coefficient (μ, day−1) and the specific nutrient consumption rate (q, mg-N or -P g-cell day−1) for a specific period were calculated using the Eqs. (1) and (2), respectively [19, 20].
where AB2 and AB1 are the algal biomass concentrations as dry cell weight (g L−1) at times t 2 and t 1, respectively. S 1 and S 2 are assimilable nutrient concentrations (mg-N or -P L−1) at time t 1 and t 2. AB′ is the average algal biomass concentration between moment time t 1 and t 2 . Therefore, the maximum value of the growth coefficient (μ max) and the maximum specific nutrient consumption rate (q max) were obtained between the closest two data points of maximum slope on the dry cell weight concentrations and the assimilated nutrient concentrations plotted against time, respectively.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to examine the differences among average values. Excel (Microsoft Office, 2013) was used for all statistical analyses, and differences in the variables were considered significant at the P < 0.05 level of confidence.
Results and Discussion
Assessment of Algal Growth on Piggery Effluent with a Wide Range of Ammonium Levels
S. accuminatus was cultivated in parallel using photobioreactors that separately contained five different growth media prepared due to SMR effluent dilution with distilled water (Fig. 2). The initial concentration of T-N ranged from 44 to 1,650 mg N L−1, where NH4-N accounted for 92 ± 1 %. The optimal level of ammonium for the bioremediation of piggery effluent using microalgae was determined by comparing both the maximum algal growth coefficients (μ max) and the maximum specific nutrient consumption rates (q max) among the six different growth media (as shown in Table 2). μ max increased as the level of T-N was decreased from 1,650 to 76 mg N L−1. However, the trends were reversed at 44 mg L−1 T-N, possibly due to the phosphorus insufficiency with the result of a microalgae growth inhibition. Similar patterns were also shown in the result from nitrogen removal, i.e., 100 mg L−1 T-N was recorded as the highest q max value among the experimental variations. The algal growth at high dilution ratios may be constrained due to phosphorus limitation. The ratio of T-N:T-P in the growth media used in this experiment increased up to 440 with an increasing dilution ratio, since the spectroscopic T-P measurement was significantly affected by the color that prominently remained after the dilution of the pretreated piggery effluent. Dilution factors <20 times were not sufficient to eliminate such color-driven interference for T-P measurement.
The growth rate of S. accuminatus was decreased with an increased concentration of T-N in the culture media. In particular, an initial lag phase was observed for a growth culture containing 163 mg L−1 T-N. This might be attributed primarily to the ammonia toxicity to microalgae assimilating inorganic carbon and also might be strongly associated with the higher turbidity of the wastewater. It was reported that high levels of ammonia can result in the inhibition of assimilating inorganic carbon into the microalgae cell for photosynthesis [21, 22]. Markou et al. [23] reported that increasing ammoniacal nitrogen concentration resulted in an increase of the degree of ammonia inhibition, especially when low biomass densities were used in the cultivation. The formation of free ammonia depends on both the ammonium level and aqueous pH of the growth media. The concentrations of free ammonia in culture media ranged from 2.5 to 22.6 mg NH3 L−1. These were calculated using the equation proposed by Ford et al. [24]. An almost complete growth inhibition was observed for a culture with 22.6 mg L−1 free ammonia, prepared with the lowest dilution rate (×4). The most highly diluted piggery wastewater (×30) contained 2.5 mg L−1 free ammonia. For this culture, the growth rate of S. accuminatus was comparable to that observed for moderately diluted piggery effluent (×20) containing 4.5 mg L−1 free ammonia and a higher level of trace metals. Table 2 also shows that significant (P < 0.05) differences were observed between the growth coefficient and the specific nitrogen consumption rate among all the experimental variations.
Additional to the ammonia toxicity, high color levels for less diluted SMR effluent might synergistically inhibit the growth of microalgae due to a poor light transmittance through the culture medium passage. The chromaticity of undiluted SMR effluent was 11,680 mg Pt-Co L−1, which absorbed 88 % of the light illumination within a path length of 1 cm. Thus, under continuous illumination, only a small amount of light could reach the algal cells for photosynthesis. The dilution of SMR effluent improved the availability of light illumination, of which 80 % penetrated at the same path length for the 20-fold diluted SMR effluent. This showed the highest growth coefficient among the experimental variations (Table 2). In general, the growth rate of microalgae can be decreased by the overshading effect causing poor light penetration due to the high population density of algal cells; however, such phenomenon was not found during the given cultivation period.
In general, the consumption of inorganic carbon in the photosynthetic activity of microalga results in the accumulation of hydroxyl ions in the aqueous phase, yielding an increase of solution pH [25]. On the contrary, a substantial pH decrease after wastewater treatment with S. accuminatus was observed for all experimental variations as shown in Table 2. Primarily, this resulted from the continuous CO2 supplements and dominantly from the ammonium metabolism during the photosynthetic activities of the microalga. Due to the NH4-N metabolism in the microalgae, hydrogen ions can be produced [26–28]. The pH decrement after 92 h of incubation was more substantial for a growth culture with the relative higher concentrations of ammonium ions among the experimental variations. However, the algal cultivation on either non-diluted or fourfold diluted SMR effluent resulted in insignificant pH changes due to an almost complete inhibition of cell growth. The algal growth can also be adversely affected by low pH levels. Li et al. [27] reported that algal incubation under acidic conditions (pH < 5) resulted in a decrease of nutrient removal and algal population density in the stable phase.
Comparison of Growth Kinetics and Ammonium Uptakes Among Microalgal Species
The results of the batch experiments with a 20-fold dilution of SMR effluent using five different microalgal species are shown in Fig. 3. The concentration of algal dry cell weight is plotted against time. The candidates can be classified into two groups in terms of cell growth; i.e., the biomass productivity of both S. accuminatus and S. quadricauda ranged from 810 to 820 mg cell L−1 day−1. This was much higher than that observed for the other species, which showed a flatted growth after the first 68 h of cultivation due to the nutrient-limited habitats. Kapdan and Aslan [29] obtained the highest removal of NH4-N from synthetic wastewater with an N/P ratio of 8:1 using C. vulgaris, while Li et al. [30] reported that the maximum growth rate of Scenedesmus sp. increased with the increasing N/P ratio (up to 20:1) at the initial T-P of 1.3 mg L−1. Ji et al. [31] reported recently that the uptake of ammonium by C. vulgaris was significantly increased by the dilution of piggery wastewater in which the extent of ammonium uptake was greater than that of either nitrite or nitrate uptake. Our result showed that S. accuminatus and S. quadricauda grew relatively well even under nutrient-limited cultivation conditions compared to the use of C. vulgaris, which has often been regarded as a reliable species for treatment of livestock wastewater.
Comparison of a growth rate and b–d nutrient uptake among S. quadricauda, S. accuminatus, B. braunii, A. gracilis, and C. vulgaris. Graphs b, c, and d show residual T-N, NH4-N, and T-P, respectively, as a function of time during the incubation of the five candidates in pretreated piggery wastewater. Monosodium phosphate was additionally used to achieve the N/P ratio of 40 for piggery effluent diluted up to 20 times in distilled water. If the error bars are larger than the symbols, then the error bars are shown
The T-N removal was governed by the extent of the reduction in NH4-N, which was the most dominant fraction among inorganic nitrogen species for all experimental variations. Both S. accuminatus and S. quadricauda converted NH4-N to their biomass through metabolic pathways to a much greater extent than achieved by the other species (Supplementary Information SI-1). Especially, B. braunii recorded the lowest q max value for nitrogen and phosphorus uptake among the examined candidates due to its preference of nitrate than ammonium for cell growth. This concurred with a previous study reporting that the B. braunii growth rate on a nitrate medium was much higher than that observed for the ammonium medium [32]. Likewise, a steep decrease of T-P was achieved by all the examined microalgal species except B. braunii at the 20-h cultivation time. Thereafter, an insignificant variation was found in the residual T-P concentration up to 92 h. Yoo et al. [33] also reported that the biomass productivity of Scenedesmus sp. was two to eight times higher than that of C. vulgaris and B. braunii for which these algal species were photoautotrophically cultivated on a synthetic growth medium supplemented with CO2 for 14 days.
Selection of the Initial Cell Concentration for Robust Algal Cultivation
Among the five candidates, S. quadricauda was selected and used to investigate the effect of different initial concentrations of algal cells inoculated into diluted SMR effluent containing 78 mg NH4-N L−1 on biomass production and nutrient uptake rate. The initial biomass density varied from 0.5 to 2.0 g L−1 as dry cell weight, and the higher initial cell concentration resulted in an increase of the production of algal biomass. Figure 4a also shows the stagnant growth of S. quadricauda, regardless of the initial cell concentration (i.e., the lowest slope was shown for 20 h of incubation) due to the transition from the lag phase to the stationary phase.
Impact of different initial algal population densities on a biomass production and b, c nutrient removal during incubation of S. quadricauda in pretreated piggery wastewater. Graphs b and c show T-N and T-P, respectively, assimilated by S. quadricauda as a function of initial cell concentration. The initial concentrations of T-N, NH4-N, and T-P were 205, 78, and 6.6 mg L−1, respectively. The T-P consumption depicted in graph c was obtained after 140 h of incubation
After the first 70 h of cultivation, the removal of nutrients by the microalga through biological assimilation was insignificant. After the cultivation test, the concentration of T-P which remained in the aqueous phase ranged from 0.1 to 0.85 mg T-P L−1 for all the experimental variations. Among these experimental variations, the highest removal of T-P was found for an initial biomass density of 1.5 g L−1 (Fig. 4c). The photoautotrophic cultivation of S. quadricauda at a biomass density of 1.5 g L−1 for 70 h resulted in a 1.0-g biomass production per unit volume (L) of pretreated wastewater. The associated removal of nitrogen also accounted for 59 %. The maximum specific nitrogen consumption rate ranged from 33 to 40 mg-N g-cell−1 day−1, which was slightly decreased when the initial biomass density in the culture media varied from 0.5 to 2.0 g L−1. Similar trends were also obtained for T-P assimilated by the microalga (data not shown). An increase of the initial biomass density in the photobioreactors resulted in a decrease of the maximum specific consumption rates for both nitrogen and phosphorus, which was attributed again to the self-shading effect of the algal cells suspending in the photobioreactors as well as in nutrient-limited habitats. The population density of cells initially inoculated into the culture media is an important factor for a better wastewater treatment performance and should be controlled after repeated algal biomass harvestings in a continuous flow cultivation system. Uggetti et al. [34] reported that biomass production positively correlated with inoculum and substrate concentrations, while microalgal growth rate was negatively affected by the self-shading phenomenon depending on the ammonium concentration. Other investigators [35, 36] reported that the low algal cell concentration in the beginning of cultivation might cause a lack of self-shading, resulting in photoinhibition. Contrarily, Markou et al. [23] suggested that higher biomass density could assimilate ammoniacal nitrogen, mitigating the ammonia inhibition effect. Figure 4 clearly shows that the cultivation duration should not be more than 70 h for the economic execution of a bioremediation strategy. This applies to the concurrent nutrient removal and biomass production from diluted SMR effluent that contains moderate levels of ammonium ions.
Fed-Batch Cultivation with Optimal Conditions
Based on the results shown in Fig. 4, both the harvesting period and initial biomass density were selected to investigate the feasibility of using microalga S. quadricauda for the treatment of piggery wastewater due to the operation with the fed-batch cultivation system. To control the cell density in the photobioreactor, the amount of the newly produced algal biomass was determined and removed from the system at 70, 110, and 170 h (Fig. 5). C. vulgaris has been considered as one of the most promising species for the robust treatment of wastewater due to its distinguished growth rate [7, 37, 38]; C. vulgaris was also examined to compare the growth coefficient with S. quadricauda under the same cultivation conditions.
The growth coefficient of S. quadricauda ranged from 0.168 to 0.270 day−1 in the fed-batch cultivation system (Table 3), which was relatively higher when compared to C. vulgaris ranging from 0.147 to 0.191 day−1 (data not shown). The nutrient consumption rate of S. quadricauda had a decreasing tendency with the periodic repetitions of algal biomass harvesting. The photoautotrophic growth was inhibited if the light illumination was blocked out. This was a result of the accumulation of color-causing materials. Similarly, the growth coefficient of the microalga also decreased with a periodical nutrient supply after the biomass harvesting due to this accumulation. Nutrients were supplied by adding the appropriate amount of non-diluted SMR effluent to the culture medium for which T-N could be reached at around 100 mg L−1 immediately after harvesting of the algal biomass. The harvest water was returned to the photobioreactor to maintain the initial volume of the growth medium. The initial chromaticity of the growth medium for the first round of cultivation was 811 mg Pt-Co L−1, which reached 2,163 mg Pt-Co L−1 for the fourth round of cultivation (Table 3). Such artificial manipulation in the lab-scale experiments resulted in the accumulation of color-causing materials in the piggery wastewater but could be readily controlled in operating a full-scale continuous flow treatment system using pretreated piggery effluent with desirable levels of color and ammonium.
Conclusions
Five different microalgae species were examined to optimize cultivation strategies for robust algal growth and thus to achieve consistent removal of nutrients from pretreated piggery effluent. The dilution of the pretreated piggery wastewater increased light transmittance and decreased free ammonia in wastewater, resulting in the improved productivity of biomass. During the semi-continuous cultivation of the best algal species, the biomass was periodically harvested and consequent removal of inorganic nutrients was achieved. With an increased initial concentration of algal cells inoculated to the anaerobically digested, microfiltered, and then diluted wastewater, the productivity of algal mass was also increased. Our results revealed that the combination of S. quadricauda and S. accuminatus could not only be a bio-agent for the remediation of piggery wastewater, but it could also be a feedstock for biomass production. The pretreatment strategies should be further strengthened to decrease free ammonia and concurrently to increase light transmittance along with reduced consumption of a relative clean diluent prior to microalgae cultivation.
References
Sánchez, E., Milán, Z., Borja, R., Weiland, P., & Rodriguez, X. (1995). Resources, Conservation and Recycling, 15, 235–244.
Montalvo, S. J. (1995). Bioresource Technology, 53, 207–210.
Rodríguez-Andara, A., & Lomas-Esteban, J. M. (1999). Biomass and Bioenergy, 17, 435–443.
Milán, Z., Sánchez, E., Weiland, P., Borja, R., Martin, A., & Ilangovan, K. (2001). Bioresource Technology, 80, 37–43.
Deng, L., Zhen, P., Chen, Z., & Mahmood, Q. (2008). Bioresource Technology, 99, 3136–3145.
Benemann, J. R., Weissman, J. C., Koopman, B. L., & Oswald, W. J. (1977). Nature, 268, 19–23.
Munoz, R., & Guieysse, B. (2006). Water Research, 40, 2799–2815.
Georgianna, D. R., & Mayfield, S. P. (2012). Nature, 488, 329–335.
Chisti, Y. (2007). Biotechnology Advances, 25, 294–306.
Harun, R., Singh, M., Forde, G. M., & Danquah, M. K. (2010). Renewable and Sustainable Energy Reviews, 14, 1037–1047.
Benemann, J. R. (2013). Energies, 6, 5869–5886.
Luque, R., Herrero-Davila, L., Campelo, J. M., Clark, J. H., Hidalgo, J. M., Luna, D., Marinas, J. M., & Romero, A. A. (2008). Energy & Environment Science, 1, 542–564.
Harun, R., Davidson, M., Doyle, M., Gopiraj, R., Danquah, M. K., & Forde, G. M. (2011). Biomass and Bioenergy, 35, 741–747.
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., & Stanier, R. Y. (1979). Journal of General Microbiology, 111, 1–61.
Kessler, E. (1991). Botanica Acta, 104, 169–171.
Aravantinou, A., Theodorakopoulos, M., & Manariotis, I. (2013). Bioresource Technology, 147, 130–134.
Park, J. M., Jin, H.-F., Lim, B.-R., Park, K.-Y., & Lee, K. S. (2010). Bioresource Technology, 101, 8649–8657.
APHA, AWWA, & WPCF. (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington: American Public Health Association.
Reynolds, T. D., & Richard, P. A. (1982). Unit operations and processes in environmental engineering (2nd ed.). Austin: International Thomson Publishing.
Wood, A. M., Everroad, R. C., & Wingard, L. M. (2005). In R. A. Andersen (Ed.), Algal culturing techniques: measuring growth rates in microalgal cultures. Oxford: Elsevier Academic Press.
Azov, Y., & Goldman, J. C. (1982). Applied and Environmental Microbiology, 43, 735–739.
Yuan, X., Kumar, A., Sahu, A. K., & Ergas, S. J. (2011). Bioresource Technology, 102, 3234–3239.
Markou, G., Vandamme, D., & Muylaert, K. (2014). Bioresource Technology, 166, 259–265.
Ford, D. L., Churchwell, R. L., & Kachtick, J. W. (1980). Journal of the Water Pollution Control Federation, 52, 2726–2746.
de Morais, M. G., & Costa, J. A. (2007). Energy Conversion Management, 48, 2169–2173.
Tam, N. F. Y., & Wong, Y. S. (1996). Bioresource Technology, 57, 45–50.
Li, X., Hu, H.-Y., Gan, K., & Yang, J. (2010). Ecological Engineering, 36, 379–381.
Kim, S., Lee, Y., & Hwang, S. (2013). International Biodeterioration and Biodegradation, 85, 511–516.
Kapdan, I., & Aslan, S. (2008). Journal of Chemical Technology & Biotechnology, 83, 998–1005.
Li, X., Hu, H.-Y., Gan, K., & Sun, Y.-X. (2010). Bioresource Technology, 101, 5494–5500.
Ji, M.-K., Kim, H.-C., Sapireddy, V. R., Yun, H.-S., Abou-Shanab, R. A. I., Choi, J. Y., Lee, W. T., Timmes, T. C., Inamuddin, & Jeon, H.-H. (2013). Applied Microbiology and Biotechnology, 97, 2701–2710.
An, J. Y., Sim, S. J., Lee, J. S., & Kim, B. W. (2003). Journal of Applied Phycology, 15, 185–191.
Yoo, C., Jun, S. Y., Lee, J. Y., Oh, C. Y., & Ahn, H. M. (2010). Bioresource Technology, 101, 571–574.
Uggetti, E., Sialve, B., Latrille, E., & Steyer, J.-P. (2014). Bioresource Technology, 152, 437–443.
Li, Y., Chen, Y., Chen, P., Min, M., Zhou, W., Martinez, B., Zhu, J., & Ruan, R. (2011). Bioresource Technology, 102, 5138–5144.
Zhu, L., Wang, Z., Shu, Q., Takala, J., Hiltunen, E., Feng, P., & Yuan, Z. (2013). Water Research, 47, 4294–4302.
Ruiz, J., Alvarez, P., Arbib, Z., Garrido, C., Barragan, J., & Preales, J. (2011). International Journal Phytoremediation, 13, 884–896.
Infante, C., Leon, I., Florez, J., Zarate, A., Barrios, F., & Zapata, C. (2013). International Journal of Environmental Studies, 70, 1–7.
Acknowledgments
This work was supported by the Eco-Innovation Technology Development Project administrated and funded by the Ministry of Environment, South Korea [405-112-037], and the Korea Institute of Science and Technology (KIST) Institutional Program (2E24732).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Kim, HC., Choi, W.J., Ryu, J.H. et al. Optimizing Cultivation Strategies for Robust Algal Growth and Consequent Removal of Inorganic Nutrients in Pretreated Livestock Effluent. Appl Biochem Biotechnol 174, 1668–1682 (2014). https://doi.org/10.1007/s12010-014-1145-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1145-2