Abstract
In this study, electron beam irradiation (EBI) assisted by a dilute acid pretreatment process was investigated to improve the glucose yield and show high selectivity in the enzymatic hydrolysis of rice straw. In the first step, EBI of rice straw was performed at various doses ranging from 50 to 500 kGy. The electron beam-irradiated rice straw was then autoclaved with 3 % dilute acid at 120 °C for 1 h. The pretreated rice straw was finally subjected to enzymatic hydrolysis at 50 °C for 24, 48, and 72 h by 70 filter paper units (FPU)/mL cellulase and 40 cellobiose units (CbU)/mL glucosidase. Glucose was obtained with a very high selectivity of 92.7 % and a total sugar yield of 80 % from pretreated rice straw after 72 h of enzymatic hydrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
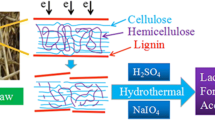
Lignocellulosic biomass has been widely investigated as a potential sustainable source of fermentable sugars that can be converted to biofuels and biochemicals [1, 2]. Lignocellulosic biomass is composed of three polymers: cellulose, hemicellulose, and lignin, which are chemically bonded to each other. Among these three components, cellulose is the dominant polymer, with relatively small proportions of hemicellulose and lignin in the lignocellulosic biomass. However, it is difficult to convert cellulose and hemicellulose to fermentable sugars because these components are tightly packed with lignin in the cell wall [3]. The structure of the cellulose-hemicellulose-lignin complex is responsible for the natural resistance of cellulose and hemicellulose to enzymatic and microbial attack, which is known as “biomass recalcitrance.” Thus, pretreatment is required to make these cellulose and hemicellulose polymers accessible to further enzymatic hydrolysis and fermentation [4].
Many researchers have developed methods to overcome the problems associated with biomass recalcitrance to convert lignocellulosic biomass to fermentable sugars such as glucose and xylose. Acid pretreatment can be a single-stage process in which the biomass is treated with H2SO4 or HCl at varying concentrations, operating times, and reaction temperatures. Acid pretreatment solubilizes hemicellulose and increases the accessibility of cellulose in the presence of lignin to enzymatic or microbial attack [5, 6]. However, acid pretreatment is limited by the presence of lignin and a tendency toward low sugar yields. To overcome these drawbacks, combined pretreatment or multi-stage pretreatment processes have been widely studied [7–9].
Irradiation pretreatment is a physical method that induces molecular chain scission of the glycosidic bonds of cellulose in the presence of lignin. The technique is also commonly used to increase the accessible surface area and pore size for enzymatic and microbial attack because irradiation disorders the crystalline region in the cellulose [10]. The advantages of irradiation pretreatment include (1) short pretreatment times; (2) high selectivity; (3) no requirement for toxic chemicals, making it environmentally friendly; and (4) ease of control [11].
Similar to wheat and corn, rice straw is an abundant and available lignocellulosic material. Rice straw can be easily hydrolyzed into fermentable sugars due to its high cellulose and hemicellulose content (32–47 % cellulose, 19–27 % hemicellulose) and low lignin content (5–24 %) [12]. Thus, rice straw has several advantages that make it a potential candidate for bioethanol production. In this study, a simple two-step pretreatment process for rice straw was developed that produced a high yield of glucose for further fermentation. The two-step pretreatment process involved electron beam irradiation (EBI) at various doses, followed by autoclaving with dilute sulfuric acid under mild conditions. In the first step, EBI induced the partial disordering of the crystalline and amorphous regions of the cellulose-hemicellulose-lignin complex. Electron beam-irradiated rice straw was separated into cellulose, hemicellulose, and lignin components by autoclaving with dilute acid in the second step. Using this two-step process, the relative content of cellulose in rice straw was determined by controlling the EBI dose in the first step. Glucose was obtained in high yield and selectivity without loss of cellulose after enzymatic hydrolysis due to the mild conditions and the controlled two-step pretreatment process.
Materials and Methods
Materials
Rice straw was harvested by the Korea Atomic Energy Research Institute (Jeongeup Province, Korea) in the fall of 2013. Rice straw was ground with an experimental grinder. The ground straw was sieved, and powders that passed through a 500-um sieve were collected. The powder was dried at 40 °C for 1 day in a vacuum oven prior to further pretreatment. The main composition of the rice straw was 39.5 % cellulose, 26.7 % hemicellulose, 19.5 % lignin, and 14.3 % ash, respectively.
Pretreatment Methods
Electron Beam Irradiation
The dried rice straw samples in an ambient condition were irradiated using an ELV-8-type electron beam accelerator (EB tech Co., Korea) with an acceleration voltage of 2.5 MeV, an electron beam current of 25 mA, and a conveyer speed of 10 m/min, which provided a dose of 25 kGy of radiation per pass. The absorbed doses used were 50, 100, 200, 300, and 500 kGy.
Autoclaving with Dilute Acid
Mixtures of electron beam-irradiated rice straw samples and 3 % dilute sulfuric acid at a ratio of 1:19 (w/w) were placed in 1-L glass bottles. The mixtures were autoclaved at 120 °C for 1 h under 1 bar pressure. Dark brown slurry was obtained after autoclaving. The slurry was washed with deionized (DI) water and filtered several times with Whatman No.1 filter paper to remove the residue. After washing and filtering, the remaining solid materials were dried at 40 °C for 1 day in a vacuum oven prior to enzymatic hydrolysis. The pretreated rice straw samples were denoted E50A (EBI 50 kGy + autoclaving), E100A, E200A, E300A, and E500A, respectively.
Enzymatic Hydrolysis
The effects of pretreatment on enzymatic hydrolysis of pretreated rice straw samples were investigated in accordance with the National Renewable Energy Laboratory (NREL) standard procedure [13]. Pretreated and untreated rice straw samples were mixed with 0.1-M sodium citrate buffer solution (pH: 4.8) at a ratio of 1:19 (w/v). The average activities of the enzymes were 70 filter paper units (FPU)/mL of Celluclast 1.5 L (cellulase, Novo Co., Denmark) and 40 cellobiose units (CBU)/mL of Novozyme-188 (β-glucosidase, Novo Co., Denmark). The enzymatic hydrolysis was performed at 50 °C in an incubator with shaking (150 rpm) for 24, 48, and 72 h. The resulting rice straw hydrolysates were obtained using three processes (EBI, autoclaving with dilute acid, and enzymatic hydrolysis).
Analytical Methods
Compositional Analysis
Changes in the composition of carbohydrates and lignin in the enzymatically hydrolyzed rice straw were determined according to Laboratory Analytical Procedures from NREL [14], Technical Association of the Pulp and Paper Industry [15]. Briefly, composition of rice straw before and after pretreatment was determined by dried weight basis. (1) Cellulose and hemicellulose composition: rice straw (0.5 g) was added into NaClO/glacial acetic acid/DI water (0.2 g/0.04 mL/30 mL) solution and stirred at 75 °C for 1 h repeatedly. The obtained cellulose was washed by DI water and then dried. This procedure is similar to the bleaching of biomass. (2) Lignin composition: rice straw was treated with 72 % (w/w) sulfuric acid at 30 °C for 1 h. The mixture was diluted by adding DI water to give an acid content of 4 % and then autoclaved at 120 °C for 1 h. The resulting hydrosate was filtered with Whatman No. 1 filter paper, and then dried.
Sugar Analysis
Substrates produced by the enzymatic hydrolysis were collected from the supernatant liquid through a 0.45-μm syringe filter and placed into a glass vial. The released sugars were analyzed using a high-performance liquid chromatography (HPLC, Shimazu Co., Japan) system equipped with a refractive index detector (410 RI detector, Waters, USA). The samples were separated on an Aminex HPX-87P column (Bio-Rad, USA) at 65 °C with DI water (B&J HPLC grade, SK chemical, Korea) as the mobile phase at a flow rate of 0.6 mL/min. The sugar yields were calculated using the following equation:
Also, glucose selectivity was defined with the following equation: glucose selectivity (%) = [glucose yield / total sugar yield] × 100.
Fourier Transform Infrared Spectroscopic Analysis
Fourier transform infrared (FTIR) spectroscopic analysis was performed to detect changes in the chemical structure of the untreated and pretreated rice straw samples. FTIR spectra were recorded at 4 cm−1 spectral resolution with 64 scans and a wavelength range of 500–4,000 cm−1 using an FTIR spectrometer (Bruker Vertex 70 spectrometer, Germany).
X-ray Diffraction Analysis
X-ray diffraction (XRD) analysis was used to determine the physical properties of the rice straw. An X’Pert Powder unit (PANalytical, Netherlands) was employed for XRD analysis. The untreated and pretreated rice straw samples were measured over the range of 2θ = 5–50° with Cu Kα (λ = 1.54 Å) radiation at 40 kV and 30 mA and a scan step size of 0.03°. The crystallinity of each rice straw was determined as the crystallinity index (CrI, %) using the method of Segal [16]. I 002 is the intensity of crystalline portion (cellulose) at 2θ = 22°, and I am is the intensity of the amorphous portion at 2θ = 18°.
Results and Discussion
Rice Straw Composition
The composition of the rice straw before and after pretreatment is shown in Table 1. The composition of the untreated rice straw was 39.5 % cellulose, 26.7 % hemicellulose, 19.5 % lignin, and 14.3 % ash. The composition of the rice straw after pretreatment was 68.1 % cellulose, 8.7 % hemicellulose, 18.1 % lignin, and 5.1 % ash. The change in the composition of the pretreated rice straw compared to the untreated rice straw demonstrates that the pretreatment process increased the accessible surface area of the rice straw for enzymatic hydrolysis due to the removal of or a decrease in other components. Autoclaving with dilute acid as the first step resulted in a notable decrease in the relative content of hemicellulose from 26.7 to 10.8 %. This result indicates that the hemicellulose fraction in rice straw is easily separated from cellulose by autoclaving with dilute acid. The resulting relative content of hemicellulose in the pretreated E500A rice straw (8.7 %) was significantly lower than that in the untreated sample (26.7 %). In addition, the relative content of the cellulose-hemicellulose-lignin complex increased due to the decrease in ash content. Cellulose must not be lost during pretreatment because it can be directly converted to fermentable sugars. After two-step pretreatment, the relative content of cellulose was increased in pretreated E500A (68.1 %) compared with untreated rice straw (39.5 %). Moreover, the relative content of cellulose was proportional to the EBI dose under the same autoclaving conditions. The relative lignin content was maintained after combined pretreatment. However, the relative content of lignin (19.5 %) can be divided into acid-soluble lignin (2.3 %) and acid-insoluble lignin (17.2 %). Acid pretreatment generally has a greater effect on the removal of hemicellulose than cellulose and lignin. Acid-insoluble lignin mostly remained after pretreatment, and acid-soluble lignin was dissolved under acidic conditions after EBI [17]. These results are similar to the surface area of the rice straw that was increased by EBI, allowing the dilute acid solution to easily infiltrate the lignocellulose [18]. Finally, the synergic effects of EBI and autoclaving with dilute acid increased the accessibility of the rice straw to enzymes and reduced the biomass recalcitrance to enhance the separation of hemicellulose.
Sugar Yields
The sugar yields resulting from the two rice straw pretreatment processes were determined. As shown in Table 2, reducing sugars such as glucose, xylose, arabinose, mannose, and galactose were released during enzymatic hydrolysis. The illustration in Fig. 1 shows the sugar yields resulting from untreated rice straw and from rice straw pretreated by autoclaving in dilute acid, EBI, of the two-step pretreatment after 72 h of enzymatic hydrolysis. A greater yield of released sugars was obtained from the electron beam-irradiated rice straw than the rice straw autoclaved with dilute acid. However, a sugar yield of 80 % was obtained from the rice straw subjected to the two-step pretreatment process and the same enzymatic hydrolysis time. This enhanced hydrolysis demonstrates the synergetic effects of the two-step pretreatment on the disordering of the crystalline and amorphous cellulose-hemicellulose-lignin complex, depolymerization of lignin, and degradation of hemicellulose. Interestingly, EBI was strongly selective for the hemicellulose of rice straw and could affect the degree of polymerization, which could enhance the subsequent degradation of hemicellulose by autoclaving with acid [19, 20]. The total amount of released sugars after enzymatic hydrolysis is shown in Fig. 2. The total sugar yields gradually increased with increasing electron beam dose ranges of 50–300 kGy when coupled with autoclaving with dilute acid under identical conditions. The resulting sugar yields were 75 % for glucose, 5.2 % for mannose, and 0.3 % for arabinose in the case of the E500A rice straw. However, xylose was not released with more hemicellulose removal at electron beam dosages higher than 200 kGy. This result confirmed that EBI pretreatment selectively increased the removal of hemicellulose prior to the subsequent autoclaving with dilute acid pretreatment. The selectivity of the glucose yields compared to the total sugar yields is described in Fig. 3. Maximums of 41.3 % for the glucose yield and 79 % for glucose selectivity were obtained from rice straw autoclaved with dilute acid. By comparison, the maximum yield of glucose was 75 % with a selectivity of 92.7 % from E500A rice straw. The glucose selectivity from untreated rice straw after 24, 48, and 72 h of enzymatic hydrolysis was only 68.7, 67.5, and 65.9 %, respectively. The glucose selectivity after 24, 48, and 72 h of enzymatic hydrolysis of rice straw pretreated only by autoclaving with dilute acid decreased slightly, in the range of 80.6–79.5 %. For the two-step pretreated rice straw, the glucose selectivity was proportional to the EBI dose, which ranged from 50 to 200 kGy. However, EBI pretreatment at lower doses of 10–40 kGy was insufficient to induce significant changes in the glucose yield, and the yields plateaued at 92 % in the range of 200–500 kGy. These results suggest that the EBI pretreatment is an effective and easily controllable method to obtain high glucose yields and selectivity.
Fourier Transform Infrared Spectroscopic Analysis
We investigated the changes in the chemical structure and composition of the two-step pretreated rice straw using FTIR spectrum analysis as shown in Table 3. The broad peak in the 3,338 cm−1 region was due to O–H stretching, and the two sharp peaks at 1,101 and 898 cm−1 were due to C–O–C stretching in the β-1,4-glycosidic linkages of cellulose [21]. The peak in the 3,338 cm−1 region for the untreated rice straw was similar to that in the spectrum of pretreated rice straw. In addition, the peaks associated with O–H stretching shifted to a higher wave number (from 3,338 to 3,342 cm−1) due to the increase in the content of free hydroxyl groups. These results demonstrated that most of the cellulose in the rice straw remained during the two-step pretreatment. The peaks in the 2,918 cm−1 region were associated with C–H stretching within cellulose and were stronger after the two-step pretreatment [22]. The peaks between 1,200 and 900 cm−1 were typically associated with the structures of cellulose and hemicellulose (C–O–H stretching of primary and secondary alcohols at 1,033 cm−1, C–O–C stretching at 1,153 cm−1). Moreover, the peak at 898 cm−1 was due to the β-1,4-glycosidic bond (C–O–C), which was not easily cleaved during the two-step pretreatment [23]. After the two-step pretreatment, the peaks in this region were stronger than those in the spectrum of untreated rice straw. These results indicated that the cellulose content in pretreated rice straw increased because the hemicellulose in the untreated rice straw was removed by the two-step pretreatment.
X-ray Diffraction Analysis
Table 4 shows the X-ray diffractograms of untreated and rice straw subjected to the two-step pretreatment. Spectral changes are apparent in the range of 5° < 2θ < 30°. The intensities of the amorphous and crystalline regions were measured at 2θ = 18° and 2θ = 22°, respectively. The intensity of the crystalline region peaks at 22° was increased, while the intensity of the amorphous region peaks at 18° was decreased. The overall crystallinity index of the pretreated rice straw was calculated to have increased to 54.5 from 41.1 % for untreated rice straw. This increase in the crystallinity index occurred because the high-energy EBI strongly attacked the cellulose-hemicellulose-lignin complex, while autoclaving with dilute acid significantly enhanced the separation of the hemicellulose from the electron beam-irradiated rice straw. In the present study, the crystallinity index of the rice straw autoclaved with dilute acid was 50.1 %. However, this value increased slightly to 54.5 % after the two-step pretreatment process due to the exposure of the crystalline and amorphous regions of the rice straw in response to EBI dose. These results indicate that the removal of hemicellulose may be responsible for the increase in the crystallinity index during the two-step pretreatment. The amorphous region was more depolymerized than the crystalline region due to the effects of EBI. This increase in the degree of crystallinity indicates that the effect of EBI was greater in the amorphous region than in the crystalline zone [8, 24]. Cellulose is protectively surrounded by hemicellulose and lignin. Hence, EBI might have primarily disordered the structure of hemicellulose and lignin, resulting in the exposure of cellulose. The sugar yields were proportional to the crystallinity index. If the pretreatment was highly effective (without loss of cellulose), hemicellulose were removed in the amorphous region, thereby increasing the crystallinity of the lignocellulose. In this study, the two-step pretreated rice straw gave the highest crystallinity index, indicating that the combined pretreatment process is highly effective.
Conclusion
Glucose yields were easily controlled by adjusting the EBI dose. With the synergetic two-step pretreatment, the yield and selectivity of glucose were 75 and 92.7 %, respectively. This method could be used to improve bioethanol production from lignocellulosic waste materials. The addition of EBI as the first step compared to dilute acid pretreatment only increased the removal of hemicellulose from the rice straw, thereby increasing the accessibility of the cellulose for enzymatic hydrolysis. The two-step pretreatment thus represents a strategy for overcoming the biomass recalcitrance of lignocellulosic biomass.
References
Chiaramonti, D., Prussi, M., Ferrero, S., Oriani, L., Ottonello, P., Torre, P., & Cherchi, F. (2012). Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass and Bioenergy, 46, 25–35.
Mood, S. H., Golfeshan, A. H., Tabatabaei, M., Jouzani, G. S., Najafi, G. H., Gholami, M., & Ardjmand, M. (2013). Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renewable and Sustainable Energy Reviews, 27, 77–93.
Binod, P., Sindhu, R., Singhania, R. R., Vikram, S., Devi, L., Nagalakshmi, S., Kurien, N., Sukumaran, R. K., & Pandey, A. (2010). Bioethanol production from rice straw: an overview. Bioresource Technology, 101, 4767–4774.
Himmel, M. E., Ding, S. Y., Johnson, D. K., Adney, W. S., Nimlos, M. R., Brady, J. W., & Foust, T. D. (2007). Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science, 315, 804–807.
Hendriks, A. T. W. M., & Zeeman, G. (2009). Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresource Technology, 100, 10–18.
Guo, G. L., Chen, W. H., & Hwang, W. S. (2010). Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresource Technology, 101, 4907–4913.
Fuying, M. N., Yang, C., Xu, H., Yu, J., & Wu, X. Z. (2010). Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Bioresource Technology, 101, 9600–9604.
Karthika, K., Arun, A. B., Melo, J. S., Mittal, K. C., Kumar, M., & Rekha, P. D. (2013). Hydrolysis of acid and alkali presoaked lignocellulosic biomass exposed to electron beam irradiation. Bioresource Technology, 129, 646–649.
Chen, W. H., Tu, Y. J., & Sheen, H. K. (2011). Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Applied Energy, 88, 2726–2734.
Driscoll, M., Stipanovic, A., Winter, W., Cheng, K., Manning, M., Spiese, J., Galloway, R. A., & Cleland, M. R. (2009). Electron beam irradiation of cellulose. Radiation Physics and Chemistry, 78, 539–542.
Cheng, J., Su, H., Zhou, J., Song, W., & Cen, K. (2011). Microwave-assisted alkali pretreatment of rice straw to promote enzymatic hydrolysis and hydrogen production in dark- and photo-fermentation. International Journal of Hydrogen Energy, 36, 2093–2101.
Karimi, K., Kheradmandinia, S., & Taherzadeh, M. J. (2006). Conversion of rice straw to sugars by dilute acid hydrolysis. Biomass Bioenerg, 30, 247–253.
Selig, M., Weiss, N., & Ji, Y. (2008). Enzymatic saccharification of lignocellulosic biomass. NREL Laboratory Analytical Procedure. Technical Report NREL/TP-510-42629, NREL, Colorado, USA. http://www.nrel.gov/docs/gen/fy08/42629.pdf.
Sluiter, A., Hames, B., Ruiz, R., Scarlate, C., Sluiter, J., Templeton, D., & Crocker, D. (2011). Determination of structural carbohydrates and lignin in biomass. Technical Report NREL/TP-510-42618, NREL, Colorado, USA. http://www.nrel.gov/biomass/pdfs/42618.pdf
TAPPI T 222 OM-02. (2002). TAPPI standards and suggested methods, Technical Association of the Pulp and Paper Industry, USA.
Segal, L., Creely, J. J., Martin, A. E., & Conrad, C. M. (1959). An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research Journal, 29, 788–793.
Cardona, C. A., Quintero, J. A., & Paz, I. C. (2009). Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresource Technology, 101, 4754–4766.
Xin, L. Z., & Kumakura, M. (1993). Effect of radiation pretreatment on enzymatic hydrolysis of rice straw with low concentrations of alkali solution. Bioresource Technology, 43, 13–17.
Shin, S. J., & Sung, Y. J. (2008). Improving enzymatic hydrolysis of industrial hemp (Cannabis sativa L.) by electron beam irradiation. Radiation Physics Chemical, 77, 1034–1038.
Yang, C., Shen, Z., Yu, G., & Wang, J. (2008). Effect and affect of γ radiation pretreatment on enzymatic hydrolysis of wheat straw. Bioresource Technology, 99, 6240–6245.
Cao, Y., & Tan, H. (2004). Structural characterization of cellulose with enzymatic treatment. Journal of Molecular Structure, 705, 189–193.
Kumar, R., & Wyman, C. E. (2009). Cellulase adsorption and relationship to feature of corn stover solid produced by leading pretreatment. Biotechnology and Bioengineering, 103, 252–267.
Xiao, B., Sun, X. F., & Sun, R. C. (2001). Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polymer Degradation and Stability, 74, 307–319.
Binod, P., Satyanagalakshmi, K., Sindhu, R., Janu, K. U., Sukumaran, R. K., & Pandey, A. (2012). Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renewable Energy, 37, 109–116.
Acknowledgment
This study was supported by the Nuclear R&D program of the Korea Science and Engineering Foundation, which is funded by the Ministry of Science, ICT and Future Planning of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
1. A facile two-step method was developed for producing fermentable sugars.
2. The synergic effect of dilute acid pretreatment on the EBI of rice straw was assessed.
3. A 75 % yield and 92.7 % selectivity were obtained for glucose.
Rights and permissions
About this article
Cite this article
Lee, BM., Lee, JY., Kang, PH. et al. Improved Pretreatment Process Using an Electron Beam for Optimization of Glucose Yield with High Selectivity. Appl Biochem Biotechnol 174, 1548–1557 (2014). https://doi.org/10.1007/s12010-014-1138-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1138-1