Abstract
Myoglobin is a primary iron, and oxygen-binding protein of muscle tissues and levels can be an important diagnostic biomarker for acute myocardial infarction, myocardial necrosis, or other cardiac diseases. The establishment of myoglobin recognition systems is important because of its protein’s structural and functional values in physiology, biochemistry, and diagnostic value in some damaged muscle tissue and cardiac diseases. For this purpose, we used molecular imprinting technique for myoglobin recognition from aqueous solutions and human plasma. In the first step, myoglobin-imprinted poly(hydroxyethyl methacrylate) (PHEMA) cryogels (MGb-MIP) were prepared, and optimum myoglobin adsorption conditions were determined. Selectivity experiments have been done with the competitive proteins, and myoglobin adsorption from IgG and albumin-free human plasma was studied. The purity of the desorbed samples was determined with SDS-PAGE. The desorption efficiency and reusability of the MGb-MIP cryogels were tested, and it was shown that without any significant loss in the adsorption capacity, MGb-MIP cryogels can be used a number of times for myoglobin recognition and separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myoglobin is a 17.6-kDa monomeric hemeprotein found in the heart and skeletal muscles. Distinguished as the first protein for which a three dimensional structure was determined, myoglobin has been one of the most studied proteins in biology in relation to its structural, functional, and diagnostic value in biochemistry, physiology, and medicine. It plays important roles in partial oxygen pressure (PO2) buffering, O2 storage, and facilitated O2 diffusion. Because of its relatively small molecular weight, myoglobin is often used in electrophoretic techniques as a molecular weight marker [1]. When there is muscle damage, myoglobin is released into the bloodstream. The released myoglobin is filtered by the renal tubular epithelium of the kidneys into the urine. In large amounts, myoglobin can damage the kidneys because of its toxicity. Myoglobin is one of the important biological markers for some diseases, such as acute myocardial infarction (AMI), myocardial necrosis, or other cardiac diseases [2–4]. It is not a cardiac-specific protein, but it is one of the very early known markers released into the blood when there is a heart muscle damage and an increase after AMI [5, 6]. Myoglobin is present in the muscles in large concentrations, and the human heart contains about 0.1 % myoglobin by weight. This high concentration provides a good source for purification. Separation, purification, detection, and quantification of myoglobin have been employed using various conventional methods, including enzyme-linked immunosorbent assay (ELISA) and chromatographic [7–9] or spectrophotometric methods [10–13].
Molecular imprinting is the technology of creating artificial counterparts having affinity constants as high as the natural ones of the interested molecules [14]. In this approach, interested template molecules are complexed with functional monomers; then, this complex is converted into solid matrix via a cross-linking agent [15, 16]. In this way, an imprint of the target molecule is introduced into the polymer, which is capable of recognizing the template with high affinity and specificity [17]. The resulting molecularly imprinted polymers (MIPs) offer high stability in extreme conditions, higher mechanical strength, lower cost, reusability, and good reproducibility [18]. MIPs are extensively used in sensing applications, analytical separations, drug delivery systems, solid-phase extractions, and library screening tools [19–27]. The imprinting of small molecules is well developed, and tailor-made molecular imprints are now commercially available. While numerous selective MIPs against small molecules have been prepared, relatively little progress has been made in the field of macromolecular imprinting [28]. Due to their importance, proteins are the most extensively studied templates in macromolecular imprinting. The inherent properties of proteins such as size, complexity, conformational instability, and solubility have been the main hindrances for protein imprinting [29]. Because of these challenges, proteins are used as template molecules in less than 2 % of the published works in the area of molecular imprinting [30–32].
In the study presented here, we used myoglobin as the template protein for the preparation of MIPs. In this purpose, we used supermacroporous poly(hydroxyethyl methacrylate) (PHEMA) cryogels as the support material. Cryogels are prepared under freezing point of the diluents, and they have large, interconnected flow channels that reduce the flow resistance and diffusion limitations of the columns. Two major advantages of cryogels make these polymers promising candidates for imprinting of biological macromolecules like proteins (lysozyme, cytochrome c, bilirubin, hemoglobin, albumin, and interferon α-2b) [20, 30–36]. The water-soluble monomers can be used for the cryogels’ preparation, and the polymerization, namely cryogelation, occurred under freezing point of water, especially between −12 and −20 °C. This restricts the molecular motions in biomolecules, which allows imprinting them more easily and specifically, meanwhile preventing them to denature [37]. In our study, as the first step, myoglobin-imprinted PHEMA cryogels (MGb-MIP) were synthesized. Second, to determine the optimum adsorption conditions, system parameters such as pH of the adsorption medium, adsorption rate, myoglobin concentration, temperature, and ionic strength were studied. After this step, we did the selectivity experiments with the competitive proteins, hemoglobin (Hb) and lysozyme (Lyz), to show the specificity of the MGb-MIP cryogel against myoglobin. The reusability of the MGb-MIP cryogel was tested, and as the last step, myoglobin adsorption from human plasma was studied. The purity of the desorbed myoglobin sample was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Experimental
Materials
2-Hydroxyethyl methacrylate (HEMA), N,N′-methylene bisacrylamide (MBAAm), ammonium persulfate (APS), and l-tryptophan methyl ester were obtained from Sigma-Aldrich (St. Louis, USA). N,N,N′,N′-tetramethylene ethylenediamine (TEMED) was purchased from Fluka A.G. (Buchs, Switzerland). Myoglobin (MGb) from the human heart (Sigma-Aldrich, cat. no. M6036), Lyz from chicken egg white (Sigma-Aldrich, cat. no. L6876), and Hb from bovine blood (Sigma-Aldrich, cat. no. H2500) were obtained from Sigma-Aldrich. All other chemicals were reagent grade and purchased from Merck A.G. (Darmstadt, Germany). All the water used in the experiments was purified using a Barnstead (Dubuque, IA, USA) ROpure LP® reverse osmosis unit with a high flow cellulose acetate membrane (Barnstead D2731) followed by a Barnstead D3804 NANOpure® organic/colloid removal and ion exchange packed bed system.
Preparation of Myoglobin-Imprinted Poly(hydroxyethyl methacrylate) (PHEMA) Cryogels (MGb-MIPs)
N-methacryloyl-(l)-tryptophan (MATrp) was selected for myoglobin imprinting because of hydrophobic interaction between aromatic ring of tryptophan amino acid and hydrophobic amino acids in myoglobin structure. Details of the preparation and characterization of the MATrp functional monomer were reported in our previous article [38]. For the preparation of MGb-MIP, different amounts of template molecule (MGb, 10–50 mg) and functional monomer (MATrp, 90 μL) were dissolved in 2 mL and 0.1 M Na2CO3-NaOH solution, as the first step. The mixtures were rotated for 2 h for precomplexation of MGb and MATrp monomers. After this step, HEMA (1.3 mL), MBAAm (0.283 g), and the prepared MATrp-MGb complex (2 mL) were dissolved in deionized water. This mixture was degassed under vacuum for about 5 min to eliminate soluble oxygen. The MGb-MIP cryogels were prepared by free radical polymerization initiated by TEMED (25 μL) and APS (20 mg). After the addition of initiators, the reaction mixture was cooled down in an ice bath for 2–3 min and poured into a plastic syringe (5 mL, i.d. 1.3 cm) with closed outlet at the bottom. The polymerization solution in the plastic syringe was frozen at −12 °C for 24 h and then thawed at room temperature. After washing with 200 mL of water, the prepared MGb-MIP cryogels were stored in a buffer containing 0.02 % sodium azide (NaNO3) at 4 °C until use. MGb-MIP cryogels were prepared with different amounts of myoglobin (MGb-MIP1, MGb-MIP2, MGb-MIP3, and MGb-MIP4), and also, nonimprinted (NIP) cryogel was synthesized in the absence of myoglobin using the same polymerization procedure given above. All of the cryogel columns (MGb-MIP and NIP) were made in plastic syringes.
After the preparation step, MGb-MIP cryogels were washed with water and 0.1 M Na2CO3-NaOH solution to remove the template protein (myoglobin). The amount of myoglobin extracted from the cryogels was determined at 410 nm.
Characterization of Cryogels
The synthesized cryogels were characterized by swelling test and scanning electron microscopy (SEM) observations. The swelling degree (S) of the cryogel was determined as follows: Cryogel was washed with ethyl alcohol and distilled water until washing solution was clear. Then, the cryogel was squeezed to remove unbound water, and then, it was transferred to a preweighed vial and weighed out (m wet gel). The wet cryogel was dried in an oven at 60 °C, and it was weighed out again to determine the mass of the dried cryogel (m dry gel). The swelling degree was calculated as follows:
The total volume of macropores in the wet cryogel was roughly estimated as follows: The cryogel was weighed out after it was incubated in 40 mL of water (m swollen gel), and then, the weight of the cryogel was determined after squeezing (m squeezed gel). The porosity was calculated as follows:
The morphology of the cryogel was investigated by SEM. The cryogel sample initially dried was mounted on a SEM sample holder and sputtered for gold film coating for 2 min. The sample was then placed in a SEM (JEOL Ltd., JSM 5600, Tokyo, Japan). The surface of the cryogel was scanned at desired magnification to investigate the topography of cryogel.
Adsorption Studies
In order to show the myoglobin adsorption performance of MGb-MIP cryogel, the adsorption studies were performed from aqueous solutions of myoglobin as continuous setup by feeding the solutions with a peristaltic pump (Watson-Marlow, 430U/R1, Wilmington, MA, USA). The MGb-MIP cryogel column was washed with 50 mL of water and then equilibrated with 20-mM acetate buffer (pH 4.0). Then, the freshly prepared myoglobin solution was passed through the cryogel column. The adsorption was monitored by following the decrease in UV absorbance between the initial and final myoglobin solutions at 410 nm. Effects of pH of the medium, flow rate, initial myoglobin concentration, temperature of the medium, and ionic strength on the adsorption capacity were studied. To determine the effect of pH on the adsorption, pH of the solution was changed between 3.0 and 8.0. The flow rate was changed in the range of 0.5 and 3.0 mL/min. To observe the effects of initial myoglobin concentration on adsorption, myoglobin concentration was changed between 0.1 and 3.0 mg/mL. To observe the effects of temperature on the adsorption, adsorption studies were carried out between 4 and 37 °C. To obtain the effect of ionic strength, the adsorption experiments were first carried out in salt-free solutions, and then, they were repeated in solutions containing NaCl, (NH4)2SO4, and Na2SO4 salts. The concentration of salts was changed between 0.01 and 1.0 M.
Selectivity Experiments
In order to show the myoglobin selectivity and imprinting efficiency of the MGb-MIP cryogel, we also performed the adsorption studies by using potential competitive proteins. In this purpose, the adsorptions of Hb and Lyz onto the cryogel were evaluated from aqueous solutions under optimal conditions determined previously. The protein solutions were circulated through the cryogel column in singular manner. In addition, the adsorptions of MGb, Hb, and Lyz onto NIP cryogel were performed for control purpose, and the data obtained were used to evaluate the selectivity parameters.
Myoglobin Adsorption from Human Plasma
Human blood was collected in EDTA-containing vacutainers, and red blood cells were separated from plasma by centrifugation at 4,000 g for 30 min at room temperature. Then, plasma sample was filtered (3-μm Sartorius filter) and frozen at −20 °C. Before application, the plasma was diluted with 25-mM phosphate buffer containing 1.0 M NaCl (pH 7.4). Plasma dilution ratio was 1:2. Immunoglobulin G (IgG) and albumin-free human plasma (50 mL) were pumped through the cryogel column at a flow rate of 1.0 mL/min for 1 h. The purity of myoglobin was assayed by SDS-PAGE using 10 % separating gel (18 × 16 cm) and 0.25 % (w/v) Coomassie brillant R250 in acetic acid-methanol-water (1:5:5, v/v/v) and destained in ethanol-acetic acid-water (1:4:6, v/v/v). Electrophoresis was run for 3 h with a voltage of 120 V.
Result and Discussion
Characterization Studies
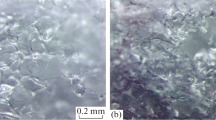
The SEM images of the MGb-MIP and NIP cryogels are given in Fig. 1. As seen in the figure, the MGb-MIP cryogel has interconnected macroporous structure that allows easy transport of the proteins through the column and convective mass transport of the analyte molecules. The macropores in the size range of 50–150 μm cause low-pressure drop (ΔP) which allows studying at higher flow rates. These macropores also permit the viscous solutions like plasma to pass through freely without clogging.
Cryogel codes, imprinted amount of myoglobin, percentage of removed template molecule from MGb-MIP cryogels, adsorbed amount of myoglobin onto the MGb-MIP, and NIP cryogels are reported in Table 1. In all the experimental studies, the MIP2 coded MGb-MIP cryogel was used because of the highest amount of template molecule removal.
The swelling ratios of the imprinted and NIP cryogels were determined as 65 and 75 % for NIP and MGb-MIP cryogels, respectively. According to the data obtained, the water contents of the cryogels in swollen state were calculated as 7.87 g H2O/g cryogel for NIP and 8.92 g H2O/g cryogel for MGb-MIP cryogel.
Effect of Template Removal Conditions on Template Protein Structure
Fluorescence spectrophotometry was employed to evaluate the effects of template removal conditions on myoglobin structure (Fig. 2). The fluorescence spectra of myoglobin samples obtained from the template removal step were recorded for MGb-MIP1 and MGb-MIP4 coded cryogels. Different imprinted amounts of myoglobin were demonstrated with code numbers in Table 1. The fluorescence spectra of native and heat-denatured myoglobin samples were taken too. A clear difference between the fluorescence spectra of native and heat-denatured myoglobin samples was observed. An appreciable shift was recorded in the maximum wavelength of denatured myoglobin according to the native one. On the other hand, the fluorescence spectra of the samples withdrawn from the template removal step were very close to the native myoglobin sample, and no significant shift of maximum wavelength was detected in the spectra of these samples relative to that of native myoglobin. This is an important result because it can be concluded that MGb-MIP can be applied for myoglobin recognition and purification without causing significant conformational change and denaturation problem to the template protein during the template molecule removal step.
Adsorption of Myoglobin from Aqueous Solutions
Myoglobin adsorption capacity of MGb-MIP cryogel was studied in a pH range of 3.0 and 8.0. As shown in Fig. 3a, maximum myoglobin adsorption was observed as 22.1-mg/g cryogel at pH 4.0. Below and over the maximum adsorption pH value, myoglobin adsorption capacity decreased significantly.
Figure 3b shows the myoglobin adsorption amount of MGb-MIP cryogel at different flow rates. As seen from the results, myoglobin adsorption capacity of MGb-MIP cryogel decreased as the flow rate through the column increased. With the increase at the flow rate from 0.5 to 3.0 mL/min, the myoglobin adsorption capacity decreased from 38.53 to 12.13 mg/g for MGb-MIP cryogel. The underlying reason is that, at higher flow rates, the contact time between the myoglobin molecules and the MGb-MIP cryogel decreases. At the lower flow rates, the myoglobin molecules have more time to diffuse into the large pores of the cryogel and to bind to the molecular cavities. Because of this, maximum adsorption capacity is observed in the lowest flow rate (0.5 mL/min).
The equilibrium concentration of myoglobin dependence of the adsorbed amount of the myoglobin onto the MGb-MIP cryogel is shown in Fig. 3c. With the increase in the myoglobin concentration, the adsorption value increased too. But, at 2.0 mg/mL of myoglobin concentration, a saturation value was reached. This is due to the saturation of the binding cavities on the MGb-MIP cryogel. As seen in Fig. 3c, maximum adsorption capacity is found as 36.08-mg/g polymer for MGb-MIP cryogel and 14.59-mg/g polymer for NIP cryogel. As expected, the nonspecific interactions between the NIP cryogel and the target molecule, myoglobin, should be minimum in order to consider the protein-adsorbent interaction as specific. As seen from the results, the MGb-MIP cryogel is approximately 2.5 times higher than the NIP cryogel on myoglobin adsorption from aqueous solutions.
Different salts including NaCl, (NH4)2SO4, and Na2SO4 salts were used to investigate the effect of ionic strength and salt type on myoglobin adsorption. The ionic strengths of all salt types were studied between the values of 0.01 and 0.5 M. As shown in Fig. 4a, the amount of adsorbed myoglobin increased with the increasing ionic strength for the three of the salt types. Salts such as sodium, potassium, or ammonium sulfates are the most effective salts to promote the hydrophobic interactions. This explains the higher protein binding at higher salt concentrations and the effective elution by decreasing the salt concentration for the hydrophobic interactions [39]. As seen from the figure, maximum myoglobin adsorption was observed in the 0.5 M (NH4)2SO4 as 31.81-mg/g polymer, which explains the stronger effect of this salt on myoglobin binding to the MGb-MIP cryogel.
The influence of temperature on myoglobin adsorption was studied at various temperatures (4–37 °C) in order to determine the most effective temperature. As seen in Fig. 4b, the adsorption capacity significantly increased with the increasing temperature. Due to this result, we can say that the most effective interaction force controlling the adsorption process is hydrophobic interaction. In hydrophobic interactions, increasing the temperature enhances protein retention, and decreasing the temperature generally promotes the protein elution. Van der Waals forces, which are important interactions in hydrophobicity, also increase by the increasing temperature [40]. The interaction between myoglobin molecules and MGb-MIP cryogel is mainly hydrophobic, and because of this, the adsorption is promoted by the increasing temperature.
Desorption and Repeated Use
The reusability is an important parameter to control the process cost of the adsorbents. It depends on the interaction of the analyte molecule with the adsorbent and the desorption efficiency of the adsorbed molecule. In order to show the desorption efficiency and reusability of the MGb-MIP cryogel, we repeated the adsorption-desorption cycle for ten times using 0.1 M Na2CO3-NaOH solution as desorption agent. At the end of the tenth cycle, the adsorption capacity was determined as 34.20 mg/g. As seen in Fig. 5, the cryogel maintained its adsorption capacity at a constant value of 94.8 %, and it remained very stable during the adsorption studies.
Selectivity Experiments
One of the most crucial experiments for determining the selectivity of MGb-MIP is the competitive adsorption experiments. In this respect, the selectivity parameters describe the imprinting efficiency of the adsorbents and the interactions of analyte molecules with the imprinted cavities. In these experiments, the MGb-MIP cryogel was tested for the binding of Hb (isoelectric point (pI) 6.5), which has a pI similar to that of myoglobin (pI 7.2), and for the binding of Lyz (molecular weight (MW) 14.31 kDa), which has a similar molecular weight to that of myoglobin (MW 17.7 kDa). The adsorption amounts of these proteins onto the MGb-MIP and NIP cryogels were determined. Initial protein concentration, flow rate, and temperature for all species were kept constant as 0.8 mg/mL, 1 mL/min, and 25 °C, respectively, while pH of the solution was adjusted to 4.0. The selectivity parameters were calculated as follows:
where k D is the distribution constant (mL/g), k is the selectivity constant, k′ is the relative selectivity constant, C i is the initial protein concentration (mg/mL), C f is the protein concentration at equilibrium (mg/mL), V is the volume of the solution (mL), and m is the weight of the cryogel (g), respectively.
The effect of imprinting is shown in Fig. 6, and the calculated parameters are summarized in Table 2. A comparison of the k D values for the MGb-MIP samples with the control (i.e., NIP) samples showed an increase in k D for MGb while k D decreased for Hb and Lyz. The relative selectivity coefficient is an indicator to express protein adsorption affinity of molecular recognition sites to the imprinted myoglobin molecules. The MGb-MIP cryogel showed a high specificity degree for the reference protein with respect to the competitive proteins (k D for MGb is 101.42 vs k D = 13.18 for Hb and k D = 7.68 for Lyz). The relative selectivity coefficients of MGb-MIP cryogel for Mgb/Hb and Mgb/Lyz were 2.7 and 8.1 times greater than those of the NIP cryogel, respectively (Table 2). Results showed that the cavities formed on the MGb-MIP cryogel are complementary in size and shape to the template molecule, myoglobin, resembling the “lock and key” paradigm of enzymes. The maximum adsorption capacities of the MGb-MIP cryogel were 22.88 mg/g for myoglobin, 9.85 mg/g for Hb, and 5.69 mg/g for Lyz in the order of Mgb/Hb/Lyz.
Myoglobin Adsorption from Human Plasma
IgG and albumin-free human plasma were used as natural sources for myoglobin adsorption. The purity of the myoglobin eluted from MGb-MIP cryogel was determined by SDS-PAGE as described before. As seen in Fig. 7, MGb in human plasma (lane 2) disappeared in lane 1 after adsorption onto the MGb-MIP cryogel. Furthermore, the presence of only one band at lane 3 indicates the purity of myoglobin after desorption of MGb-MIP cryogel. The purity of the desorbed myoglobin was about 91 %. This result shows that the MGb-MIP cryogel can be used as an efficient method to purify myoglobin from human plasma with high adsorption capacity, high purity, and high selectivity.
SDS-PAGE of plasma fractions. The fractions were assayed by SDS-PAGE using 10 % separating gel (18 × 16 cm). Separating gels were stained with 0.25 % (w/v) Coomassie brillant R250 in acetic acid-methanol-water (1:5:5, v/v/v) and destained in ethanol-acetic acid-water (1:4:6, v/v/v). Lane 1 1:2 diluted plasma (IgG and albumin free) after adsorption onto the MGb-MIP cryogel; Lane 2 1:2 diluted plasma (IgG and albumin-free); Lane 3 eluted sample; Lane 4 protein biomarker (Sigma-Aldrich). Equal amounts of samples were applied to each line. (Color figure available online)
Conclusion
Polymer films, sensors, silica beads, and composite polymers were used as materials for myoglobin imprinting in literature [7, 27, 41–43]. Protein recognition in molecular imprinting technology was combined with new generation cryogels with main advantages such as large pores, short diffusion path, low-pressure drop, and very short residence time for both adsorption and elution. In this study, we have benefited from these advantages of cryogels. Myoglobin adsorption capacity of MIP cryogel is found as approximately 2.5 times greater than that of NIP cryogel, and maximum adsorption capacity was observed as 35.96-mg/g polymer. The coefficients for the MIP cryogel were evaluated as 2.72 (MGb/Hb) and 8.16 (MGb/Lyz) times greater than those for NIP cryogel, respectively. Repeated adsorption-desorption studies did not show a decrease in adsorbing capacity. The results obtained are encouraging and indicate that molecular imprinting technique can be considered as a potential technique for myoglobin recognition from aqueous solutions and human plasma with high adsorption capacity and selectivity without any significant decrease in binding capacity.
References
Shihabi, Z. K. (1999). In S. M. Palfrey (Ed.), Clinical applications of capillary electrophoresis (pp. 53–57). New York: Humana.
Wu, A. H. (2005). Scandinavian Journal of Clinical and Laboratory Investigation Supplement, 240, 112–121.
Rozenman, Y., & Gotsman, M. S. (1994). Annual Review of Medicine, 45, 31–44.
Murphy, M. J., & Berding, C. B. (1999). Critical Care Nurse, 19, 58–66.
Adams, J. E., & Miracle, V. A. (1998). American Journal of Critical Care, 6, 418–423.
Montague, C., & Kircher, T. (1995). American Journal of Clinical Pathology, 4, 472–476.
Lin, H., Rick, J., & Chou, T. (2007). Biosensors and Bioelectronics, 22, 3293–3301.
Powell, S. C., Friedlander, E. R., & Shihabi, Z. K. (1984). Journal of Chromatography, 28, 87–92.
Han, D., McMillin, K. W., & Godber, J. S. (1994). Journal of Food Science, 59, 1279–1282.
Kelner, M. J., & Alexander, N. M. (1985). Clinical Chemistry, 31, 112–114.
Schuder, S., Wittenberg, J. B., Haseltine, B., & Wittenberg, B. A. (1979). Analytical Biochemistry, 92, 473–481.
Modi, S., Shedbalkar, V. P., & Behere, D. V. (1989). Indian Journal of Biochemistry and Biophysics, 26, 84–86.
Boesken, W. H., Boesken, S., & Mamier, A. (1977). Experimental Medicine, 171, 71–78.
Shiomi, T., Matsui, M., Mizukami, F., & Sakaguchi, K. (2005). Biomaterials, 27, 5564–5571.
Rao, T. P., Daniel, S., & Gladis, J. M. (2004). Trends in Analytical Chemistry, 23, 28–35.
Mosbach, K., & Ramström, O. (1996). Nature Biotechnology, 14, 163–170.
Haupt, K., & Mosbach, K. (1998). Tibtech, 16, 468–475.
Kryscio, D. R., & Peppas, A. N. (2012). Analytica Chimica Acta, 718, 109–115.
Moreira, F. T. C., Kamel, A. H., Guerreiro, J. R. L., & Sales, F. G. M. (2010). Biosensors and Bioelectronics, 26, 566–574.
Bereli, N., Andaç, M., Baydemir, G., Say, R., Galaev, I. Y., & Denizli, A. (2008). Journal of Chromatography A, 1190, 18–26.
Ertürk, G., Uzun, L., Tümer, M. A., Say, R., & Denizli, A. (2011). Biosensors and Bioelectronics, 28, 97–104.
Şener, G., Uzun, L., Say, R., & Denizli, A. (2011). Sensors and Actuators B, 160, 791–799.
Uzun, L., Say, R., Ünal, S., & Denizli, A. (2009). Journal of Chromatography B, 877, 181–188.
Uzun, L., Say, R., Ünal, S., & Denizli, A. (2009). Biosensors and Bioelectronics, 24, 2878–2884.
Ge, Y., & Turner, A. P. F. (2009). Chemistry - A European Journal, 15, 8100–8107.
Türkoğlu, E. A., Yavuz, H., Uzun, L., Akgöl, S., & Denizli, A. (2013). Artificial Cells Nanomedicine, 41, 213–221.
Osman, B., Uzun, L., Beşirli, N., & Denizli, A. (2013). Materials Science and Engineering C, 33, 3609–3614.
Kryscio, D. R., & Peppas, N. A. (2012). Acta Biomaterialia, 8, 461–473.
Hansen, D. E. (2007). Biomaterialia, 28, 4178–4191.
Tamahkar, E., Bereli, N., Say, R., & Denizli, A. (2011). Journal of Separation Science, 34, 3433–3440.
Andaç, M., Galaev, I. Y., & Denizli, A. (2013). Colloids and Surfaces B, 109, 259–265.
Derazshamshir, A., Baydemir, G., Andaç, M., Say, R., Galaev, I. Y., & Denizli, A. (2010). Macromolecular Chemistry and Physics, 211, 657–668.
Bereli, N., Ertürk, G., & Denizli, A. (2012). Separation Science and Technology, 47, 1813–1820.
Kumar, A., Plieva, F. M., Galaev, I. Y., & Mattiasson, B. (2003). Journal of Immunological Methods, 283, 185–194.
Ertürk, G., Bereli, N., Tümer, M. A., Say, R., & Denizli, A. (2013). Journal of Molecular Recognition, 26, 633–642.
Baydemir, G., Bereli, N., Andaç, M., Say, R., Galaev, I. Y., & Denizli, A. (2009). Colloids and Surfaces B, 68, 33–38.
Lozinsky, V. I., Galaev, I. Y., Plieva, F. M., Savina, I. N., Jungvid, H., & Mattiasson, B. (2003). Trends in Biotechnology, 21, 445–451.
Aslıyüce, S., Uzun, L., Say, R., & Denizli, A. (2013). Reactive and Functional Polymers, 73, 813–820.
Altıntaş, E. B., & Denizli, A. (2009). Materials Science and Engineering C, 29, 1627–1634.
Arakawa, T., & Timasheff, S. N. (1984). Biochemistry, 23, 5912–5923.
Yıldırım, E., Turan, E., & Çaykara, T. (2012). Journal of Materials Chemistry, 22, 636–642.
Moreira, F. T. C., Dutra, R. A. F., Noronha, J. P. C., & Sales, M. G. F. (2013). Electrochimica Acta, 107, 481–487.
Moreira, F. T. C., Dutra, R. A. F., Noronha, J. P. C., & Sales, M. G. F. (2011). Biosensors and Bioelectronics, 26, 4760–4766.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ertürk, G., Bereli, N., Ramteke, P.W. et al. Molecularly Imprinted Supermacroporous Cryogels for Myoglobin Recognition. Appl Biochem Biotechnol 173, 1250–1262 (2014). https://doi.org/10.1007/s12010-014-0844-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0844-z