Abstract
In this study, culture conditions, including dissolved oxygen (DO) content, presence of osmoprotectants, residual glucose concentration, and ammonium sulfate-feeding strategies, were investigated for decreasing the inhibition effects of acetic acid, ammonium, and osmotic stress on l-lysine fermentation by Escherichia coli. The results revealed that higher DO content and lower residual glucose concentration could decrease acetic acid accumulation, betaine supplementation could enhance osmotic stress tolerance, and variable speed ammonium sulfate-feeding strategy could decrease ammonium inhibition. Thus, with 25 % DO content, 0–5.0 g/L of residual glucose concentration, and 1.5 g/L of betaine supplementation, 134.9 g/L of l-lysine was obtained after 72 h of culture, with l-lysine yield and productivity of 45.4 % and 1.9 g/(L · h), respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-Lysine is currently predominantly used as an additive in animal feed, pharmaceutical industry, and cosmetics [1]. The global market volume of l-lysine monohydrochloride was more than one million tons per year in 2009 [2], with a predicted market growth of about 8 % every year [3, 4]. Similar to the production of most of the other l-amino acids, fermentation is the current dominant method for the industrial production of l-lysine. l-Lysine can be produced by microbial fermentation using a number of microorganisms, including Bacillus subtilis [5], Brevibacterium flavum [6], Corynebacterium glutamicum [7], and Escherichia coli [8]. Traditionally, E. coli has been used as a workhouse to develop new processes for the biosynthesis of many valuable chemicals owing to the extensive knowledge of its metabolism, available genetic tools, and good growth characteristics, combined with low nutrient requirements [9, 10]. Several studies have mentioned the use of E. coli for the production of amino acids such as l-phenylalanine [11], l-tyrosine [12], and l-tryptophan [13]. However, only a few reports on l-lysine production using E. coli have been noted. Imaizumi focused on the modification of the rmf gene, which encodes the ribosome modulation factor protein, to improve the production of l-lysine [14], and obtained more than 4 g/L of l-lysine. In addition, Nadeem et al. achieved 13–15 g/L of l-lysine titer by exposing an E. coli mutant to N-methyl-N′-nitro-N-nitrosoguanidine [8]. Furthermore, Yuri et al. improved l-lysine production by E. coli by increasing the glyceraldehyde 3-phosphate dehydrogenase activity, and achieved 14.1–16.9 g/L of l-lysine with a yield of 35.0–41.8 % using glucose as the carbon source [15]. Nevertheless, to date, only less than 100 g/L of l-lysine had been obtained by using E. coli, which can be easily achieved by employing C. glutamicum [8].
Many culture conditions, such as osmotic stress [16], ammonium concentration [17], and acetic acid (as a byproduct) [18], exert negative effects on E. coli culture. In the present study, the inhibition effects of osmotic stress, ammonium concentration, and acetic acid concentration on l-lysine fermentation were studied, and several culture conditions were optimized for decreasing these inhibition effects for efficient production of l-lysine by using engineered E. coli.
Materials and Methods
Strain and Cultivation
The engineered E. coli NT1003 was kindly provided by Prof. Long Yu, and has been preserved in China Center for Type Culture Collection (No. M 2013239). This lysine-production strain is a threonine- and methionine-deficient E. coli and is imported pSC101 plasmid-linking ppc gene encoding phosphoenolpyruvate carboxylase, pntB gene encoding pyridine nucleotide transhydrogenase, and aspA gene encoding aspartate ammonia-lyase.
The medium for inoculum culture was composed of (g/L): glucose, 5.0; tryptone, 5.0; yeast extract, 4.0; (NH4)2SO4, 5.0; K2HPO4, 1.5; MgSO4, 0.5; FeSO4, 0.01; MnSO4, 0.01; kanamycin, 0.025; and tetracycline, 0.025. The medium was heat sterilized at 121 °C for 15 min. The cells were grown in 500-mL shake flask containing 50 mL of medium. Subsequently, the shake flask was inoculated with 1 mL of glycerol stock culture stored at −70 °C and incubated at 37 °C and 200 rpm for 12 h.
Fed-batch fermentation was carried out in a 7.5-L fermentor (BioFio 115, New Brunswick Scientific, Edison, NJ, USA) with an initial broth volume of 3 L. The medium for fermentation culture was composed of the following (g/L): glucose, 30.0; (NH4)2SO4, 10.0; K2HPO4, 3.0; MgSO4, 1.5; KCl, 1.2; FeSO4, 0.035; MnSO4, 0.035; tryptone, 10.0; yeast extract, 5.0; threonine, 0.2; methionine, 0.2; kanamycin, 0.025; and tetracycline, 0.025. The culture pH was monitored by using a Mettler electrode and controlled at 7.0 with the addition of concentrated ammonium hydroxide (25 % w/w NH3). The aeration in the fermentation broth was maintained at 1.0 vvm. The dissolved oxygen (DO) content was monitored by using a Mettler oxygen electrode and was maintained at about 5 % by automatically adjusting the agitation speed. Glucose was heat sterilized at 121 °C for 15 min before being supplemented to the medium. Residual glucose concentration was monitored offline and maintained by manually adjusting the feed pump. The initial culture conditions were carried out with DO of 5 % and residual glucose concentration between 5.0 and 15.0 g/L.
Ammonium Sulfate-Feeding Strategies
Two kinds of ammonium sulfate-feeding strategies were employed. One was constant speed feeding of 10 mL of 400 g/L ammonium sulfate solution per hour, and the other was variable speed feeding, with the speed calculated as follows:
where R ammonium sulfate and R nitrogen represent ammonium sulfate and nitrogen-feeding rate, respectively; Nlysine represents nitrogen in l-lysine, calculated from l-lysine accumulated during the time interval; Ncell represents nitrogen in the cell, calculated as 40 % of the dry cell weight (DCW) accumulated during the time interval; Nammonia represents nitrogen in ammonia water, calculated from ammonia water consumption during the time interval; T represents the time interval, which was 4 h for fermentation before 24 and 8 h for fermentation after 24 h; and the coefficient 1.2 represents other nitrogen that could not be detected.
Analytical Methods
The DCW was computed from a curve of optical density measured at 562 nm (OD562) with respect to dry weight. An OD562 of 1.0 represented 40 mg dry weight per liter. The concentrations of glucose and l-lysine were analyzed by using a SBA-40C biosensor analyzer [19] (Shandong Province Academy of Sciences, China). The organic acids were evaluated by using high-performance liquid chromatography (1290, Agilent Technologies, USA) equipped with an ion-exchange column (prevail organic acid 5 u, 250 × 4.6 mm, Grace, USA), and 25 mM KH2PO4 (adjusted to a pH of 2.5 by H3PO4) was used as a mobile phase with a flow rate of 1 mL/min. The yield of l-lysine was defined as the final amount of l-lysine produced from 1 g of glucose, and expressed as a mass ratio. Utilization of nitrogen was defined as the nitrogen in l-lysine accumulated in broth (g) divided by the nitrogen in ammonia and ammonium sulfate consumed (g), and expressed as percent.
Results and Discussion
Inhibition Effect on l-Lysine Fermentation by E. coli
During l-lysine fermentation by E. coli NT1003, l-lysine sulfate, ammonium sulfate, and acetic acid were the main components found in the culture medium. To investigate the inhibition effects of these compounds on l-lysine fermentation, 70.0 g/L of l-lysine sulfate, 40.0 g/L of ammonium sulfate, and 5.0 g/L of acetic acid were respectively added to the initial medium.
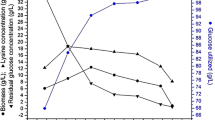
The results showed that l-lysine sulfate, ammonium sulfate, and acetic acid had inhibition effects on l-lysine fermentation (Fig. 1). As shown in Fig. 1, when l-lysine sulfate, ammonium sulfate, and acetic acid were added to the medium, the cell growth sharply decreased by 79.2, 83.4, and 92.3 %, respectively, when compared with the control, along with 83.2, 79.2, and 89.6 % decrease in l-lysine yield, respectively. Therefore, to decrease these inhibition effects, the influences of culture conditions, including DO content, osmoprotectants, residual glucose concentration, and ammonium sulfate-feeding strategies, on l-lysine fermentation were investigated.
l-lysine concentration and DCW when different inhibitions appears, including 70 g/L l-lysine sulfate which might cause excessive osmotic stress, 40 g/L ammonium sulfate, and 5 g/L acetic acid which might inhibited the cultivation. Cells were grown in a 1-L bioreactor for 72 h; glucose were controlled below 20 g/L with a initial concentration of 20 g/L
Effect of DO Content on l-Lysine Production
It is known that E. coli produces acetic acid when the level of DO in the culture medium is insufficient [20]. In the present study, the effect of 5–25 % DO content on l-lysine production was investigated (Fig. 2). As shown in Fig. 2, the l-lysine yield and cell growth increased with the increasing level of DO from 5 to 25 %. On the other hand, the concentration of acetic acid increased with decreasing DO content; a maximum acetic acid concentration of 6.8 g/L was noted when the DO content was 5 % at 72 h of culture, which was more than sufficient to inhibit the cell growth of E. coli [21] [22]. However, when the DO level was maintained at 15 and 25 %, the maximum concentration of acetic acid at 20 h was 3.2 and 4.0 g/L, respectively; subsequently, acetic acid was consumed and the final acetic acid concentration was less than 1.0 g/L at 25 % DO content. These findings are also in agreement with those reported in a previous study [23]. Furthermore, the maximum DCW at a DO content of 25 % was 13.9 g/L at 34 h, which was 51.4 and 38.9 % higher than that observed at 5 and 15 % DO content, respectively. In addition, a maximum l-lysine concentration of 79.1 g/L was obtained at 25 % DO content. However, because of the limitations of aeration and agitation, the highest level of DO that could be achieved in the present study was 25 %.
Effect of Supplementation of Osmoprotectants on l-Lysine Production
Osmotic stress due to high substrate or product concentrations is a challenge during industrial fermentation [24]. Osmoprotectants are substances either absorbed or synthesized by bacteria, which allow them to grow in high-osmolality medium [25]. In the present study, the effects of four different commonly used osmoprotectants, namely, trehalose, glycine, betaine, and proline, on l-lysine production by E. coli in shake-flask cultivation were investigated (data not shown). The result revealed that betaine was the best osmoprotectant for E. coli with respect to both l-lysine production and cell growth. In a previous study, it was reported that betaine was the most effective and important osmoprotectant for C. glutamicum, and that addition of this compound to the fermentation medium had a positive effect on cell growth and l-lysine production [15]. In the present study, the effect of 0–6.0 g/L of betaine supplementation on l-lysine production was investigated at a DO level of 25 %. As shown in Table 1, l-lysine concentration and cell growth increased with increasing betaine concentration from 0 to 1.5 g/L. On the other hand, within the range of 1.5–6.0 g/L, increasing betaine concentration had little influence on l-lysine production and cell growth. A maximum l-lysine concentration of 85.6 g/L was obtained at a betaine concentration of 1.5 g/L, which was 10 % higher than that observed in the control, with an l-lysine yield and productivity of 37.5 % and 1.2 g/(L · h), respectively.
Effect of Residual Glucose on l-Lysine Production
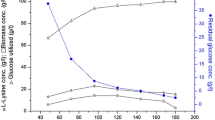
It has been indicated that excessive amounts of glucose in the culture medium might result in the production of acetic acid [23], which may inhibit cell growth and product fermentation. Therefore, in the present study, the effects of three different ranges of residual glucose concentrations (0–5.0, 5.0–10, and 10.0–15.0 g/L) in the culture medium with 25 % DO content and 1.5 g/L of betaine were investigated. As shown in Fig. 3, an increase in residual glucose concentration in the culture medium led to an increase in acetic acid concentration, but a decrease in l-lysine concentration and cell growth. A maximum l-lysine concentration of 108.0 g/L was obtained at a residual glucose concentration of 0–5.0 g/L, with an l-lysine yield and productivity of 41.9 % and 1.5 g/(L · h), respectively, while the acetic acid concentration was less than 0.8 g/L.
Fed-batch fermentation under the condition of different residual glucose concentration. a l-lysine concentration under the condition of different residual glucose concentration. b DCW under the condition of different residual glucose concentration. c Acetic acid concentration under the condition of different residual glucose concentration (black square residual glucose concentration of 0–5.0 g/L, empty circle residual glucose concentration of 5.0–10.0 g/L, filled triangle residual glucose concentration of over 10.0 g/L). d Different conditions of residual glucose concentration (filled square residual glucose concentration of 0–5.0 g/L; empty circle residual glucose concentration of 5.0–10.0 g/L; filled triangle residual glucose concentration of over 10.0 g/L)
Effect of Ammonium Sulfate Supplementation on l-Lysine Production
l-Lysine, a basic amino acid, has two amino groups, indicating that two moles of ammonium are required to produce 1 mol of l-lysine. On the other hand, other l-amino acids, such as l-phenylalanine, have only one amino group, and can be produced using concentrated ammonium hydroxide employed for controlling the pH during fermentation [26]. As ammonium in the culture medium is not sufficient for the production of l-lysine, addition of a nitrogen source, most preferably, ammonium sulfate, to the medium is necessary [1]. As mentioned earlier, high concentration of ammonium sulfate could obviously inhibit both cell growth and l-lysine production. Therefore, in the present study, constant and variable speed ammonium sulfate-feeding strategies were employed. As shown in Fig. 4, the maximum cell growth achieved with variable speed ammonium sulfate-feeding strategy was 23.3 g/L, which was 2.4-fold higher than that obtained using constant speed ammonium sulfate-feeding strategy. Furthermore, the utilization of nitrogen under variable speed ammonium sulfate-feeding strategy was 89.9 %, which was 33.7 % higher than that under constant speed ammonium sulfate-feeding strategy (only 59 % nitrogen utilization). As shown in Table 2, after 72 h of culture, the maximum l-lysine concentration was 134.9 g/L, with l-lysine yield and productivity of 45.4 % and 1.9 g/(L · h), respectively.
Fed-batch fermentation using two different ammonium sulfate supplement strategies. a l-lysine concentration using two different ammonium sulfate supplement strategies. b DCW using two different ammonium sulfate supplement strategies (filled square l-lysine production using variable speed supplement of sulfate ammonium; empty circle l-lysine production using constant speed supplement of sulfate ammonium). c Cumulative ammonium concentration added to the fermenter in both feeding strategies (constant speed and variable speed) (filled square cumulative ammonium concentration by variable speed supplement; empty circle cumulative ammonium concentration by constant speed supplement)
Conclusion
Ammonium concentration, acetic acid concentration, and osmotic stress in the culture medium could have inhibition effects on l-lysine fermentation by E. coli. In the present study, for decreasing these inhibition effects, the influences of culture conditions, including DO content, presence of osmoprotectant, residual glucose concentration, and ammonium sulfate-feeding strategy, on l-lysine fermentation by E. coli were investigated. The results indicated that under optimized culture conditions, l-lysine production could be enhanced. These findings could be significant for the industrial production of l-lysine using engineered E. coli.
References
Brautaset, T., & Ellingsen, T. E. (2011). Industrial biotechnology and commodity products. In M. Y. Murray (Ed.), Comprehensive Biotechnology (Vol. 3, pp. 541–554). New York: Elsevier.
Becker, J., Klopprogge, C., & Wittmann, C. (2009). Applied and Environmental Microbiology, 75, 7866–7869.
Eggeling, L., & Sahm, H. (1999). Applied Microbiology and Biotechnology, 52, 146–153.
Wittmann, C., and Becker, J. (2007). Biosynthesis—pathways, regulation and metabolic engineering. In: Volker, F.W. (eds). Microbiology monographs, vol. 5: Amino acid. Springer: New York. pp. 39–70.
Lee, G. H., Hur, W., & Flickinger, M. C. (1996). Biotechnology and Bioengineering, 49, 639–653.
Xu, D. Q., Tan, Y. Z., & Huan, X. J. (2009). Journal of Microbiological Methods, 80, 86–92.
Sassi, A. H., Fauvart, L., & Deschamps, A. M. (1998). Biochemical Engineering Journal, 1, 85–90.
Nadeem, S., Ikaram, A., & Yaqoob, N. (2001). International Journal of Agriculture and Biology, 4, 448–450.
Mey, M. D., Maeseneire, S. D., & Sortaert, W. (2007). Journal of Industrial Microbiology, 34, 689–700.
Hanning, G., & Makrides, S. C. (1998). Trends in Biotechnology, 16, 54–60.
Khamduang, M., Packdibamrung, K., & Chutmanop, J. (2009). Journal of Industrial Microbiology & Biotechnology, 36, 1267–1274.
Patnaik, R., Zolandz, R. R., & Green, D. A. (2008). Biotechnology and Bioengineering, 99, 741–752.
Zhao, Z. J., Zou, C., & Zhu, Y. X. (2011). Journal of Industrial Microbiology & Biotechnology, 38, 1921–1929.
Imaizumi, A., Takikawa, R., & Koseki, C. (2005). Journal of Biotechnology, 117, 111–118.
Nagai, Y., and Masumitsu, Y. (2008). EP2202299.
Mika, J. T. (2012). Molecule diffusion in bacteria and consequences of osmotic stress (pp. 10–14). Groningen: University of Groningen.
Thompson, B. G., Kole, M., & Gerson, O. (1985). Biotechnology and Bioengineering, 27, 818–824.
Han, K., Lim, H. C., & Hong, J. (1992). Biotechnology and Bioengineering, 39, 663–671.
Saurina, J., Cassou, S. H., & Alegtet, S. (1999). Biosensors & Bioelectronics, 14, 211–220.
Suarez, D. C., & Kilikian, B. V. (2000). Process Biochemistry, 35, 1051–1055.
Korz, D. J., Rinas, U., & Hellmuth, K. (1995). Journal of Biotechnology, 39, 59–65.
Padan, E., & Krulwich, T. A. (2000). Sodium stress (pp. 117–130). Washington DC: ASM Press.
Shiloach, J., Kaufman, J., & Fass, R. (1996). Biotechnology and Bioengineering, 49, 421–428.
Ronsch, H., Kramer, R., & Morbach, S. (2003). Journal of Biotechnology, 104, 87–97.
Kunte, H. J., Crane, R. A., & Culham, D. E. (1999). Journal of Bacteriology, 181, 1537–1543.
Mutsumi, T., Yoshinori, N., & Gyuseop, O. (1996). Biotechnology and Bioengineering, 52, 653–660.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (grant no. 21106066, 21106068), National Key Technology Support Program (grant no. 2012BAI44G00), “973” program of China (grant no. 2011CBA00807), and Nature Science Foundation of Jiangsu High School (11KJB530003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ying, H., He, X., Li, Y. et al. Optimization of Culture Conditions for Enhanced Lysine Production Using Engineered Escherichia coli . Appl Biochem Biotechnol 172, 3835–3843 (2014). https://doi.org/10.1007/s12010-014-0820-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0820-7