Abstract
This investigation was carried out with the aim of determining the effect of paclobutrazol (PBZ) (0 and 2 mg l−1) and polyethylene glycol (PEG) (0, 2, 4 and 6 % w/v of PEG 6000) treatments on antioxidant system of Stevia rebaudiana Bertoni under in vitro condition. Analysis of data showed that PEG treatment significantly increased hydrogen peroxide (H2O2) and phenolic contents, while PBZ treatment limited the effect of PEG on them. Our data revealed that PEG treatment significantly increased total antioxidant capacity, catalase (CAT), ascorbate peroxidase (APX), polyphenol oxidase (PPO) and peroxidase (POD) activity, while it inversely decreased glutathione reductase (GR) activity. The superoxide dismutase (SOD) activity was not affected by PEG treatment. PBZ treatment induced significantly higher levels of CAT and GR activity and lower levels of SOD activity in PEG-treated plants. PBZ in combination with PEG resulted in no significant difference on APX activity with PEG treatment alone. PBZ treatment prevented the effect of PEG on the PPO activity. PEG (with or without PBZ) treatment increased the ascorbate pool, whereas total glutathione level was not affected by PEG. Our finding indicated that PBZ reduced the negative effect of PEG treatment by quenching H2O2 accumulation and increasing the CAT activity. Collectively, the antioxidant capacity of S. rebaudiana in PEG treatment condition was associated with active enzymatic and non-enzymatic defence systems which partly could be improved by the PBZ treatment. In addition, a higher accumulation of phenolic compounds leads to a more potent reactive oxygen species scavenging activity in S. rebaudiana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stevia rebaudiana Bertoni is a perennial shrub of the Asteraceae (Compositae) family native to Paraguay and Brazil. It is often referred to as the sweet herb of Paraguay [21]. Steviol glycosides are the compounds responsible for the sweet taste of S. rebaudiana. Stevioside and rebaudioside A are the major steviol glycosides in the leaves of S. rebaudiana. Stevioside is about 300 times sweeter than sucrose (0.4 % solution; [11]). S. rebaudiana is a drought-sensitive plant [13] that its cultivation has spread to other regions of the world, including Canada and some parts of Asia and Europe. The International Water Management Institute predicts that by the year 2025, one third of the world's population will live in regions that will experience severe water scarcity (www.iwmi.org). Plants grown under osmotic stress conditions are seriously affected by oxidative stress. Therefore, it has become imperative for us to understand the antioxidant mechanisms by which S. rebaudiana responses to water deficit.
Drought stress promotes the production of reactive oxygen species (ROS), including superoxide (O2−), singlet oxygen (1O2), hydroxyl (·OH) and hydrogen peroxide (H2O2). ROS react with lipids, proteins and DNA, resulting in lipid peroxidation, protein denaturation and DNA damage [39]. Plants have evolved in both enzymatic and non-enzymatic defence systems for scavenging and detoxifying ROS. In enzymatic systems, superoxide dismutase (SOD) scavenges O2− to H2O2, whereas peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), mono-dehydroascorbate reductase and dehydroascorbate reductase decompose H2O2 to H2O at different cellular locations ([28]; Illustration 1). Polyphenol oxidase (PPO) oxidizes phenolics and takes part in the regulation of the phenolic concentration in plants (Illustration 1). Some studies indicated that phenol-oxidizing enzymes participate in the response to drought stress [42, 46]. The balance between ROS production and activities of antioxidant enzymes determines whether oxidative signalling and/or damage will occur [26].
Metabolites such as ascorbate, glutathione, phenolic compounds and tocopherol control the levels of ROS in plant tissues. A major component of these defence mechanisms is the ascorbate–glutathione cycle involving four enzymes: APX, dehydroascorbate reductase, mono-dehydroascorbate reductase and GR participate in the removal of H2O2 via recycling of the reduced glutathione (GSH) pool, thus playing a central role during stress ([39]; Illustration 1). Ascorbate and glutathione are not consumed in this pathway but participate in a cyclic transfer of reducing equivalents, involving four enzymes, which permits the reduction of H2O2 to H2O using electrons derived from nicotinamide adenine dinucleotide phosphate (NADPH) [28].
Leaf extract of S. rebaudiana has a great potential to use as a natural antioxidant agent [14]. In Stevia leaves, some compounds other than steviol glycosides, e.g. folic acid, pyrogallol, phenolic and flavonoid, have ROS scavenging activity [18]. Kim et al. [18] suggested that the high antioxidant activity of S. rebaudiana is due to the presence of phenolic compounds. With this knowledge, we examined the phenolic compounds beside ascorbate and glutathione pools to evaluate their antioxidant capacity in Stevia.
According to our previous experiments, polyethylene glycol (PEG) treatment reduced plant growth, photosynthetic pigments, carbohydrates and proline amounts, while it increased electrolyte leakage, MDA, α-tocopherol and glycine betaine contents in S. rebaudiana [13]. Paclobutrazol (PBZ), a plant growth regulator from triazole family, has been reported to protect plants against drought stress [3, 22, 23, 45]. Also, Hajihashemi and Ehsanpour [13] reported that the negative effects of PEG treatment in S. rebaudiana could partly be restored by PBZ treatment. PBZ treatment reduced lipid peroxidation while it increased glycine betaine, α-tocopherol, proline and protein contents in PEG-treated S. rebaudiana [13].
Maintaining a high level of antioxidants in Stevia may contribute to drought tolerance. Therefore, the present investigation was carried out with the objective to study the effect of PBZ and PEG treatments on antioxidant systems by measuring some enzymatic and non-enzymatic antioxidants. To this end, we examined total antioxidant capacity; the amounts of H2O2 and phenolic compounds; the activity of CAT, APX, PPO, SOD, GR and POD enzymes; and ascorbate and glutathione pools.
Materials and Methods
Seeds of S. rebaudiana Bertoni were provided from Prairie Oak Publishing, 221 South Saunders Street, Marville MO 64468. They were surface-sterilized in 70 % (v/v) ethanol for 1 min and 20 % (v/v) sodium hypochlorite solution for 15 min before being washed three times in sterile distilled water. All subsequent operations were performed under aseptic conditions in a laminar flow cabinet.
Culture Media Preparation and Culturing Conditions
The culture medium used for these initiation and multiplication experiments was Murashige and Skoog (MS) [27]. Each was supplemented with 30 g l−1 sucrose. After the pH was adjusted to 5.8, 8 g l−1 agar was added, after which the medium was dispensed into jars and autoclaved. Four seeds were cultured per jar under aseptic condition. All cultures were incubated at 26 ± 1 °C under a 16-h photoperiod produced by white fluorescent lamps at 120 μmol photons m−2 s−1. The rooted plantlets were sub-cultured into the new MS medium.
PEG and PBZ Treatments
The study of the effects of PEG as drought stress on the performance of plants grown under in vitro tissue condition can allow growing plants in rapid and under controlled conditions to carry out experiments in identical conditions all year round. Although the addition of PEG to in vitro culture is not a real drought stress comparable to open field conditions, our results reported in the present study provide knowledge that could be used for field culture. Some prior characterization of multiple parameters was done to select a meaningful level of PBZ (0–10 mg l−1) and PEG (0–20 % w/v) (results not shown; [13]). The results (not shown) demonstrated that 2 mg l−1 PBZ and 0–6 % w/v PEG were the most effective concentrations [13]. Therefore, the subsequent experiments were done with PBZ treatment at 0 and 2 mg l−1 and PEG 6000 (molecular weight 6,000) at concentrations of 0, 2, 4 and 6 % (w/v). The osmotic potential of 2, 4 and 6 % (w/v) of PEG at 25 °C is respectively −10.4 × 10−2, 20.6 × 10−2 and 30.7 × 10−2 bar [25]. Similar rooted plants on basal medium of about 10-cm height were transferred to medium supplemented with PBZ and PEG without the gelling agent (liquid medium). Plants could not survive for long under PEG treatment, so the cultures were kept for 14 days to study their growth potential and regeneration capacity under different treatments. All measurements were performed 2 weeks after transferring the plants to the described media.
H2O2 Content
Fresh leaves (0.25 g) were homogenized in an ice bath with 2.5 ml of 0.1 % (w/v) trichloroacetic acid. The homogenate was centrifuged at 12,000 × g for 15 min. Then, 0.5 ml of the supernatant was added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) with 1.0 ml of 1.0 M potassium iodide. The absorbance of supernatants was recorded at 390 nm. The contents of H2O2 were calculated using a standard curve [41].
Total Antioxidant Capacity (FRAP Assay)
One gram of fresh leaves was homogenized in 9 ml of 0.1 M phosphate buffer (pH 7.6) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA). The homogenate was centrifuged at 13,000 rpm at 4 °C for 10 min. The supernatant was used for the measurement of “antioxidant power” according to Szôllôsi and Varga et al. [37]. The ferric reducing antioxidant power (FRAP) mixture was consisted of 25 ml of 300 mmol l−1 acetate buffer (pH 3.6), 2.5 ml of 10 mmol l−1 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mmol l−1 hydrochloric acid and 2.5 ml of 20 mmol l−1 ferric chloride. Then, 1.5 ml of FRAP mixture was added to 0.5 ml of plant extract, and samples were monitored at 593 nm at 25 °C for 5 min against the FRAP reagent as a blank. The relative activities of the samples were assessed by comparing their activities with that of l-ascorbic acid as a standard curve.
Protein Extraction and Enzyme Activity
Fresh leaf samples were used for protein and enzyme analysis. Leaves were frozen immediately in liquid nitrogen after harvesting and stored at −80 °C until further use. One gram of leaves was homogenized in 3 ml of 50 mM sodium phosphate buffer (pH 7.8) including 1.0 mM EDTA, 4 mM dithiothreitol, 5 mM magnesium sulphate, 10 % glycerol and 2 % (w/v) polyvinyl polypyrrolidone. The homogenate was centrifuged at 13,000 rpm at 4 °C for 40 min. The supernatant was used for protein analysis and enzyme activity assays. All assays were done at 4 °C. Proteins were determined by the method of Bradford [7] using bovine serum albumin (Sigma-Aldrich Co., USA) as a standard.
Superoxide Dismutase (SOD) (EC 1.15.1.1) Activity
The SOD activity assay was based on the method of Beauchamp and Fridovich [6] which measures the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT) spectrophotometrically at 560 nm. One unit of enzyme activity was defined as the quantity of SOD required to produce a 50 % inhibition of NBT reduction. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8), 33 mM NBT, 10 mM methionine and 1.17 μM riboflavin. Three millilitres of the reaction medium was added. Reactions were carried out at 25 °C under a light intensity of about 40 W for 30 min. Identical solutions were kept in the dark and served as blanks. The absorbance was read spectrophotometrically at 560 nm against the blank. SOD activity was expressed in unit per milligram protein.
The SOD activity gel assay carried out was also based on the inhibition of NBT reduction by SOD. Plant extracts containing equal amounts of proteins were subjected to discontinuous polyacrylamide gel electrophoresis (PAGE) under non-denaturing and non-reducing conditions. Native PAGE of SOD was performed on a 12 % resolving gel. After electrophoretic separation, the staining for SOD activity was performed by a modified method of Beauchamp and Fridovich [6]. The gel was soaked in a solution of 50 mM potassium phosphate buffer (pH 7.8), 2.5 M NBT, 28 mM riboflavin and 28 mM N,N,N′,N′-tetramethylethylenediamine (TEMED) in darkness for 20 min and then exposed to a light box until the SOD activity bands became visible. The enzymes appeared as colourless bands in a purple background.
Catalase (CAT) (EC 1.11.1.6) Activity
In order to determine CAT activity, the assay medium contained 50 mM potassium phosphate buffer (pH 7.0), 15 mM H2O2 and 50 μl enzyme extract in a final volume of 3 ml. The decrease in the absorbance of H2O2 was recorded at 240 nm for 3 min. One unit of activity was defined as the amount of enzyme catalyzing the decomposition of 1.0 μmol H2O2 min−1 mg−1 protein, calculated from the extinction coefficient (36 M−1 cm−1) for H2O2 [1].
Peroxidase (POD) (EC 1.11.1.7) Activity
POD activity was determined as described by Plewa et al. [31]. The assay mixture was consisted of 50 μl of the enzyme extract, 100 mM phosphate buffer (pH 7.0), 0.1 μM EDTA, 10 mM guaiacol and 15 mM H2O2 in a total volume of 2 ml. Guaiacol oxidation (tetraguaiacol formation) was monitored by reading the absorbance at 470 nm at the moment of H2O2 addition and continued for 3 min. The difference in absorbance (ΔA470) was divided by the molar extinction coefficient of tetraguaiacol (26.6 mM−1 cm−1). The enzyme activity expressed as micromole per minute per milligram protein.
Ascorbate Peroxidase (APX) (EC 1.11.1.1) Activity
APX activity was determined according to Asada and Takahashi [2]. The reaction mixture (1.0 ml) contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 1.0 mM H2O2 and 50 μl of enzyme extract. The absorbance was read at 290 nm against the blank (extinction coefficient 2.9 mM−1 cm−1) for 3 min. The enzyme activity was expressed in micromole ascorbate (ASC) per minute per milligram protein.
Glutathione Reductase (GR) (EC 1.6.4.2) Activity
GR activity was measured according to Foyer and Halliwell [10], with minor modifications, which depends on the rate of decrease in the absorbance of oxidized glutathione (GSSG) at 340 nm. The assay mixture was consisted of 50 μl of the enzyme extract, 100 mM phosphate buffer (pH 7.8), 0.1 μM EDTA, 0.05 mM NADPH and 3.0 mM GSSG in a total volume of 1.0 ml. The rate of NADPH oxidation was monitored by reading the absorbance at 340 nm at the moment of H2O2 addition and was carried out for 3 min. GR activity was calculated from the reduced GSSG concentration (extinction coefficient 6.2 mM−1 cm−1). The enzyme activity was expressed in micromole NADPH per minute per milligram protein.
Polyphenol Oxidase (PPO) (EC 1.10.3.1) Activity
PPO activity was determined according to a modified version of the method as described by Flurkey and Jen [9]. The assay mixture was consisted of 50 mM potassium phosphate buffer (pH 6.5), 0.2 M catechol solution as a substrate and 50 μl enzyme extract in a total volume of 3 ml. Quinone formation was monitored by reading the increase of absorbance at 420 nm for 3 min. The enzyme activity was expressed as micromole quinone per minute per milligram protein.
Phenol Content
The amount of phenolic compounds in leaves was measured using Folin and Ciocalteu's reagent [36]. One hundred milligrams of fresh leaves was homogenized in 1.5 ml of 95 % ethanol and kept for 24 h in the dark. The homogenates were centrifuged at 13,000 rpm for 10 min. To 1.0 ml of supernatant, 1.5 ml of 95 % ethanol and 5 ml of distilled water were added. To this mixture, 0.5 ml of 50 % Folin's reagent and 1.0 ml of 5 % sodium carbonate were added. After vortexing, the mixture was kept in the dark for 1 h before reading each sample at 725 nm. Samples were quantified using a standard curve based on a gallic acid.
Analysis of Ascorbate and Glutathione Pools
Two hundred milligrams of fresh leaves was ground with 1.5 ml of 5 % metaphosphoric acid containing 1.0 mM EDTA and centrifuged at 14,000 rpm at 4 °C for 10 min. The ASC and dehydroascorbate (DHA) content were measured according to a modified version based on the method of Kampfenkel et al. [17]. Total glutathione, GSSG, and GSH were assayed according to the method of Griffith [12].
Total ASC was determined in a reaction mixture of 1.0 ml containing 200 μl plant extract, 200 μl of 0.2 M phosphate buffer (pH 7.4) and 200 μl of 10 mM dithiothreitol. After shaking and incubating at room temperature for 30 min, 100 μl of 0.5 % N-ethylmaleimide was added, followed by incubation for 10 min at room temperature. ASC levels were determined in the same way, except that dithiothreitol and N-ethylmaleimide were substituted with water. Colouring was obtained by adding 200 μl of 10 % trichloroacetic acid, 200 μl of 42 % phosphoric acid, 200 μl 2,2′dipyridyl dissolved in 70 % ethanol and 100 μl of 3 % ferric chloride. After shaking, the mixture was incubated at 42 °C for 40 min, after which the absorbance was recorded at 525 nm [17]. The concentration of DHA was calculated as the difference between total ascorbate and ASC. A standard curve in the range of 0–100 μg ml−1 of ASC was prepared.
The levels of GSH were measured spectrophotometrically by monitoring the reduction of 5,5-dithio-bis-(2-nitrobenzoic acid) at 412 nm. In order to determine the glutathione pool, 400 μl of the supernatant was neutralized with 600 μl of 0.5 M phosphate buffer (pH 7.5). For the GSSG assay, GSH was made by adding 20 μl 2-vinylpyridine to the neutralized extract, whereas 20 μl water was added to the aliquots for the total glutathione pool (GSH + GSSG) assay. Tubes were mixed until an emulsion was formed. The glutathione content in 1.0 ml of reaction mixture containing 0.2 mM NADPH, 100 mM phosphate buffer (pH 7.5), 5 mM EDTA, 0.6 mM 5,5′-dithiobis(2-nitrobenzoic acid) and 100-μl sample was obtained as described above. The reaction was started by adding 3 units of GR and was monitored by measuring the changes in the absorbance at 412 nm for 1 min. The concentration of GSH was calculated as the difference between total glutathione and GSSG. A standard curve was developed based on GSH in the range of 0–50 μM.
Statistical Analysis
The experiment included three biological replicates and each replication comprised up to 12 plants. The experimental set-up was designed by using a randomized complete block designs with three replications. The data were analyzed by the Duncan test's SPSS (version 16) statistical package to assess significant differences (at 5 % level) among means.
Results
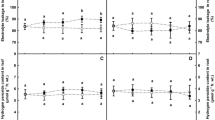
To compare the effect of PEG and PBZ on the antioxidant system of Stevia, first, we measured the H2O2 content. Data indicated that H2O2 content significantly increased in PEG-treated plants, and the highest increase was observed in 6 % PEG, by about 64 % higher than that of control plants without treatment (Fig. 1a). PBZ treatment alone did not increase H2O2 content. As expected, PBZ treatment limited the effect of PEG on H2O2 content. Interestingly, the highest H2O2 content in PBZ-treated plants was observed in 6 % PEG + PBZ treatment, but it was only by about 37 % higher than that of control plants without PBZ and PEG treatments. Next, we were interested to know how the total antioxidant activity of plant was affected by treatments, so we measured the total antioxidant capacity of plants with the FRAP method. Results of the evaluations revealed a significant increase in the total antioxidant capacity of plants in 4 and 6 % PEG (Fig. 1b). Surprisingly, PBZ treatment holds the total antioxidant capacity of Stevia plants the same as PEG treatment alone, and no significant differences were observed between PEG treatment and PEG + PBZ treatment.
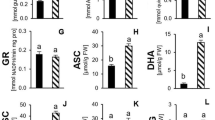
In the next step of our study, APX, POD, SOD, CAT, PPO and GR were the selected enzymes to study the effect of PEG and PBZ treatments on the enzymatic antioxidant defence system of S. rebaudiana. Measurement of the activity of key enzyme of SOD, involved in the first step of ROS detoxification processes, revealed that PEG treatment had no significant effect on it (Fig. 2a). PBZ (with or without PEG) treatment significantly decreased SOD activity as compared with PEG treatment alone. In PBZ + PEG-treated plants, SOD activity was almost the same in different concentrations of PEG. Additionally, the native PAGE analysis did not reveal any clear variations in SOD activities in different treatments of PEG and PBZ (Illustration 2). Also, the noted reduction of SOD activity in PBZ-treated plants was not detectable on the gel.
As the enzymes CAT, POD and APX catalyze dismutation reactions of H2O2 into H2O and are indispensable for ROS detoxification during stress conditions, we measured their activities. At first, we started with CAT because it is an important enzyme in the removal of H2O2 generated in peroxisomes. PEG treatment induced a significant enhancement in the CAT activity, and the highest CAT activity was observed at 4 % PEG (48 % higher than that of control plants without treatment). CAT activity significantly improved in PBZ treatment with or without PEG. PBZ treatment increased CAT activity in the PEG-treated plants to a level higher than that of PEG treatment alone. Regarding CAT, the greatest activity was observed in 4 % PEG + PBZ, by about 65 % higher than control plants without treatment.
As suspected, the activity of POD significantly increased in PEG treatment with the highest activity observed at 4 and 6 % PEG (Fig. 2c). Although PBZ treatment alone and 2 % PEG + PBZ treatment had no significant effect on POD activity, PBZ treatment with 4 and 6 % PEG significantly increased it more than that in control plants without treatment. APX activity significantly increased in PEG-treated plants higher than that of control plants without treatment (Fig. 2d). PBZ treatment gave no significant effect on APX activity in PEG-treated plants comparing with the PEG treatment alone. GR enzyme belongs to ascorbate–glutathione cycle that is a metabolic pathway to detoxify H2O2. PEG treatment showed no significant effect on GR activity, with the exception of 6 % PEG which significantly decreased GR activity, by about 30 % less than that of control plants without treatment (Fig. 2e). PBZ treatment prevented the effect of PEG on GR activity. In PBZ + PEG treatment, GR showed a significant higher activity than the same level of PEG without PBZ.
As phenols are important criterions to evaluate stress tolerance in plants, we examined the activity of PPO and the phenol contents. The activity of PPO significantly increased under PEG treatment, but there were no significant differences between different concentrations of PEG (Fig. 2f). PBZ treatment significantly inhibited the effect of PEG treatment on PPO activity because PPO activity in PBZ-treated plants was almost the same as in control plants without treatment. Analysis of phenolic contents revealed a considerable increase under PEG treatment (Fig. 3). The highest amount of phenols was observed at 6 % PEG, by about 55 % more than that in control plants. PBZ treatment by itself had no significant effect on phenols. Interestingly, PBZ treatment limited the effect of PEG on phenols. In PBZ-treated plants, the highest phenol content was detected in 6 % PEG + PBZ treatment which was by about of that in 2 % PEG without PBZ.
To understand how PEG and PBZ treatments affect non-enzymatic antioxidants, the ascorbate and glutathione pools were measured. Considering the ascorbate pool, a significant increase was observed in the PEG-treated plants (Fig. 4). For example, in the 4 % PEG, the increase in ASC (Fig. 4a), DHA (Fig. 4b) and total ascorbate (Fig. 4c) levels was about 59, 50 and 52 % more than that in the control plants without treatment respectively. In PBZ-treated plants, the ascorbate pool was significantly higher than that in control plants, and it was almost the same as that in plants treated with PEG alone. The value of the ASC/DHA ratio showed no significant changes under PEG and PBZ treatments (Fig. 4d).
The total glutathione (Fig. 5c) and GSH (Fig. 5b) contents were not affected by PEG and PBZ treatments (Fig. 5). The level of GSSG (Fig. 5a) did not show significant changes in PEG treatment, while a significant reduction was observed in PBZ with or without PEG treatment. The GSH/GSSG value significantly increased in PBZ-treated (with or without PEG) plants, but PEG treatment had no significant effect on this ratio (Fig. 5d).
Discussion
Oxidative stress arises from an imbalance in the generation and metabolism of ROS, with more ROS (such as H2O2) being produced than metabolized. H2O2 generation via superoxide during electron transport was increased in response to environmental stresses such as excess excitation (light) energy, drought and cold [4]. There have been many reports of the increased activities of SOD under abiotic stresses induced with tissue culture techniques, including heavy metals, salinity, and drought [5, 30, 32–34, 43]. As suspected, the higher H2O2 observed in PEG treatment was slightly scavenged by PBZ treatment in S. rebaudiana. Surprisingly, a correlation was observed between SOD activity and H2O2 contents in treated plants because SOD activity similar to the H2O2 levels in PBZ + PEG treatment was less than that in PEG treatment alone. Kraus and Fletcher [20] proposed that PBZ increased SOD activity, but in our results, no such increase was observed. Therefore, it seems that plants do not apply the same mechanisms against oxidative stress. Shukla et al. [35] have demonstrated that S. rebaudiana is an accessible source of natural antioxidants. It is interesting that the total antioxidant capacity was the same in both groups of PEG treatment and PEG + PBZ treatment, while lower H2O2 was observed in PEG + PBZ treatment. These data suggested that the reduction of H2O2 accumulation in S. rebaudiana in the PBZ + PEG-treated plants might be correlated to a lower SOD activity or higher scavenging activity by antioxidants. In other words, these data strongly suggested that PBZ prevented the increase of H2O2 induced by PEG.
The accumulation of H2O2, if accompanied by enhanced scavenging mechanisms like CAT and POD enzyme activities, has been considered as an important antidrought mechanism to cope with oxidative stress during water deficit conditions [24]. Our data showed that CAT activity of the leaf extract of Stevia plants is more than POD and APX activities, so probably, CAT is the most critical enzyme in Stevia in detoxifying H2O2. PBZ treatment improved CAT activity in PEG-treated plants to a level higher than that of the same PEG treatment without PBZ. In agreement with our finding, triadimefon (a triazole plant growth regulator) increased CAT activity in Catharanthus roseus [16]. POD activity was significantly induced by PEG treatment, while PBZ treatment slightly changed the effect of PEG on POD. In conclusion, CAT is more active in PBZ-treated plants than in POD, so the lower accumulation of H2O2 in PBZ-treated plants might be correlated to a higher CAT activity.
PPO is responsible for the oxidative browning which accompanies plant senescence, wounding and responses to pathogens [38]. PPO activity and phenolic levels significantly increased in PEG treatment, whereas PBZ treatment prevented the increase of phenolic compounds and PPO activity stimulated by PEG. The present results support the correlation between PPO enzyme and phenol contents. The role of induced leaf PPO activity is concomitant with increases in total phenolic content during drought stress [38]. Phenols are very important plant constituents because of their scavenging ability, which is mainly due to their hydroxyl groups [15]. PPO overexpression increases photo-oxidative damage during drought stress, but its suppression had an increase in stress tolerance in tomato [38]. According to our finding, a linear relationship between the H2O2 content and phenolic compounds in PEG and PBZ treatments supports the tendency of the phenolic compounds and antioxidant activity in S. rebaudiana.
The ascorbate–glutathione cycle has been shown to be of great importance in multiple stress reactions [8]. Since APX and GR are key enzymes of the ascorbate–glutathione cycle [28], this pathway could be a potential mechanism for the adaptation of S. rebaudiana to PEG treatment. The present analyses demonstrated critical differences in response to PEG between APX and GR activities. APX is involved in scavenging of H2O2 in the ascorbate–glutathione cycle and utilizes ascorbic acid as the electron donor to produce DHA [28]. PEG treatment increased APX activity which was followed by an increased ascorbate pool. Comparing ASC and DHA contents, S. rebaudiana seemingly prefers to accumulate DHA rather than ASC. In contrast with PEG, PBZ treatment alone had no significant effect on the APX activity and the ascorbate pool. From our results, it can be deduced that the ascorbate pool acts as an effective ROS scavenger in S. rebaudiana. It should be noticed that PBZ treatment was not effective to limit the effect of PEG on the ascorbate pool and APX activity.
The total glutathione and GSH are stable under PEG and/or PBZ treatment, but a significant reduction was observed in GSSG under PBZ treatment. Zhang and Kirkham [44] reported that drought stress decreased GSSG level in Sorghum. The activity of GR significantly decreased in PEG treatment. This was also observed in drought-sensitive Lycopersicon esculentum plants under drought stress, whereas GR activity significantly increased in drought-tolerant Lycopersicon peruvianum [40]. From our results, a correlation between GSSG and GR might be inferred. The reduction of GR activity in PEG-treated plants was not followed by any change in GSSG accumulation. The GSSG content in PBZ-treated plants decreased because GR activity was constant and consumed GSSG. Comparison of two maize cultivars differing in drought resistance showed that the more tolerant cultivar had a twofold higher GR activity when leaf discs were exposed to H2O2 and paraquat [29]. The results show that the reduction in GSSG had no effect on the glutathione pool because Stevia plants would rather accumulate GSH almost 100 times more than GSSG. In sunflower, GSSG was not generally detectable [44]. DHA and GSH are the two major water soluble antioxidants which scavenge ROS to maintain the integrity of cell structures and the proper functions of various metabolic pathways [19]. In summary, the correlation between the ascorbate and glutathione pools cannot be easily explained, and further research is warranted to explain some of our results. As mentioned before, S. rebaudiana seemingly prefers to accumulate DHA and GSH. At this moment, it is not known why the accumulation of ascorbate and glutathione did not follow the same trend in S. rebaudiana.
In summary, the present study provided data that S. rebaudiana mobilized APX, CAT, PPO and POD enzymes together with the ascorbate pool to response to oxidative stress. Furthermore, under these stresses, Stevia accumulated higher levels of phenolic compounds to scavenge free radicals. PBZ reduced the negative effect of PEG treatment by limiting SOD activity and H2O2 accumulation and increasing CAT activity. PBZ also decreased the effect of PEG on PPO and phenols. The interaction between the production and the scavenging of ROS maintains the plants in a relatively stable state. It should be noticed that the described results in this investigation are limited to in vitro culture, and more research on PEG treatment of plants growing in pots is warranted to draw a more reasonable conclusion. Our results reported in the present study also provide knowledge that could be applied for improvement of drought tolerance in Stevia using biotechnological approaches.
Abbreviations
- ASC:
-
Ascorbate
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DHA:
-
Dehydroascorbate
- EDTA:
-
Ethylenediaminetetraacetic acid
- POD:
-
Peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- MS:
-
Murashige and Skoog
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NBT:
-
Nitroblue tetrazolium
- PAGE:
-
Polyacrylamide gel electrophoresis
- PBZ:
-
Paclobutrazol
- PEG:
-
Polyethylene glycol
- PPO:
-
Polyphenol oxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TEMED:
-
N,N,N′,N′-tetramethylethylenediamine
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Asada, K., & Takahashi, M. (1987). Production and scavenging of active oxygen in photosynthesis. In D. J. Kyle, B. Osmond, & C. J. Arntzen (Eds.), Photoinhibition. pp. 227–287. Amsterdam: Elsevier.
Asare-Boamah, N. K., Hofstra, G., Fletcher, R. A., & Dumbroff, E. B. (1986). Triadimefon protect bean plants from water stress through its effect on abscisic acid. Plant and Cell Physiology, 27, 383–390.
Bartosz, G. (1997). Oxidative stress in plants. Acta Physiologiae Plantarum, 19, 47–64.
Basu, S., Roychoudhury, A., Saha, P. P., & Sengupta, D. N. (2010). Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regulation, 60, 51–59.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Bradford, M. (1976). A rapid and sensitive method for the quantification of microgram quantities in utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 284–254.
Drazkiewicz, M., Skorzynska-Polit, E., & Krupa, Z. (2003). Response of the ascorbate–glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Science, 164, 195–202.
Flurkey, W. H., & Jen, J. J. (1980). Purification of peach polyphenoloxidase in the presence of added protease inhibitors. Journal of Food Biochemistry, 4, 29–41.
Foyer, C. H., & Halliwell, B. (1976). The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta, 133, 21–25.
Geuns, M. C. J. (2003). Molecules of interest: stevioside. Phytochemistry, 64, 913–921.
Griffith, O. W. (1985). Glutathione and glutathione disulfide. In H. U. Bergmeyer (Ed.), Methods of enzymatic analysis (pp. 521–529). Weinheim: Verlagsgesellschaft mhH.
Hajihashemi, S., & Ehsanpour, A. A. (2013). Influence of exogenously applied paclobutrazol on some physiological traits and growth of Stevia rebaudiana under in vitro drought stress. Biologia, 68, 1–7.
Hajihashemi, S., Geuns, J.M.C. Radical scavenging activity of steviol glycosides, steviol glucuronide, hydroxytyrosol, metformin, aspirin and leaf extract of Stevia rebaudiana. Free Radicals Antioxidant. In Press, 2014.
Hatano, T., Edamatsu, R., Mori, A., Fujita, Y., & Yasuhara, E. (1989). Effect of interaction of tannins with co-existing substances VI. Effects of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chemical Pharmaceutical Bulletin, 37, 2016–2021.
Jaleel, C. A., Gopi, R., Alagu Lakshmanan, G. M., & Panneerselvam, R. (2006). Triadimefon induced changes in the antioxidant metabolism and ajmalicine production in Catharanthus roseus (L.) G. Don. Plant Science, 171, 271–276.
Kampfenkel, K., Van Montagu, M., & Inzé, D. (1995). Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochemistry, 225, 165–167.
Kim, I. S., Yang, M., Lee, O. H., & Kang, S. N. (2011). The antioxidant activity and the bioactive compounds content of Stevia rebaudiana water extracts. Food Science and Technology, 44, 1328–1332.
Kocsy, G., Galiba, G., & Brunold, C. (2001). Role of glutathione in adaptation and signaling during chilling and cold acclimation in plants. Plant Physiology, 113, 158–164.
Kraus, T. E., & Fletcher, R. A. (1994). Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxification of active oxygen involved? Plant and Cell Physiology, 35, 45–52.
Lemus-Mondaca, R., Vega-Galvez, A., Zura-Bravo, L., & Ah-Hen, K. (2012). Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical, nutritional and functional aspects. Food Chemistry, 132, 1121–1132.
Marshall, J. G., Rutledge, R. G., Blumwald, E., & Dumbroff, E. (2000). Reduction in turgid water volume in jack pine, white spruce and black spruce in response to drought and paclobutrazol. Tree Physiology, 20, 701–707.
Marshall, J. G., Scarrat, J. B., & Dumbroff, E. B. (1991). Induction of drought resistance by abscisic acid and paclobutrazol in jack pine. Tree Physiology, 8, 415–421.
McKersie, B. D., Bowley, S. R., & Jones, K. S. (1999). Winter survival of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiology, 119, 839–848.
Michel, B. E., & Kaufmann, M. R. (1973). The osmotic potential of polyethylene glycol 6000. Plant Physiology, 51, 194–196.
Moller, I. M., Jensen, P. E., & Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annual Review of Plant Biology, 58, 459–481.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology, 15, 473–479.
Noctor, G., & Foyer, C. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology, 49, 249–279.
Pastori, G. M., & Trippi, V. S. (1993). Cross resistance between water and oxidative stresses in wheat leaves. Journal of Agricultural Science, 120, 289–294.
Patade, V. Y., Bhargava, S., & Suprasanna, P. (2012). Effects of NaCl and iso-osmotic PEG stress on growth, osmolytes accumulation and antioxidant defense in cultured sugarcane cells. Plant Cell, Tissue and Organ. Culture, 108, 279–286.
Plewa, M. J., Smith, S. R., & Wanger, E. D. (1991). Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutation Research, 247, 57–64.
Sakthivelu, G., Devi, M. K. A., Giridhar, P., Rajasekaran, T., Ravishankar, G. A., Nedev, T., et al. (2008). Drought-induced alterations in growth, osmotic potential and in vitro regeneration of soybean cultivars. General and Applied Plant Physiology, 34(1–2), 103–112.
Sen, A., & Alikamanoglu, S. (2011). Effect of salt stress on growth parameters and antioxidant enzymes of different wheat (Triticum aestivum L.) varieties on in vitro tissue culture. Fresenius Environmental Bulletin, 20, 489–495.
Shehab, G. G., Ahmed, O. K., & El-Beltagi, H. S. (2010). Effects of various chemical agents for alleviation of drought stress in rice plants (Oryza sativa L.). Notulae Botanicae Horti Agrobotanici Cluj, 38(1), 139–148.
Shukla, S., Mehta, A., Mehta, P., & Bajpai, V. K. (2012). Antioxidant ability and total phenolic content of aqueous leaf extract of Stevia rebaudiana Bert. Experimental Toxicologic Pathology, 64(7–8), 807–811.
Singleton, V. L., & Rossi, J. R. (1965). Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagent. American Journal of Enology and Viticulture, 16, 144–158.
Szôllôsi, R. (2002). Varga, I.S. Total antioxidant power in some species of Labiatae (Adaptation of FRAP method). Proceedings of the 7th Hungarian Congress on Plant Physiology. Acta Biologica Szegediensis, 46, 125–127.
Thipyapong, P., Kelkonian, J., Wolfe, D. W., & Steffens, J. C. (2004). Suppression of polyphenol oxidases increases stress tolerance in tomato. Plant Science, 167, 693–703.
Torres-Franklin, M. L., Contour-Ansel, D., Zuily-Fodil, Y., & Pham-Thi, A. T. (2008). Molecular cloning of glutathione reductase cDNAs and analysis of GR gene expression in cowpea and common bean leaves during recovery from moderate drought stress. Journal of Plant Physiology, 165, 514–521.
Ünyayar, S., Keleş, Y., & Çekiç, F. Ö. (2005). The antioxidative response of two tomato species with different drought tolerances as a result of drought and cadmium stress combinations. Plant, Soil and Environment, 51, 57–64.
Velikova, V., Yordanov, I., & Edrava, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Science, 151, 59–66.
Veljovic-Jovanovic, S., Kukavica, B., Stevanovic, B., & Navari-Izzo, F. (2006). Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. Journal of Experimental Botany, 57, 1759–1768.
Xu, X. Y., Shi, G. X., Wang, J., Zhang, L. L., & Kang, Y. N. (2011). Copper-induced oxidative stress in Alternanthera philoxeroides callus. Plant Cell, Tissue and Organ. Culture, 106, 243–251.
Zhang, J., & Kirkham, M. B. (1996). Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist, 132, 361–373.
Zhu, L. H., Peppal, A., Li, X. Y., & Welander, M. (2004). Changes of leaf water potential and endogenous cytokinins in young apple trees treated with or without paclobutrazol under drought conditions. Scientia Horticulturae, 99, 133–141.
Živković, S., Popović, M., Dragišić-Maksimović, J., Momčilović, I., & Grubišić, D. (2010). Dehydration-related changes of peroxidase and polyphenol oxidase activity in fronds of the resurrection fern Asplenium ceterach L. Archives of Biological Science Belgrade, 62(4), 1071–1081.
Acknowledgments
The authors wish to thank the University of Isfahan and the Plant Stress Center of Excellence (PSCE) for their support and Stijn Ceunen for the help with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajihashemi, S., Ehsanpour, A.A. Antioxidant Response of Stevia rebaudiana B. to Polyethylene Glycol and Paclobutrazol Treatments Under In Vitro Culture. Appl Biochem Biotechnol 172, 4038–4052 (2014). https://doi.org/10.1007/s12010-014-0791-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0791-8