Abstract
Spent coffee ground (SCG), a present waste stream from instant coffee production, represents a potential feedstock for mannooligosaccharides (MOS) production. MOS can be used in nutraceutical products for humans/animals or added to instant coffee, increasing process yield and improving product health properties. The SCG was evaluated for MOS production by steam pretreatment and enzymatic hydrolysis with a recombinant mannanase and a commercial cellulase cocktail (Acremonium, Bioshigen Co. Ltd, Japan). The mannanase was produced using a recombinant strain of Yarrowia lipolytica, used to produce and secrete endo-1,4-β,d-mannanase from Aspergillus aculeatus in bioreactor cultures. Endo-1,4-β,d-mannanase was produced with an activity of 183.5 U/mL and 0.23 mg protein/mL. The enzyme had an optimum temperature of 80 °C, and the activity in the supernatant was improved by 150 % by supplementation with 0.2 % sodium benzoate and 35 % sorbitol as a preservative and stabiliser, respectively. The steam pretreatment of SCG improved the enzymatic digestibility of SCG, thus reducing the required enzyme dosage for MOS release. Combined enzymatic hydrolysis of untreated or steam-pretreated (150, 190 and 200 °C for 10 min) SCG with mannanase and cellulase cocktail resulted in 36–57 % (based on mannan content) of MOS production with a degree of polymerization of up to 6. The untreated material required at least 1 % of both mannanase and cellulase loading. The optimum mannanase and cellulase loadings for pretreated SCG hydrolysis were between 0.3 and 1 and 0.4 and 0.8 %, respectively. Statistical analysis suggested additive effect between cellulase cocktail and mannanase on MOS release, with no indication of synergism observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of prebiotic products with potential benefits for immunity seems to be a promising alternative as growth promoter in animal feed, as well as a component of nutraceutical supplements for humans. Prebiotic compounds are nondigestible carbohydrates that stimulate the growth and/or activity of beneficial bacteria in the digestive system. Oligosaccharides with a degree of polymerization (DP) between 2 and 12 has been attributed to have prebiotic effect [1]; some probiotic microorganisms such as Bifidubacterias are grown preferentially using low DP oligomers (DP 2 and 3) [2]. Several prebiotics have been studied, including frutooligosaccharides [3, 4], xylooligosaccharides [5, 6], galactooligosaccharides [7] and mannooligosaccharides (MOS) [8–11]. According to this property, oligosaccharides are widely applied in animal feed to improve gastrointestinal health, growth performance, reduce hepatosomatic index and modulate the intestinal microbiota [12–15]. Such prebiotics could replace antibiotics as a growth promoter in livestock nutrition, where the misuse of antibiotics for nontherapeutic purposes has arisen to increase the resistant microorganism [16], resulting in its prohibition in EU [17].
MOS can be extracted from the SCG by means of enzymatic hydrolysis of the mannan polysaccharides. Mannan polysaccharides can be found in the structure of spent coffee ground (SCG), a residue generated in the production of instant coffee, with an estimated worldwide annual production of 6 million tons [18]. Mannan in green coffee beans is water insoluble and showed a crystalline structure, corresponding approximately 20–30 % of dry weight of SCG [19]. These polysaccharides comprise a backbone containing mannose (homo-polymer) or a combination of glucose and mannose residues (hetero-polymer, glucomannan) that are linked by β-1,4-linkages [20, 21]. The backbone can be substituted by α-d-galactopyranosyl (1,6-α linked) and O-acetyl groups at C-2 and C-3 positions [22]. The complex structure of mannan polysaccharides requires a mixture of enzymes for the extensive hydrolysis required for MOS production. The main enzyme involved in MOS release from SCG is endo-β-1,4-mannanase (β-mannanase; 1,4-β-d-mannanmannohydrolase, EC 3.2.1.78), which has an endo-action on the polysaccharides, thus cleaving the backbone at random locations to release MOS from galactomannan, glucomannan, galactoglucomannan and mannan [23, 24].
The structural organisation of mannan and cellulose is likely to impact on SCG recalcitrance and hence in the MOS production by enzymatic hydrolysis. The SCG carbohydrate fraction are composed of galactomannan, arabinogalactan and cellulose structures [23, 25], suggesting a structure of galactomannan–cellulose linked in the carbohydrate fraction of SCG [26]. Cellulose is composed of β-1,4-linked glucose residue forming a linear structure characterised by crystalline and amorphous regions based on intra- and intermolecular hydrogen bonds [27]. In addition to the intrinsic characteristic of each polysaccharide, the structural organisation between mannan and cellulose can play an important effect on the material recalcitrance. The application of a pretreatment to disrupt this structure could improve MOS release by enzymatic hydrolysis, as well as reduce the enzyme requirements.
MOS can be obtained from industrial residues such as SCG, which is attractive because of its abundance and high mannan content. In this context, the main aim of this work was to evaluate the production of MOS by a combination of mannanase and cellulase for hydrolysis of untreated and steam-pretreated SCG. Mannanase and cellulase cocktail loadings were evaluated for improvement of MOS release, taking into account the biochemical properties of the heterologous mannanase produced by recombinant Yarrowia lipolytica.
Methodology
Chemicals
All chemicals, media components and supplements were of analytical-grade standard. Cellulase was purchased from Acremonium (Bioshigen Co.Ltd - China) and MOS standards from Megazyme (Ireland). Acremonium cellulase cocktail was purchased from Bioshigen Co. Ltd (Japan). Lignin and carbohydrate contents were determined based on NREL standard procedures [28]. Monosaccharide quantification (glucose, mannose, galactose, arabinose and xylose) from untreated and pretreated SCG was carried out by high-performance liquid chromatography (HPLC) in a Dionex Ultimate 3000 system using an Aminex HPX-87P column.
Substrate
The SCG was supplied by National Brands Limited (Isando, South Africa). The SCG, with an initial moisture content of 75 %, was pressed to remove the excess water. The resulting cake (50 % dry matter (DM); w/w) was dried at 30 °C up to 90 % DM and stored in air-tight containers at 4 °C until use.
Inoculum and Culture Medium for β-mannanase Production
The development of the Y. lipolytica ManA/HmA strain for expression of the endo-1,4-β,d-mannanase from Aspergillus aculeatus was reported elsewhere [29] and was obtained from CSIR Biosciences (Pretoria, South Africa). Conical flasks (2 L) containing 200 mL medium consisting of 15 g/L yeast extract, 8.9 g/L malt extract and 6.67 g/L glucose (pH 5.5) were inoculated with the Y. lipolytica ManA/HmA and incubated on a rotary shaker at 220 rpm for 18 h. The 200-mL culture from the flask was then used to inoculate 10 L of medium in the NBS BioFlo (14 L) fermenters. The fermentation medium consisted of the following: 20 g/L yeast extract and 40 g/L glucose. The fermentation was run at 28 °C with an initial stirrer speed of 200 rpm. The pH was maintained at 6.8 with either 20 % (v/v) (NH4)OH or 25 % (v/v) H2SO4. The airflow rate was set at 1.0 (v/v) for the NBS BioFlo fermenters. The pO2 was controlled at 30 % by cascading to the agitation speed. Samples (2 mL) were taken every 3 h and centrifuged, and the supernatant was analysed for enzyme activity. The pellet was used for dry cell weight determination by drying (overnight) to a constant weight at 110 °C. Supernatant from the Y. lipolytica fermentation was purified and concentrated by ultrafiltration (Millipore) using a 0.22-μm and 5 kDa cut-off membranes, respectively.
Enzyme Assays and Protein Determination
Mannanase activity was determined using the DNS method [30]: 50 μL β-mannanase solution diluted in 50 mM sodium citrate buffer (pH 4.8) was mixed with 450 μL of 0.25 % locust bean gum at 60 °C for 5 min. The hydrolysis was terminated after 5 min by adding 750 μL DNS solution (1 % 3,5-dinitro-salicyclic acid, 20 % potassium sodium tartrate, 1 % NaOH, 0.2 % phenol and 0.05 % Na2SO3). The reactions were boiled at 100 °C in a water bath for 5 min, and colour development in cooled samples was measured as absorbance at 540 nm. The reducing sugars released at 5 min were determined from standard curves, which were plotted using mannose as standard. One unit (U) of enzyme activity is defined as the amount of enzyme producing 1 μmol of reducing sugars (mannose)/min under the given conditions.
The enzyme activity of commercial cellulase (Acremonium, Bioshigen Co. Ltd, Japan) was measured in a similar procedure but using 1 % carboxymethyl–cellulose or beech wood xylan as substrate for endo-glucanase and endo-xylanase activities determination, respectively. Additionally, cellulase activity in terms of filter paper units (FPU) was determined according to IUPAC [31]. Protein concentration in mannanase and cellulase cocktail was carried out by BCA method using bovine serum albumin as protein standard (Kit BCA-Compact-Able Protein Assay kit, ref 23229, Pierce, Rockford, IL).
Effect of Temperature and Various Reagents on β-mannanase Activity
The effect of temperature on β-mannanase activity was determined by incubating the purified enzyme with the substrate at temperatures ranging from 30 to 90 °C in 50 mM citrate buffer at pH 4.8 for 30 min. Thermal stability of the enzyme was determined by assaying for residual enzyme activity after incubation. The assays were performed in duplicates, and average values of residual activity are shown. The standard deviation was between 1.5 and 10 %.
The effect of stabilising/preservative reagents on the thermal stability of β-mannanase was determined by assaying for residual activity after incubating the enzyme with reagents dissolved in 50 mM sodium citrate buffer (pH 4.8). β-mannanase solution was incubated in a 0.2 % (w/v) solution of sodium benzoate or potassium sorbate with either 30 to 40 % sorbitol or 30 to 40 % glycerol from 30 to 80 °C, according to a previous optimization study [32]. Samples of the enzyme were collected every 30 min to measure the residual enzymatic activity.
Steam Explosion Pretreatment
The steam explosion pretreatment was performed at 150 °C/10 min, 190 °C/12 min and 200 °C/10 min. For each batch, about 700 g (dry weight) was weighed and fed into a 19-L pressure reactor (“steam gun”); saturated steam at 30 bar was then injected into the pressure reactor, and the temperature of the vessel was controlled through manipulation of the vessel pressure by means of a steam injection control valve. Following injection of steam, the pressure reactor took approximately 2 min to heat up, upon which the timing of the pretreatment commenced. At the end of the residence time, an automatic ball valve capable of opening within less than 0.5 s was automatically opened. Subsequently, an explosive expansion of the steam occurred, and exploded samples were collected in a cyclone-type vessel with the excess steam escaping to the atmosphere. After pressing, the liquor fraction was analysed for MOS content. The solid fraction was thoroughly washed with deionised water and vacuum filtered to remove water-soluble solids, which was also considered for total MOS production. The pretreated material, water-insoluble solids (WIS), was dried at 40 °C for 3 days and stocked until use in enzymatic hydrolysis.
Enzymatic Hydrolysis of Spent Coffee Ground
About 2 g of dried untreated or steam-pretreated SCG was pre-wetted at 60 °C for 5 h with agitation using 100 mL sodium citrate buffer (50 mM, pH 4.8), resulting in a solid loading of 2 % (w/v). Enzymes were added for hydrolysis with dosages according to protein weights (in milligrammes protein) and/or enzyme activity. Sodium azide (0.02 %) was added to enzymatic hydrolysis reactions to prevent microbial contamination. SCG was hydrolysed by incubation with agitation of 100 rpm at temperatures of 60 °C for 18 h. Supernatant was filtered through filter papers (Whatman paper No. 1) to separate the spent ground from the liquid fraction (filtrated). The filtrate was subjected to protein precipitation by addition of perchloric acid and KOH and filtered by a 0.22-μm filter prior to HPLC analysis. The samples were analysed for MOS content by high-performance anion exchange chromatography using a Dionex Ultimate 3000 Series chromatograph with pulsed amperometric detection (Coulochem III electrochemical detector) and a CarboPac PA1 column (4 × 250 mm and precolumn 4 × 50 mm). Samples (10 μL) were eluted at 1 mL/min using the following sodium acetate (NaOAc) gradient in 100 mM NaOH: 0–2.5 min, 100 mM NaOH; 2.5–23 min, slow linear gradient of 0–50 mM NaOAc; 23–23.1 min sharp gradient 50–500 mM NaOAc; 26–26.1 min, sharp down gradient 500–0 mM NaOAc; and 26.1–34 min, 100 mM NaOH. Enzymatic hydrolysis yield was reported based on untreated and pretreated substrate used on reaction (g/100 g WIS) and based on mannan content (in per cent).
Statistical Analysis of Enzymatic Hydrolysis
An experimental design with two-level, two-variable, mannanase (X 1) and cellulase (X 2) loadings was applied to determine the best combination of enzymes for the MOS release from pretreated and untreated SCG. Three assays at the centre point of the experimental design were carried out to estimate the random error for the analysis of variance and to check the presence of curvature in the response surface. The total MOS yield (in per cent; g/100 g WIS) was taken as dependent variable or response of the experimental design. STATISTICA (Version 10) was used for regression and graphical analyses of the data obtained. The fitness of the polynomial model equation was expressed by the coefficient of determination R 2, and its statistical significance was checked by probability (p) of 0.1 and 0.05 (90 and 95 % of confidence, respectively).
Results and Discussion
Enzyme Production and Thermal Stability
The maximum biomass concentration in cultures of recombinant Y. lipolytica was observed at 24 h, while glucose depletion occurred after 48 h (Fig. 1). The supernatant was harvested after 48 h and filtered (0.22 μm), with the latter having an endo-mannanase (β-mannanase) activity of 183.5 UI/mL (0.23 g protein/mL). After concentration/washing of the enzyme using a 5-kDa membrane, the endo-mannanase activity was increased to 930.9 UI/mL. The enzymatic activities of commercial cellulase cocktail (Acremonium, Bioshigen Co. Ltd, Japan) were: endo-glucanase 770 UI/g, mannanase 86.4 UI/g and xylanase 30.6 UI/g of powder and 350 FPU/g, with 80 % of protein content in the powder extract.
The activity of the purified endo-mannanase was measured at various temperatures, from 30 to 100 °C for 5 min, according to the assay described elsewhere. The maximum activity was found at 80 °C (Fig. 2), indicating that the enzyme is thermoactive and similar temperature optima were previously reported for other fungal mannanases. Endo-1,4-β-mannanase from Sclerotium rolfsii has been reported to have optima temperature range of 72 to 74 °C [33]. The optimum temperature of mannanases from A. aculeatus was 75 °C, while mannanase from Thermotoga neapolitana displayed a higher activity at 92 °C [34, 35]. Overall, most mannanases have been reported to be active at pH 5–7 and temperature between 30 and 60 °C [36].
Effect of Additives on the Mannanase Activity
Enzyme processes are generally more productive when applying high temperatures that favour higher conversion rates. Although the maximum mannanase activity was displayed at 80 °C (Fig. 2), the experimental assay used to determine optimum temperature for activity was determined for short periods of time (5 min). However, the enzyme may be deactivated when operating at longer incubation periods required in industrial processes. The addition of preservatives in enzyme preparations and during the industrial process is a common approach to preserve enzyme stability. Furthermore, the addition of stabilisers could be of benefit for reducing enzyme dosage and enzyme recycling [36]. Thermal stability of the purified mannanase enzyme was therefore determined at 50, 60, 70 and 80 °C, in the presence of various potential stabilisers/preservatives (glycerol, potassium sorbate/sodium benzoate and sorbitol) that would typically be added to industrial enzyme products (Figs. 3 and 4). Results are expressed in terms of relative mannanase activity calculated as percentage of the activity determined in the control enzyme solution with no additives.
Influence of stabilisers on activity of purified mannanase. Enzyme solution containing stabiliser (as indicated) was assayed by the standard method. The mixtures were incubated for 30 min at 60 °C in 50 mM sodium citrate buffer, pH 4.8. The relative activity was expressed as percentage of the original activity of enzyme (without stabiliser)
a Stability of purified mannanase in sodium benzoate mixtures incubated at 60 °C for 240 min. b Stability of purified mannanase in potassium sorbate mixtures incubated at 60 °C for 240 min. c Stability of purified mannanase in sodium benzoate mixtures incubated at 80 °C for 240 min. d Stability of purified mannanase in potassium sorbate mixtures incubated at 80 °C for 240 min. The residual activity in each mixture was measured every 30 min in 50 mM sodium citrate buffer, pH 4.8. The relative activity expressed as percentage of the original activity of enzyme (without stabiliser) at the beginning of the incubation
The residual activities of endo-mannanase at various concentrations of additives (30 to 40 % glycerol, 30 to 40 % sorbitol, 0.2 % potassium sorbate and 0.2 % sodium benzoate) were determined at 60 °C for an incubation period of 30 min (Fig. 3). This temperature was selected based on the optimum temperature for Acremonium cellulase which will be combined with mannanase in the hydrolysis reactions of SCG. As shown in Fig. 3, only 40 % sorbitol did not decrease the mannanase activity, whereas the rest of the additives exerted deactivation effects. The performance of 40 % sorbitol could be attributed to the stabilisation effects of the enzyme structure benefiting the commercial extract preparation for stocking. While some researchers have shown that sorbitol may improve the stability of enzymes [37], the additive might also have negative influence on other proteins if added at higher concentration [38]. The fact that sorbitol did not deactivate the endo-mannanase activity motivated new experiments combining additives for stabilisation effects at a wider range of temperatures and longer incubation periods.
Several works have shown that a combination of additives in some cases can improve the activity much better than a single additive [32, 37]. The mannanase activity was evaluated in the presence of combined additives. Six different combinations of additives were obtained using 0.2 % sodium benzoate, 30 to 40 % glycerol and 30 to 40 % sorbitol. As shown in Fig. 4a, the combination of mannanase with 0.2 % sodium benzoate and 30 or 35 % sorbitol presented the highest relative activity of about 150 % when incubated at 60 °C for 4 h, while the control retained 63 % of the initial activity. The enhancement in mannanase activity could be explained as the synergistic effect between the two additives, which finally led to stable enzyme structure. Based on these results, it could be observed that the combination of additive agents have better preservative effect on the enzyme activity than the single-additive agents because more stabilisation is promoted in the enzyme structure [32].
In terms of combining stabilisers/preservatives, substituting sodium benzoate with potassium sorbate strongly enhanced the enzyme activity at 60 °C (Fig. 4b). The relative activity after 4 h was 180, 200 and 220 % with 0.2 % potassium sorbate when combined with sorbitol at 30 or 35 % or with 35 % of glycerol, respectively. This result might certainly indicate that the improvements of mannanase activity are directly related with increasing amounts of sorbate ions. When increasing the incubation temperature to 80 °C (Fig. 4, d), the mannanase activity was strongly reduced regardless of the stabiliser combination. This result suggests that the effect of stabilisers is only adequate at reasonable temperatures and unable to protect the enzyme in very harsh temperature conditions.
Kinetics of Thermal Deactivation of Mannanase in the Presence of Stabilisers
The experimental profiles (not shown) achieved for the residual activity of endo-mannanase after incubation for 4 h were fitted to the first-order Arrhenius model (A) t /A o = exp(−k t ) for the calculation of the deactivation constant (k d) and half-life (t 1/2). Table 1 summarises the values obtained for the endo-mannanases in the presence of various stabilisers at different temperatures including 50, 60 and 70 °C. At 50 °C, endo-mannanase in the presence of the stabilisers showed similar deactivation profiles to that of the control (mannanase without stabiliser) with estimated half-life of 2,310 min. The stabilisers slightly increased the half-life of endo-mannanase at 70 °C. The more pronounced effect was observed at 60 °C, where the half-life for the stabilised and control were 1,732.5 and 533.1 min, respectively.
Spent Coffee Ground Steam Pretreatment
The steam pretreatment was performed at temperature similar of instant coffee extraction (150 °C) and temperatures over this industrial range (190 and 200 °C). The aim of this pretreatment was to increase the enzymatic digestibility of the SCG, a recalcitrant material. The insoluble solids recovery and the carbohydrates composition of the pretreated solids from the different conditions are listed in Table 2. It can be observed that the recovery of pretreated solids was similar among the three pretreatment conditions, ranging from 90 to 95 %. Regarding the carbohydrates content, only the arabinan and galactan contents were reduced in the pretreated solids with respect to the untreated material for the harshest conditions tested.
Although steam pretreatment solubilised 5 to 10 % of the SCG (Table 2), no MOS were detected in the liquid fraction (including wash water) after any of the pretreatment conditions studied. No oligomers were detected even when the pretreatment was performed at low temperature, i.e. 150 °C. The temperature of steam treatment (150, 190 and 200 °C) probably caused degradation of oligomers in the liquid fraction, which can also be associated with longer reaction time. It has been reported that the increase of pretreatment temperature release preferentially low DP oligomers and monosaccharides from lignocellulosic materials [39]. According to Carvalheiro et al. [40], for oligomer release, the steam pretreatment should be conducted from seconds to a few minutes. However, depending also on the materials used, oligomers were released by steam explosion of wood chip at 180 °C/10 min [39] and at 175 °C for 7.5 min [41]. Early studies showed oligomers are released from SCG using a thermal plug flow reactor catalysed with acid (1 %) during seconds to 8 min [42]. However, the use of steam explosion for oligomer production from SCG has received little attention.
Enzymatic Hydrolysis of Pretreated SCG
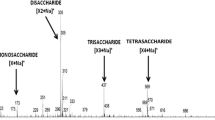
Both untreated and steam-pretreated SCG were subjected to enzymatic hydrolysis with combinations of mannanase and cellulase to evaluate if the pretreatment favours oligomers release by means of enzymes. Enzymatic hydrolysis yield was reported based on untreated and pretreated substrate used on reaction (g/100 g WIS) and showed on tables based on mannan content (in per cent). The DP of the MOS released is an important factor to consider, as it is the determinant in the beneficial health effects or in the stimulation of the probiotic microorganisms [43]. For example, Bifidubacterias are grown preferentially using low DP oligomers, such as DP 2 and 3 [2]. Oligomers with DP of more than 6 were not observed in the chromatograms (data not shown). The enzymatic hydrolysis of untreated SCG released MOS with a DP of up to 6. The main oligosaccharide present in the supernatant was with a DP of 2, followed by DP of 3, 4 and 5 (Table 3). MOS with a DP of 6 was found only in the hydrolysis of untreated SCG, whereas oligomers with a DP of 5 was found in lower percentage (<2.7 g/100 g WIS) in the hydrolysis of untreated and steam-pretreated SCG. The pretreatment temperature increases improved the material for enzymatic hydrolysis related to the amount of MOS with a DP of 5. MOS with a DP of 6 was not found from enzymatic hydrolysis of pretreated materials. Moreover, MOS with a DP of 5 was found on hydrolysis mainly of the pretreated material at 190 °C. The release of MOS with a DP of 5 was only observed at low enzyme loading; it was not detected when applying intermediate and higher loading of enzymes. Oligomers with a DP of 3 and 4 were not influenced by mannanase and cellulase loadings, allowing maximisation of the MOS yield from SCG hydrolysis within the studied range.
The steam pretreatment generated a SCG substrate that was more susceptible to enzymatic hydrolysis for MOS release, compared with untreated SCG. The enzymatic hydrolysis of untreated SCG released a total of 8.76 % (g/100 g WIS–36.24 % based on mannan content) of MOS (DP of 2 to 6) applying 1 % loadings of mannanase and cellulase (Table 4). The enzymatic hydrolysis of SCG pretreated at 150 °C for 10 min released 10.1 % (g/100 g WIS–45.21 % based on mannan content) of MOS when applying the same loadings (1 %) of mannanase and cellulase.
The steam pretreatment was also effective in decreasing the loading of needed enzymes for MOS release. The enzymatic hydrolysis of pretreated SCG at 190 and 200 °C released 10.45 and 12.46 % (g/100 g WIS–47.44 and 57.79 % based on mannan content, respectively) of MOS, respectively, applying intermediate loading of enzymes (0.51 % for both mannanase and cellulase) (Table 3). A higher pretreatment temperature increased MOS yield only by 2 %. However, this yield was reached by half amounts of enzymes required to release the same amounts of MOS from SCG without or with lower temperature treatments. The SCG is generated after thermal treatments (roasting and thermal extraction) where there is a partial degradation and depolymerisation of polysaccharides [25], and partial extraction of arabinogalactan proteins [43], compared with the unprocessed green beans. However, further modification of the SCG structure by steam explosion pretreatment was effective to improve accessibility of the substrate to enzymatic hydrolysis.
Statistical Analysis of Enzymatic Hydrolysis
A statistical analysis of enzymatic hydrolysis of untreated and steam-pretreated SCG was performed, to determine optimum enzyme loadings for MOS release. The effect of mannanase and cellulase loadings on the enzymatic hydrolysis of SCG for MOS release was evaluated by Pareto charts (Fig. 5), where the bars length beyond the vertical line correspond to effects statistically significant at 90 % confidence level.
The variable cellulase was positive and significant for enzymatic hydrolysis of untreated (Fig. 5a) and pretreated SCG (Fig. 5b–d). The effect of enzymes was more pronounced by increasing the temperature of the pretreatment. However, the Pareto of the 190 °C does not follow this trend, and the effect of the enzymes is even lower than the pretreatment at 150 °C. The variable mannanase was positively significant only in the hydrolysis of steam-pretreated SCG at 200 °C for 10 min (Fig. 5d). The significance of mannanase at highest pretreatment condition shows the effect of steam explosion in exposing the polysaccharides.
The cellulase cocktail showed composition of cellulase enzymes (endoglucanase, exo-glucanase and β-glucosidase) and lower mannanase. The lower content of mannanase associated to higher cellulase enzymes activities was beneficial for MOS release. However, application of a single enzyme, mannanase or cellulase cocktail, generated lower yield in MOS than when applied both enzymes (Table 4; assays 8 to 13). For SCG untreated and pretreated at 150 °C for 10 min, the intermediate loading of mannanase or cellulase (0.51 %) was enough for reaching the highest yield of MOS (Table 4; assay 9 compared with assays 8 and 10, lower and higher loading, respectively). The hydrolysis of SCG pretreated at 190 °C/10 min and 200 °C/10 min with single enzyme showed better hydrolysis at higher level of mannanase or cellulase cocktail (Table 4; assays 10 and 13, respectively), although the increase in the enzyme loading did not justify the improved yield.
As central point condition (0.51 % mannanase and cellulase cocktail) showed a higher yield of MOS release from enzymatic hydrolysis of SCG pretreated at 190 °C/10 min and 200 °C/10 min, a full-factorial design was evaluated to determine the best combination of mannanase and cellulase cocktail for MOS release. Moreover, curvature analysis was significant for enzymatic hydrolysis of SCG pretreated at 150 °C/10 min, 190 °C/10 min and 200 °C/10 min, indicating that a second-order model could fit the experimental data better.
Enzymatic hydrolysis of untreated SCG indicated only linear terms and interaction between variables that were significant in the model (Table 5). This result confirmed the observation of no significant curvature from statistical analysis of the standard design (Fig. 5). For enzymatic hydrolysis of SCG pretreated at 150 °C/10 min, only the linear term for the cellulase loading was significant. These results indicated that higher loading of enzymes would always provide a higher yield. However, the statistical analysis was limited to maximum enzyme dosage of 1 % (in grammes protein per grammes substrate) considering the enzyme cost and typical loadings for industrial enzymes. The model obtained for enzymatic hydrolysis of pretreated SCG at 150 °C/10 min was not reliable because only one variable (cellulase loading) was significant; however, it was used to compare the effect of steam pretreatment of SCG in the enzymatic hydrolysis considering the loading of enzymes used.
The enzymatic hydrolysis of pretreated SCG at 190 °C/10 min and 200 °C/10 min indicated that both the mannanase and cellulase terms in the model were significant. The models for hydrolysis of pretreated SCG at 190 °C/10 min and 200 °C/10 min were significant at 95 and 99 % confidence level with correlation coefficients of 0.83 and 0.96, respectively (Table 5). Considering that the lack of fit was not significant, the models were accepted as representative of the enzymatic hydrolysis of pretreated SCG for comparing the enzyme loading applied.
The response surface of enzymatic hydrolysis of untreated SCG showed a combination of 1 % loading for both mannanase and cellulase to reach the maximum yield (Fig. 6). The surface response for pretreated SCG at 150 °C/10 min showed the best combination of loadings between 0.5 and 1 % for mannanase and between 0.6 and 1 % of cellulase, thus confirming a reduction in the enzyme dosage requirements for pretreated SCG. For pretreated SCG at 190 °C/10 min, the best combination of enzyme was between 0.3 and 0.7 % of mannanase and 0.7 and 1 % of cellulase. For pretreated SCG at 200 °C/10 min, the best combination of enzyme was between 0.8 and 1 % of mannanase and 0.4 and 0.8 % of cellulase. For practical implementation, the ratio between enzymes can be varied within the range proposed by the response surface, to achieve the minimum overall cost of hydrolysis (Fig. 6).
The combination of cellulase cocktail and mannanase showed additive effect, i.e. no interaction was observed (Fig. 5a–c), except for pretreated material at 200 °C/10 min (Fig. 5d). However, the interaction among enzymes at several pretreatments was negative, indicating that the enzyme level should be lower than 1 % to maximise the MOS release from this material. Furthermore, the negative interaction between enzymes for pretreated material at 200 °C/10 min confirms that the higher enzyme loading (1 %) is not the best enzyme combination.
A resume of possible conditions of enzyme combination, models and predicted yields is shown in Table 6. The significance of the variables was determined at least at 90 % confidence level for all the studied cases, except for the pretreated at 150 °C. This was done to minimise the error of determination by considering more variables in the model. The statistical analysis confirmed the effectiveness of steam pretreatment in generating a substrate easily hydrolysable needing lower enzyme loading for MOS release from SCG.
Conclusions
SCG, a residue from instant coffee production, is a source of cellulose and galactomannans, polysaccharides that remain unextractable during the process for instant coffee production. These polysaccharides can be hydrolysed to oligosaccharide molecules, such as MOS, which have potential application as prebiotic products in human and animal feed. The addition of MOS, obtained from SCG, to instant coffee can be suggested, collaborating to a health product and reducing the amount of SCG generated as residue.
The action of endo-mannanase for MOS release from mannan present in SCG could be enhanced by the use of enzyme stabilisers and pretreatment of SCG to improve accessibility. Up to 12 % (g/100 g WIS–55.66 % based on mannan content) of MOS could be released from untreated SCG by enzymatic hydrolysis using 1 % of mannanase and cellulase loading. The steam pretreatment was effective in generating a substrate amenable to enzyme action and allowed the reduction of the mannanase and cellulase cocktail loading while keeping the same yield obtained with higher enzyme loadings for hydrolysis of untreated SCG.
References
Nabarlatz, D., Montané, D., Kardosová, A., Bekesová, S., Hríbalová, V., & Ebringerova, A. (2007). Almond shell xylo-oligosaccharides exhibiting immunostimulatory activity. Carbohydrate Research, 342, 1122–1128.
Okazaki, M., Fujikawa, S., & Matsumoto, N. (1990). Effect of xylooligosaccharides in the growth of bifidobacteria. Bifidobacteria Microflora, 9, 77–86.
Sangeetha, P. T., Ramesh, M. N., & Prapulla, S. G. (2005). Recent trends in the microbial production, analysis and application of fructooligosaccharides. Trends in Food Science and Technology, 16, 442–457.
Patti, L., Cipriano, P., & Rivellese, A. A. (2004). Effects of short-chain fructooligosaccharides on glucose and lipid metabolism in mild hypercholesterolaemic individuals. Clinical Nutrition, 23, 331–340.
Brienzo, M., Carvalho, W., & Milagres, A. M. F. (2010). Xylooligosaccharides production from alkali-pretreated sugarcane bagasse using xylanases from Thermoascus aurantiacus. Applied Biochemistry and Biotechnology, 162, 1195–1205.
Christakopoulos, P., Katapodis, P., Kalogeris, E., Kekos, D., Macris, B. J., Stamatis, H., & Skaltsa, H. (2003). Antimicrobial activity of acidic xylo-oligosaccharides produced by family 10 and 11 endoxylanases. International Journal of Biological Macromolecules, 31, 171–175.
Grisdale-Helland, B., Helland, S. J., & Gatlin, D. M., III. (2008). The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture, 283, 163–167.
White, L. A., Newman, M. C., Cromwell, G. L., & Lindemann, M. D. (2002). Brewers dried yeast as a source of mannan oligosaccharides for weanling pigs. Journal of Animal Science, 80, 2619–2628.
Mourão, J. L., Pinheiro, V., Alves, A., Guedes, C. M., Pinto, L., Saavedra, M. J., Spring, P., & Kocher, A. (2006). Effect of mannan oligosaccharides on the performance, intestinal morphology and cecal fermentation of fattening rabbits. Animal Feed Science and Technology, 126, 107–120.
Tang, Z. R., Yin, Y. L., Nyachoti, C. M., Huang, R. L., Li, T. J., Yang, C., Yang, X. J., Gong, J., Peng, J., Qi, D. S., Xing, J. J., Sun, Z. H., & Fan, M. Z. (2005). Effect of dietary supplementation of chitosan and galacto-mannan-oligosaccharide on serum parameters and the insulin-like growth factor-I mRNA expression in early-weaned piglets. Domestic Animal Endocrinology, 28, 430–441.
Hooge, D. M. (2004). Meta-analysis of broiler chicken pen trials evaluating dietary mannan oligosaccharide, 1993–2003. International Journal of Poultry Science, 3, 163–174.
Dimitroglou, A., Merrifield, D. L., Spring, P., Sweetman, J., Moate, R., John, S., & Davies, S. J. (2010). Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture, 300, 182–188.
Dimitroglou, A., Merrifield, D. L., Moate, R., Davies, S. J., Spring, P., Sweetman, J., & Bradley, G. (2009). Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Animal Science, 87, 3226–3234.
Castillo, M., Martin-Orue, S. M., Taylor-Pickard, J. A., Perez, J. F., & Gasa, J. (2008). Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: effects on microbiota and gut function. Journal of Animal Science, 86, 94–101.
Genc, M. A., Aktas, M., Genc, E., & Yilmaz, E. (2007). Effects of dietary mannan oligosaccharide on growth, body composition and hepatopancreas histology of Penaeus semisulcatus (de Haan, 1844). Aquaculture Nutrition, 13, 156–161.
WHO (2009) Report of the 1st Meeting of the Advisory Group on Integrated Surveillance of Antimicrobial Resistance. Copenhagen 15–19 June, 2009. Online: available from http://apps.who.int/medicinedocs/en/m/abstract/Js16735e.
Regulation EU (2005) Ban on antibiotics as growth promoters in animal feed enters into effect (1831/2003/EC) In: safety, E.f. (Ed.), Europa, Brussels.
Tokimoto, T., Kawasaki, N., Nakamura, T., Akutagawa, J., & Tanada, S. (2005). Removal of lead ions in drinking water by coffee grounds as vegetable biomass. Journal of Colloid and Interface Science, 281, 56–61.
Bradbury, A. G., & Halliday, D. J. (1990). Chemical structures of green coffee bean polysaccharides. Journal of Agricultural and Food Chemistry, 38, 389–392.
Liepman, A. H., Nairn, C. J., Willats, W. G. T., Sørensen, I., Roberts, A. W., & Keegstra, K. (2007). Functional genomic analysis supports conservation of function among cellulose synthase-like A gene family members and suggest diverse roles of mannans in plants. Plant Physiology, 143, 1881–1893.
Petkowicz, C. L. O., Reicher, F., Chanzy, H., Taravel, F. R., & Vuong, R. (2001). Linear mannan in the endosperm of Schizolobium amazonicum. Carbohydrate Polymers, 44, 107–112.
Ishurd, O., Kermagi, A., Elghazoun, M., & Kennedy, J. F. (2006). Structural of a glucomannan from Lupinus varius seed. Carbohydrate Polymers, 65, 410–413.
Arya, M., & Rao, L. J. M. (2007). An impression of coffee carbohydrates. Critical Reviews in Food Science, 47, 51–67.
McCleary, B. V., & Matheson, N. K. (1986). Enzymic analysis of polysaccharide structure. In R. S. Tipson & D. Horton (Eds.), Advances in carbohydrate chemistry and biochemistry (pp. 147–276). UK: Academic Press.
Redgwell, R. J., Trovato, V., Curti, D., & Fischer, M. (2002). Effect of roasting on degradation and structural features of polysaccharides in Arabica coffee beans. Carbohydrate Research, 337, 421–431.
Bradbury, A. G. W. (2006). Chemistry I: non-volatile compounds, 1A: carbohydrates. In R. J. Clarke & O. G. Vitzthum (Eds.), World agricultural series, coffee: recent developments (pp. 1–17). Oxford, U.K: Blackwell Science.
Fengel, D., & Wegener, G. (1984). Wood: chemistry, ultrastructure, reactions. Berlin: New York.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D. (2010) Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP).
Roth, R., Moodley, V., & van Zyl, P. (2009). Heterologous expression and optimized production of an Aspergillus aculeatus endo-1,4-beta-mannanase in Yarrowia lipolytica. Molecular Biotechnology, 43, 112–20.
Miller, G. L. (1959). Use of dinitrosalicylle acid for determination of reducing sugar. Analytical Chemistry, 11, 426–428.
IUPAC (International Union of Pure and Applied Chemistry). (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Liu, Z., Wei, Q. I., Weina, W. U., Yue, L. I. U., & Zhimin, H. E. (2008). Enhancing thermostability of β-mannanase by protective additives. Frontiers Chemical Engineering in China, 2, 439–442.
Gubitz, G. M., Hayn, M., Urbanz, G., & Steiner, W. (1996). Purification and properties of an acidic α-mannanase from Sclerotium rolfsii. Journal of Biotechnology, 45, 165–172.
Duffaud, G. D., McCutchen, C. M., Ledc, P., Parker, K. N., & Kelly, R. M. (1997). Purification and characterisation of extremely thermostable beta-mannanase, beta-mannosidase and alpha-galactosidase from the hyper thermophilic eubacterum Thermotoga neapolitana. Applied and Environmental Microbiology, 63, 169–177.
Hossain, M. Z., Abe, J., & Hizukuri, S. (1996). Multiple form of β-mannanase from Bacillus sp. KK01. Enzyme and Microbial Technology, 18, 95–98.
Howard, R. L., Abotsi, E., van Rensburg, J. E. L., & Howard, S. (2003). Lignocellulose biotechnology: issues of bioconversion and enzyme production. African Journal of Biotechnology, 2, 602–619.
Back, J. F., Oakenfull, D., & Smith, M. B. (1979). Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry, 18, 5191–5196.
Graber, M., & Comber, D. (1989). Effect of polyols on fungal alpha-amylase thermostability. Enzyme and Microbial Technology, 11, 673–677.
Shimizu, K., Sudo, K., Ono, H., Ishihara, M., Fujii, T., & Hishiyama, S. (1998). Integrated process for total utilization of wood components by steam-explosion pretreatment. Biomass and Bioenergy, 14, 195–203.
Carvalheiro, F., Duarte, L. C., & Gírio, F. M. (2008). Hemicellulose biorefineries: a review on biomass pretreatments. Journal of Scientific and Industrial Research, 67, 849–864.
Shevchenko, S. M., Chang, K., Robinson, J., & Saddler, J. N. (2000). Optimization of monosaccharide recovery by post-hydrolysis of the water-soluble hemicellulose component after steam explosion of softwood chips. Bioresource Technology, 72, 207–211.
Fulger, C., Bavha, R., Stahl, H., Turek, E.J. (1982) Production of a mannan oligomer hydrolysate. Patent 4508745.
Otieno, D. O., & Ahring, B. K. (2012). The potential for oligosaccharide production from the hemicellulose fraction of biomasses through pretreatment processes: xylooligosaccharides (XOS), arabinooligosaccharides (AOS), and mannooligosaccharides (MOS). Carbohydrate Research, 360, 84–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiyanzu, I., Brienzo, M., García-Aparicio, M.P. et al. Application of Endo-β-1,4,d-mannanase and Cellulase for the Release of Mannooligosaccharides from Steam-Pretreated Spent Coffee Ground. Appl Biochem Biotechnol 172, 3538–3557 (2014). https://doi.org/10.1007/s12010-014-0770-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0770-0