Abstract
The effects of the addition of short-chain fatty acids and lower alcohols on the production of natamycin from Streptomyces natalensis F4-245 was investigated, and propanol was found to be the most effective additive. Under the optimal condition of propanol addition, the maximal natamycin titer reached 10.38 g/l, which was 17 % higher than that of the control. Metabolites analysis showed the concentrations of amino acids and acetyl-CoA were enhanced while those of organic acids in tricarboxylic acid (TCA) cycle were reduced. This work demonstrates that the addition of propanol is an effective strategy to increase natamycin yield through regulating metabolite node and pools of precursors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natamycin, also known as pimaricin, is a polyene macrolide antibiotic which is produced by Streptomyces strains, such as S. natalensis, S. gilvosporeus, S. chattanoogensis [1] and S. lydicus sp. [2–4]. Because of its broad spectrum activity, lack of development of resistance, slightly solubility and lower toxicity, natamycin is widely used as an antifungal agent in pharmacotherapy for topical treatment. It is also a biological antifungal food preservative which is recommended by the FDA and classified as a GRAS (generally regarded as safe) compound.
Due to the important commercial value of natamycin, many medium optimization methods [5–8] and gene transformation methods [1, 9] have been applied in attempts to increase natamycin yield. But only a few reports are about precursor in natamycin fermentation. It is reported that short-chain fatty acid and lower alcohols can act as precursor of macrolide antibiotic [10]. Precursor as the raw material of antibiotic plays an important role in the biosynthesis of products. However, there are only a few studies focusing on the analysis of precursor in natamycin biosynthesis.
Recently, the biosynthetic mechanism of natamycin has gradually been revealed, and a whole gene cluster responsible for its biosynthesis in S. natalensis ATCC 27448 was cloned and identified [11, 12]. Similar to fatty acids, polyketide assembly progresses through successive decarboxylative Claisen condensation reactions of activated precursors and is catalyzed by multifunctional enzymes called polyketide synthases (PKSs) [13]. The pathway of natamycin biosynthesis has been acknowledged to some extent. And propanol has been believed to be transformed into propionate via oxidation and then isomerized into succinate and methylmalonate in 1970 [14]. Based on these studies, it is possible to analyze propanol’s effects on natamycin biosynthesis.
In this study, the suitable time and amount of propanol addition for natamycin yield by S. natalensis F4-245 were determined. Through analyzing the metabolites of the strain F4-245, the main pathway of natamycin biosynthesis was assayed. To the best of our knowledge, this is the first report to focus on propanol’s function on natamycin biosynthesis in S. natalensis.
Materials and Methods
Strain, Media, and Cultivation Conditions
S. natalensis F4-245 was provided by Lifecome Biochemistry Company, Ltd. The solid medium was used for sporulation. This medium contained per liter of distilled water/yeast extract, 4.0; malt extract, 10.0; glucose, 4.0; and agar, 20.0, with a pH of 7.4. The seed medium was comprised of 10 g/l soybean powder, 8 g/l yeast powder, 20 g/l glucose, and 2 g/l calcium carbonate, with a pH of 7.0. The batch medium contained 50 g/l starch, 30 g/l soybean powder, 8 g/l yeast powder, 0.05 g/l potassium dihydrogen phosphate, and 2 g/l calcium carbonate, with a pH of 7.0. The starch needed to be gelatinized when mediums were made up and all the mediums were sterilized at 121 °C for 30 min except glucose, which should be sterilized independently at 115 °C for 30 min.
The strain F4-245 was activated from glycerin tube by the solid medium through being incubated for 8 days at 29 °C. At least 1 cm2 bulk from solid medium was cultivated into a 250-ml shake flask containing 30 ml seed medium. After incubation at 29 °C for 44 h on a rotary shaker at 220 rpm, a 3-ml portion of the seed culture was used to inoculate 25 ml natamycin production medium in 250-ml shake flask and was shaken at the same condition for 144 h.
The Optimal Additive and Its Feeding Time and Volume
The isometric (0.3 %) short-chain fatty acids and lower alcohols, i.e., sodium formate, sodium acetate, sodium propionate, ethanol, ethanediol, propanol, isopropanol, butanol and isobutanol, were added to the batch medium at 24 h. After 144 h, the titer of each medium (each treatment had three repetitions) was used to analyze which was the optimal additive.
Next, various feeding times (0, 20, 24, 28, 32 h) and volumes (0.05 %, 0.1 %, 0.15 %, 0.2 %, 0.25 %, 0.3 %) of optimal additive (propanol) were investigated. Each group has three repetitions.
Effect of Propanol on Natamycin Biosynthesis of S. natalensis F4-245
The time curves of the medium with propanol and control were analyzed in order to explain propanol’s function on S. natalensis F4-245 fermentation. Many items were detected to assist the analysis, such as biomass, titer, pH, propanol content, organic acid, amino acid and concentration of acetyl-CoA (AcCoA). The details of these methods are described below.
Analytical Methods
The biomass concentration was determined on the basis of its volume ratio between solid volume and total volume after centrifugation at 3,000 rpm for 10 min. Natamycin in the culture medium was determined by HPLC. Subsequently, the dilution was loaded onto an Agilent 1200 series HPLC system(Agilent Technologies, USA), which contains a G1311A quaternary gradient pump, a G1313A AutoSampler, and a G1314C UV detector (wavelength was set at 303 nm) to monitor the production of natamycin. The dilution was separated by an Agilent C18 column (4.6 × 150 mm; 5 μm particle sizes) with a mobile phase of 3 g/l ammonium acetate, 1 g/l ammonium chloride, 25 % acetonitrile and 0.5 % tetrahydrofuran. The column temperature was controlled at 25 °C, and the flow rate was set at 1 ml/min.
The propanol was measured by gas chromatography (GC), which was based on the method of Zhang [15]. The GC was Trace GC Ultra, and the column was TR-WAX (30 m × 0.320 mm ID, 0.25 μm film). FID detector and split-flow mode (1:40) were adopted and the flow rate of N2 was 1.0 ml/min. The temperature programming was from 50 °C (2 min) to 220 °C (1 min) and the rate of it was 10 °C/min.
The extracellular amino acids of S. natalensis on different fermentation times were derivatized by ethyl chloroformate (ECF). The derivations were assayed by GC system (Agilent 7890A; Agilent Technologies, USA) according to Qiu et al. [16]. A CATALOG HP-5 (30 m × 0.320 mm, 0.25 μm film), FID detector and splitless mode were adopted and the flow rate of N2 was 1.5 ml/min. The temperature programming was from 70 °C (5 min) to 280 °C (5 min) and the rate of it was 10 °C/min.
The extracellular organic acids including α-ketoglutaric acid (α-KG), citric acid (Cit), succinic acid (Suc) and malic acid (Mal) were detected by GC according to Qiu et al. [16], and the method is the same as that used for amino acids analysis. Other extracellular organic acids, like formic acid (FA), acetic acid (Ac), lactic acid (Lac), pyruvic acid (Py) and oxaloacetic acid (OAA) were detected by an Agilent HPLC1260 series (Agilent Technologies USA), equipped with an Agilent C18 column (4.6 × 250 mm, 5 μm), the column temperature was maintained at 35 °C, and the UV detector was set at 210 nm. The mobile phase, which had a flow rate of 0.5 ml/min contained 20 mM Na2HPO4 solution (pH 2.0 adjusted with H3PO4) and acetonitrile in the ratio of 99:1. The fermentation sample was centrifuged at 12,000 rpm for 10 min and filtered through 0.22-μm filter.
The acetyl-CoA was assayed by HPLC according to Seker et al. [17]. The method of sample treatment was the same as that used in organic acids detection by HPLC. The equipment was an Agilent HPLC 1260 series (Agilent Technologies, USA) equipped with an Agilent C18 column (4.6 × 150 mm, 5 μm), the column temperature was 25 °C, the flow rate was 0.75 ml/min and the detection wavelength was set at 210 nm. The stock solution of 200 mM ion-pair reagent tetrabutylammonium sulfate (TBAS) was prepared, and the pH was adjusted to pH 6 with solid NaH2PO4.
Data Analysis
All HPLC and GC raw files were converted to OPJ format and were pictured via Origin 8.0 (OriginLab Cor., USA). Each experiment was repeated and yielded similar results. Photoshop CS3 and WPS 2012 were used to draw the pathway of propanol entered natamycin lactone biosynthesis.
Results and Discussion
The Optimal Additive
Various short-chain fatty acids and lower alcohols were added to the medium to choose an optimal additive. As shown in Table 1, the natamycin production could be increased by feeding propanol in cultivation process. So, propanol was selected as the most effective additive for the following experiments. The propanol concentration and feeding time were optimized.
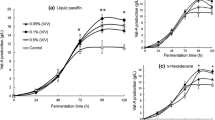
In order to seek the best feeding strategy of propanol in S. natalensis F4-245 fermentation, the effects of propanol in different concentrations and time on the natamycin production were tested. Figure 1 shows that the optimal propanol concentration and feeding time are 0.2 % and 28 h, respectively. Under the optimal condition, natamycin production reached appropriately 10.38 g/l and increased by 17 % compared to the control. It was the first finding that the addition of propanol could enhance natamycin biosynthesis in S. natalensis.
Results (Fig. 1) indicated that a slightly suppression of cell was observed in propanol addition medium. According to the different growth situation, the addition of propanol at 28 h could offer abundant time for cell growth and thus caused a higher biomass than adding propanol at inoculation time (Fig. 1a). Studies with the other additional quantity or time also indicated that overdose propanol could suppress the mycelia growth.
Effects of Propanol on the Process of S. natalensis F4-245 Fermentation
The effect of propanol on the biosynthesis of antibiotic was investigated by adding 0.2 % propanol into the medium at 28 h. Differences were observed both in the antibiotic biosynthesis and pH curves (Fig. 2). When 0.2 % propanol was added at 28 h, it stimulated the biosynthesis of the natamycin. Figure 2 shows that the accumulation of the antibiotic was stable during the fermentation and a lower increase rate of natamycin production between 60 h and 130 h in the medium without propanol. A minimal suppression of mycelia growth and a slight decrease of sugar uptake rate (not shown) of treatment were observed whereas the pH value declined obviously from 100 h to 114 h.
The time curves of the fermentation process by the S. natalensis cultivated in the medium with propanol and in the medium without propanol. The control (solid) and propanol added (open) are shown. The figure displays the biomass (square), natamycin (circle), pH (five-pointed star) and propanol ('*' type). Origin 8.0 software was used to create this artwork
Because propanol can be transformed into propionate via oxidation and then isomerized into succinate and methylmalonate [14], the content of succinate and methylmalonate would increase when propanol was added to the medium. Methylmalonate and propionate can act as precursors of natamycin biosynthesis improving natamycin yield. However, 0.2 % propanol can only improve 2.22 % natamycin in theory. Therefore, propanol may have other functions in natamycin biosynthesis.
Effects of Propanol on the Organic Acid Metabolism of S. natalensis F4-245
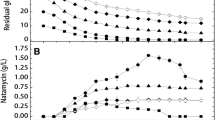
To understand how propanol stimulated the secondary metabolite synthesis, the changes in extracellular organic acid were analyzed. It was revealed that organic acids in tricarboxylic acid cycle (TCA cycle) were decreased in different extent in propanol addition medium (Fig. 3a). Among them, the α-ketoglutaric acid (α-KG) concentration during the middle stage of fermentation declined markedly.
The time curves of the organic acid concentration with the S. natalensis cultivated in the medium with propanol added and in the medium without propanol. OAA oxaloacetic acid, α-KG α-ketoglutaric acid, FA formic acid, Ac acetic acid, Lac lactic acid. The filled symbol represents control and the open symbol is 0.2 % propanol added at 28 h
The tendency of by-products (Ac, FA and Lac) in the antibiotic fermentation with propanol addition is shown in Fig. 3b, c and d. In the medium with propanol, FA had a significant growth and consumption phase in the fermentation while the control was gradually increased after 60 h. The Ac content was higher at the beginning 70 h. Since then, it fell gradually from 70 to 90 h when Ac was utilized and kept the level at about 0.3 g/l, apart from in 130 h when the concentration shot up and peaked at 1.5 g/l. Combined with natamycin synthesis, it is indicated that the accumulation and utilization of Ac were enhanced in propanol addition medium. In addition, the concentration of Lac experienced a large change and lower accumulation in the fermentation.
It was reported that natamycin production was positively associated with high OAA concentration [18]. However, this study drew the opposite conclusion. Combined with the pathway of polyene macrolide biosynthesis and related amino acids and organic acids biosynthesis, it is inferred that the lower OAA concentration leads to the higher natamycin production because propanol might be used in natamycin biosynthesis to reduce OAA concentration and TCA cycle flux. The excessive OAA would inhibit natamycin biosynthesis through strengthen TCA cycle and reduce AcCoA which can be used to synthetize polyene macrolide.
Effects of Propanol on the Amino Acid Metabolism of S. natalensis F4-245
Amino acids were assayed in the medium with and without propanol, respectively, in order to understand the effect of propanol and to explain the change of metabolites involved in the antibiotic biosynthesis. They were assorted by metabolic correlation (Fig. 4).
When the propanol was added to the medium, most of the detected amino acids (except Pro) were present at higher levels than those of the control. The changes of Pro content in treatment (Fig. 4f) was different with control especially between 60 and 140 h, and the Pro content in treatment remained at a lower level.
Gln’s concentration was the highest among all the amino acids in both culture mediums. Followed by Glu, Ser, Cys and Phe, Tyr, Asp, Lys, His and Pro were in lower levels. Other amino acids, like Asn, Orn, Trp, Ala, Val, Thr, Leu, Ile and Met, can not be detected.
Almost all amino acids detected increased during the fermentation. The higher concentration of Gln and Glu indicated abundant nitrogen sources in the propanol addition medium, so was testified by the lower α-KG concentration [19]. Moreover, the higher concentration of Glu and Gln proves that propanol addition strengthened nitrogen utilization and reduced α-KG in TCA cycle. The irregular changes of Pro are expected to be related to the changes of Gln and Glu. In brief, the higher levels of amino acids during the fermentation suggest that the primary metabolism and nitrogen utilization of S. natalensis F4-245 were more active in the propanol addition medium.
Effects of Propanol on the Acetyl-CoA of S. natalensis F4-245
Apart from amino acids and organic acids transformation, the changes of AcCoA in natamycin biosynthesis with propel addition was analyzed. The concentration of AcCoA in treatment was higher than that in control from 40 to 80 h (Fig. 5); during this time, natamycin biosynthesis rate of the treatment remained stable while the rate decreased in control (Fig. 2). It proved that propanol promotes AcCoA accumulation, and the content of AcCoA increases when the concentration of OAA is lower than control.
According to the concentrations of AcCoA in different times, it is possible to explain the changes of organic acids. The reason why AcCoA increased in propanol addition medium is that propanol leads to succinate increasing, and succinate inhibits the activity of α-ketoglutarate dehydrogenase, so it makes α-KG increase in a short time and then reduces. In this way, the activity of TCA cycle reduces and OAA concentration becomes lower than that of control, which makes it possible for AcCoA accumulation to occur. The increase of other organic acids including Ac, FA and Lac in propanol addition medium indicates the lower level of dissolved oxygen, and the increase can be used to synthesize pyruvic acid (PY), which is in favor of AcCoA accumulation.
The changes in amino acids also proved that organic acids transformation in the period of AcCoA accumulation. Reduced OAA was used to increase Asp and Lys; α-KG was used to increase Pro, Glu and Gln. Increased Phe, Tyr and His explain that AcCoA accumulation inhibits glycolysis process at the same time.
Overall, propanol improves natamycin’s production by two means. Firstly, propanol, as the precursor of natamycin, can offer three-carbon units to macrolide. Secondly, propanol can affect the metabolite node. It enhanced AcCoA accumulation and reduced TCA cycle and glycolysis (Fig. 6).
Conclusion
Propanol addition improved natamycin production. When adding 0.2 % propanol at 28 h, the optimal antibiotic production (about 10.38 g/l) could be achieved, which is 17 % higher than that produced by control. Furthermore, propanol addition regulates metabolite node and pools of precursor by enhancing AcCoA accumulation.
The current study reveals for the first time that propanol addition is useful in improving natamycin production by S. natalensis F4-245 accompanied by various responses of metabolite intermediates content. This simple and effective analysis is of practical value to antibiotic fermentation optimization and metabolic flux analysis. The results of the study are beneficial to the enhancement of the production of natamycin at a commercial scale.
References
Du, Y.-L., Chen, S.-F., Cheng, L.-Y., Shen, X.-L., Tian, Y., & Li, Y.-Q. (2009). Journal of Microbiology, 47, 506–13.
Lu, C. G., Liu, W. C., Qiu, J. Y., Wang, H. M., Liu, T., & Liu, D. W. (2008). Brazilian Journal of Microbiology, 39, 701–7.
Wang, Z., Qi, F., Lu, C., Liu, T., & Liu, W. (2010). Chinese Journal of Biological Control, 26, 335–9.
Hua, Y., Wen, C., Liu, W., Yang, J., & Lu, C. (2011). Chinese Journal of Biological Control, 27, 520–7.
El-Enshasy, H. A., Farid, M. A., & El-Sayed, E.-S. A. (2000). Journal of Basic Microbiology, 40, 333–42.
Farid, M. A., El-Enshasy, H. A., El-Diwany, A. I., & El-Sayed, E.-S. A. (2000). Journal of Basic Microbiology, 40, 157–66.
Liang, J., Xu, Z., Liu, T., Lin, H., & Cen, P. (2008). Enzyme and Microbial Technology, 42, 145–50.
Chen, G.-Q., Lu, F.-P., & Du, L.-X. (2008). Journal of Agricultural and Food Chemistry, 56, 5057–61.
Wang, T., Bai, L., Zhu, D., Lei, X., Liu, G., & Cen, P. (2012). Enzyme and Microbial Technology, 50, 5–9.
Jing, K., Hao, X., & Lu, Y. (2011). African Journal of Biotechnology, 10, 10030–3.
Martin JF, Aparicio JF. (2009). Methods in Enzymology, 459, 215–42.
Aparicio, J. F., Fouces, R., Mendes, M. V., Olivera, N., & Martin, J. F. (2000). Chemistry and Biology (London), 7, 895–905.
Wilson, M. C., & Moore, B. S. (2012). Natural Product Reports, 29, 72–86.
Potvin, J., & Péringer, P. (1993). Biotechnology Letters, 15, 455–60.
Zhang L. (2010). PhD dissertation, Shanghai: East China University of Science and Technology.
Qiu, Y., Su, M., Liu, Y., Chen, M., Gu, J., Zhang, J., & Jia, W. (2007). Analytica Chimica Acta, 583, 277–83.
Seker, T., Muller, K., & Nielsen, J. (2005). Applied Microbiology and Biotechnology, 67, 119–24.
Li D. (2004). PhD dissertation, Tianjin, China: Tianjin University of Science and Technology.
Leigh JA, Dodsworth JA. (2007). Annual Review of Microbiology, pp. 349–77.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Chen, S., Li, J. et al. Propanol Addition Improves Natamycin Biosynthesis of Streptomyces natalensis . Appl Biochem Biotechnol 172, 3424–3432 (2014). https://doi.org/10.1007/s12010-014-0766-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0766-9