Abstract
The present work explores brewery wastewater as a novel substrate for fumaric acid production employing the filamentous fungal strain Rhizopus oryzae 1526 through submerged fermentation. The effects of different parameters such as substrate total solid concentrations, fermentation pH, incubation temperature, flask shaking speed, and inoculum size on the fungal morphologies were investigated. Different morphological forms (mycelium clumps, suspended mycelium, and solid/hairy pellets) of R. oryzae 1526 were obtained at different applied fermentation pH, incubation temperature, flask shaking speed, and inoculum size. Among all the obtained morphologies, pellet morphology was found to be the most favorable for enhanced production of fumaric acid for different studied parameters. Scanning electron microscopic investigation was done to reveal the detailed morphologies of the pellets formed under all optimized conditions. With all the optimized growth conditions (pH 6, 25 °C, 200 rpm, 5 % (v/v) inoculum size, 25 g/L total solid concentration, and pellet diameter of 0.465 ± 0.04 mm), the highest concentration of fumaric acid achieved was 31.3 ± 2.77 g/L. The results demonstrated that brewery wastewater could be used as a good substrate for the fungal strain R. oryzae 1526 in submerged fermentation for the production of fumaric acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many platform chemicals are being produced from renewable biomass under moderate process condition pertaining to the depletion of conventional oil and the deterioration of the global environment. Fumaric acid (FA) and its derivatives are examples of such chemicals. The ability of FA to be converted into pharmaceutical products and act as starting material for polymerization and esterification reactions has led the US Department of Energy to designate FA among the top 12 biomass building block chemicals with potential to significantly enhance the economy [1]. FA functions as an acidulant and controls the growth of microorganisms, adjusts pH, and enhances flavors [2]. As an important platform chemical, FA is a valuable intermediate in the preparation of edible products, such as l-malic acid and l-aspartic acid, and with the increasing market share of l-aspartic acid and l-malic acid in sweeteners, beverages, and other health food areas, the worldwide demand for FA and its derivatives is growing each year [3]. Currently, the annual production of FA is estimated to be 12,000 t, and the projected market volume is 200,000 t [4]. Additionally, FA is widely used in the feed industry as an antibacterial agent and a physiologically active substance [5]. World growth prospects for FA in food and beverages are significant. The main factors behind this growth are food safety, desire for convenience, new beverage and food introduction, and growing consumption of nutritional bars (including cereal, sports, and energy bars), particularly in North America, Europe, and Asia. Food and beverages accounted for 33 % of world consumption of FA in 2009, followed by rosin paper sizes (20.0 %), unsaturated polyester resins (18.6 %), and alkyd resins (12.3 %) [6]. Moreover, two potentially new applications for FA are (a) as a medicine to treat psoriasis, and (b) as a supplement in cattle feed that reduces the methane emission up to 70 % [7]. Very recently, FA has been explored for many novel applications in the biomedical field. A number of synthetic biodegradable and injectable scaffold materials based on FA for an assortment of tissue engineering applications have been designed that can also be tailored for particular applications, ranging from cell encapsulation to gene delivery [8].
In the recent time, production of FA employing Rhizopus oryzae through submerged fermentation from different waste materials has gained tremendous importance. Low-cost carbon sources of agro-industrial origin have been explored as substrate for FA production with a good productivity. Investigation on FA production from woodchips, daily manure, and lignocellulosic biomass such as corn straw exhibited good product features [9]. With the increasing awareness of low-cost carbon option for the synthesis of value-added product, more of such substrates are being considered at large scale. In support of the “carbohydrate economy” principles, production of FA, the high value platform chemical from agro-industrial biomass is also very important in “biorefinery” prospects.
Meanwhile, the brewing industry holds a strategic economic position with an annual world beer production exceeding 1.34 × 109 hL in 2002. The brewing process uses large volumes of water in a number of different batch-type operations in processing raw materials to the final beer product. Water is a very substantial ingredient of beer, composing of 90–95 % of beer by mass [10]. An efficient brewery uses between 4 and 7 L of water to produce 1 L of beer for the brewing, rinsing, and cooling processes [11]. Thus, a large amount of brewery wastewater (BW) is discharged to the drains as effluent. This water must be disposed of or safely treated for reuse, which is often costly and problematic for most breweries. The BW contains biological contaminants (0.7–2.1 kg of BOD/barrel) [10]. The main solid wastes are spent grains, yeast, spent hops, and diatomaceous earth. BW is not toxic, does not usually contain appreciable quantities of heavy metals, and is easily biodegradable [12]. BW can be a good source of nutrition for microorganism and bioproduction of a platform chemical which is important from both carbohydrate economy and biorefinery prospects.
In the present investigation, R. oryzae NRRL 1526, the one of the best filamentous fungus strain for fumaric acid (FA) production, was employed against BW. Recently, it has been claimed that there exists a direct quantitative relation between the pellet morphology and enhanced production of FA with this strain [13]. Apart from this specific finding, it is now a consensus view that formation of fungal pellets can benefit the fermentation as they reduce the medium viscosity and also has the advantages of not wrapping into the impeller of fermenter, reuse of fungal biomass, and more mass and oxygen transfers [14, 15]. However, the optimized parameters from different studies cannot be drawn into a strong conclusion. Hence, with every new medium composition, parameters need to be optimized to control the morphology of the fungal strain. The recent trend on the development of efficient strategies for pellet formation with a reduced diameter and claim for enhanced production of FA highly encouraged us to carry out the present investigation. Moreover, the spectrophotometric method adopted for the quantification of FA in the present approach further eased the FA determination procedure compared with conventional analytical methods such as high-performance liquid chromatography (HPLC). Thus, the present work encompasses the economic, eco-friendly, and methodological advantages in the production of FA.
Materials and Methods
Microbial Culture
R. oryzae (NRRL 1526) was procured from Agricultural Research Services (ARS) culture collection, IL, USA. The obtained strain was first cultured on a potato dextrose agar (PDA) slant at 37 ± 1 °C for a maximum of 4 days to form spores. For spore inoculum preparation, spores were further propagated on PDA plated (90 mm) at 37± 1 °C for 72 h. The agar plates were washed with sterile distilled water and filtered through sterile cotton wool to obtain a spore suspension free of mycelial contamination. The suspended spores were maintained at 4 °C for future use. For long-time storage, the spore suspension was placed in 20 % glycerol solution at −80 °C. The cultures were renewed every 4 weeks. After counting with a haemocytometer, the spore concentration of the suspension was controlled to 1 × 107 spores/mL and used for inoculation.
Culture Media
Two different media were applied in this study. For preculture of R. oryzae, glucose basic salt medium was used, while for fumaric acid production, BW was exploited.
Composition of Preculture Medium and Growth Condition
Preculture medium consisted of (g/L) glucose 50, urea 2, KH2PO4 0.6, MgSO4·7H2O 0.5, ZnSO4·7H2O 0.11, and FeSO4·7H2O 0.0088. The medium pH (4.6) was not adjusted, otherwise mentioned. The medium was sterilized in two parts to avoid the Maillard reaction between the carbonyl group (>C = O) of glucose and amino group (−NH2) of urea. One part without the glucose was heat-sterilized (20 min, 15 lb, 121 ± 1 °C), while glucose was sterilized separately (20 min, 15 lb, 110 ± 1 °C). Sterilized medium was used for preculture of R. oryzae to obtain the pelletized seed. Medium was inoculated with the spore suspension (2 %, v/v). Precultures were carried out in 250-mL Erlenmeyer flasks with a final medium volume of 50 mL under the growth conditions of 30 °C and 200 rpm for 24 h. The precultured R. oryzae was used as inoculum for the fermentation of BW at a concentration of 10 % (v/v).
Procurement of Brewery Wastewater and Application for Submerged Fermentation (SmF) for Fumaric Acid Production
Brewery wastewater was utilized as fermentation medium to evaluate its potential for FA production. BW was procured from a local brewery industry (La Barberie, Quebec, Canada). To avoid microbial decay, BW was stored at 4 ± 1 °C for a maximum of 2 weeks before fermentation. For compositional data of BW, the work of Dhillon et al. was cited [16]. To prepare for the fermentation, 135 mL of BW was dispensed in a 500-mL Erlenmeyer flask and heat-sterilized (20 min, 15 lb, 121 ± 1 °C). Sterilized BS was inoculated with 15 mL (10 %, v/v) of precultured fungus (pellets + mycelium) giving a final volume of 150 mL. Flasks were incubated at 30 °C and 200 rpm in a shaker incubator for 3 days.
Different growth conditions were applied for R. oryzae in the fermentation process. Based on previous findings, the parameters chosen for the optimum production of FA were pH (4–10), rpm (100, 150, 200, 250, and 300), temperature (25 and 37 °C), total solid concentration (TSC) (10–40 g/L) of BW, and percentage (2.5, 5, 10, and 20 %) of precultured inoculum. Effects of variations in the values of each parameter were co-related with FA production. The fermentation time was maintained at 72 h for all experiments.
Neutralizing Agent
Calcium carbonate (CaCO3) was used as neutralizing agent in the fermentation medium (BW) at a fixed concentration of 20 g/L.
Analytical Methods
Fumaric Acid Concentration
FA was quantified spectrophotometrically by the modified method of Marshall, Orten, and Smith [17]. Briefly, the modified method is as follows:
-
1
Chemicals: anhydrous fumaric acid (FA), pyridine, anhydrous CuSO4, gum ghatti, anhydrous citric acid, NH4OH, and sodium diethyldithiocarbamate. All the chemicals used were of analytical grade and purchased from Fisher Scientific, Canada.
-
2
Working solutions: FA (1 mg/mL), pyridine (0.5 %, v/v), CuSO4 (20 %, w/v), gum ghatti (2 %, w/v), citric acid (20 %, w/v), NH4OH (10 %, v/v), sodium diethyldithiocarbamate (0.2 %, v/v), and copper-pyridyl reagent (20 mL of 20 % CuSO4 + 8 mL of pyridine).
-
3
The standard curve of FA: different volumes (200, 400, 600, 800, and 1000 μL) of FA were transferred to five different test tubes. To each test tube, 50 μL of copper-pyridyl reagent was added and left for 1–2 min. Development of turbidity indicated the formation of copper-pyridine-fumarate complex. To this, another 500 μL of copper-pyridyl reagent was added and vortexed. The tubes were left at 4 °C for atleast 15 min for incubation. The content of each test tube was then transferred into microcentrifuge tubes and subjected to centrifugation at 2000 rpm for 3 min. The supernatant was removed, and to each of pellet left, 2 mL cold (kept at 4 °C) pyridine solution was added and centrifuged at 2000 rpm for 3 min. Pellet recovered were dissolved in 1 mL of citric acid solution. This was followed by the addition of 1 mL of NH4OH solution and mixed properly. To this mixture solution, 200 μL of gum ghatti and 1 mL of diethyldithiocarbamate solution were added. The solutions were left for color development. After 4–5 min, all the solution developed a light blue color (formation of fumarate-copper-diethyldithiocarbamate complex). The transmittance of the solutions was measured at a wavelength of 460 nm in a 96-well plat reader. The OD (optical density) values were plotted against fumaric acid concentrations, and a standard curve was constructed.
-
4
Sample analysis for FA estimation: Concentration of FA for different broth samples were quantified taking the standard curve of FA as reference. The concentrations were expressed in grams per liter of BW.
-
5
Sample blank: A mixture of citric acid, NH4OH, gum ghatti, and diethyldithiocarbamate in the same proportion as used for the standard curve was taken as “sample blank”.
As mentioned before, the procedure mentioned in the original protocol was technically improvised to make it more time and cost effective. A detailed comparison of the original and modified protocol has been presented in Table 1.
Downstream Processing for Fumaric Acid Recovery
To recover FA from the insoluble calcium fumarate, fermented broth was heated at 90 °C with simultaneous acidification (5 N, H2SO4) until clear. Finally, the broth was centrifuged (8000×g, 10 min), and the supernatant containing the FA was collected and analyzed spectrophotometrically at 460 nm. The precipitate (fungal biomass + CaSO4) was further processed for dry mass determination.
Biomass Dry weight
Biomass dry weight (BDW) was measured by washing the mycelia three times with distilled water and then allowed to dry at 60 ± 1 °C until a constant weight was achieved.
Morphological Study
The morphological patterns displayed by R. oryzae 1526 with the variations of growth conditions during fermentation were studied with digital photography and electron microscopy. After recovering the samples from culture, they were repeatedly washed with copious amount of water and photographed with a digital camera (Canon PC 1585).
The fungal pellets obtained at the optimized growth conditions were further considered for scanning electron microscopic (SEM, Carl Zeiss EVO® 50) analysis to have a highly magnified view of the surface morphology. To prepare for SEM, cleaned fungal pellets were air-dried on a microscopic glass slide at isolated position overnight at room temperature (25 ± 1 °C). Dried samples were directly mounted on a SEM grid and sputter coated (SPI Module Sputter Coater) with gold before SEM analysis. Both size and shape were analyzed to confirm the parameter effects on R. oryzae morphology.
Statistical Analysis
Data are represented as mean ± SD of three independent experiments. Correlations were considered significant at P < 0.05 for different applied parameters.
Results and Discussion
The Advantages of Selecting the Strain R. oryzae 1526 for Fumaric Acid Production
The fungal species and the strain selected for the present study has a strong and decade-old research background. Many fungal species belonging to the genus Rhizopus were identified as the best FA producers and received industrial attention. The most important were R. nigricans, R. formosa, R. arrhizus, and R. oryzae. However, among the four species, R. oryzae was preferred over the other three due to its simple nutrient requirements and high productivity (4.25 g/L/h). After the 1990s, R. oryzae has been the frontliner in the production of FA [1]. Among different strains of R. oryzae tested for FA production, the strain NRRL 1526 is one of the best strains [18]. Thus, selection of R. oryzae 1526 for FA production meets technical requirements for a scientific investigation on FA production.
The Brewery Wastewater as Substrate for R. oryzae 1526
Brewery wastewater (BW) is the waste sludge produced by any brewery industry in large volumes. Recently, BW has been screened as the potential substrate for the production of citric acid [16]. In this study, compositional analysis of BW showed it to be rich in carbon and other vital nutrients required for culturing fungi. The results of the study suggested that BW could be a good and cheap biomass source for FA fermentation. So far, this agro-industrial waste has not been exploited for FA production.
Selection of Neutralizing Agent
It is now a well-known fact that in Rhizopus-mediated FA production, the pH value of FA production medium drops down (e.g., from 5 to 2) quickly in the first 20 to 24 h after inoculation due to production of FA. The consequence is the strong inhibitory effect on the growth of R. oryzae and FA production. This necessitates the addition of a neutralizing agent that will make complex with FA and thus maintaining the pH level at optimum for the growth of R. oryzae and FA production. After decades of research on different neutralizing agents (e.g., CaCO3, Na2CO3, NaHCO3, (NH4)2CO3, and Ca (OH)2), calcium carbonate was found to be the most efficient neutralizing agent in the commercial level production of FA [1]. The justifications made were (a) FA yield and volumetric productivity were found to be lower for other neutralizing agents than CaCO3; (b) accumulation of byproducts, such as malic acid and ethanol, was higher with other neutralizing agents; and (c) CaCO3 can supply CO2 that can be used for the formation of oxaloacetate in the tricarboxylic acid (TCA) cycle.
Thus, selection of CaCO3 as a neutralizing agent makes sure that the present study does not compromise with the growth of R. oryzae and production of FA.
Downstream Processing
In FA production, depending on the type of neutralizing agent being used, downstream processing of the fermented broth is performed with different methods. In case the neutralizing agent is CaCO3, the downstream methods employ mineral acids (HCl or H2SO4) and heat energy for the recovery of FA from the feebly soluble calcium fumarate (CaC4H2O4) formed during fermentation. In one method, heating of broth at high temperature (160 °C) followed by acidification (pH 1.0) is performed for FA recovery [19]. However, the approach consumes a large amount of energy to maintain the high temperature and also requires especially durable heating equipment, thus, the cost of FA recovery becomes high. The other available method is the simultaneous acidification and heating of broth at moderate temperature (60–90 °C) for FA recovery. No special heating equipment is required in this method [1]. Therefore, in the present study, low heating strategy was adapted to make the FA production more economically attractive.
The Improvised Spectrophotometric Method for Fumaric Acid Quantification
All the quantification of FA was performed spectrophotometrically following the method of Marshall, Orten, and Smith. However, some technical improvisations were made in the original protocol that did not cause any significant difference in the measured values of FA. The technical comparison of the procedure mentioned in the original work and followed in the present investigation is shown in Table 1. The outcome of the technical improvisation of the existing technique could be evaluated in terms of cost (volumetric reduction of reagents used) and time effectiveness (total estimation time). However, the chemical requirement was the same in both cases. As can be summarized from Table 1, there was an almost fourfold decrease in the total time of estimation in the improvised technique. Practically, there was no drastic change in the FA concentration determined following both procedures.
Previously, there was no report on the spectrophotometric determination of FA in biological sample. The method being followed in the present investigation could be a good alternative to conventional methodological approach such as HPLC for concentration measurement of FA. Literature on fermentation-based production of FA shows that the presence of (only qualitative) fumaric acid was determined by the formation of insoluble mercurous fumarate in 5 % nitric acid as no method for exact quantification of FA was available [20]. This might have urged many researchers to develop a reliable colorimetric method for the determination of FA concentration in biological samples. However, probably pertaining to the rapid development in the modernization and sophistication of analytical instruments such as HPLC, this method of FA estimation was not explored anymore. The present investigation has highlighted and improvised a method important in both economical and analytical prospective.
The Parameters Chosen for the Present Study and Their Effects
The parameters taken into consideration for the present investigation are very important from the point of view that R. oryzae is highly susceptible to these parameters and has a direct impact on the FA production. In many recently conducted studies, it is claimed that tuning of morphology of this fungal strain can work as a decisive factor in the overall performance of the fungus for FA production [13]. These investigations finally led to the conclusion that formation of pellet morphology by R. oryzae is very important to enhance the production of FA in SmF. In turn, the pellet formation could be programmed by changing the growth conditions. Based on those important previous findings on the morphological behaviors of R. oryzae, five different parameters (pH, temperature, rpm, total solid concentration, and volume of preculture) were studied. To start with, growth condition parameters of pH 5, 30 °C, and 200 rpm were initially maintained for SmF. Under these growth conditions, R. oryzae 1526 performed best in terms of FA production [13]. For BW as novel substrate, process optimization was carried out for achieving the fungal pellets of smaller diameter and higher production of FA.
Effects of Total Solid Concentration of BW
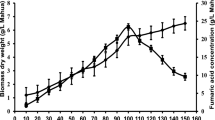
Different total solid concentrations were tested to optimize for the maximum production of FA. With the increase of TSC, there was a corresponding increase in the body dry weight of R. oryzae 1526, but FA production was lowered (Fig. 1). The inverse relation between TSC versus FA concentration was due to the broth rheology. The broth viscosity was highly prone to TSC and fungal morphology [16]. The TSC concentration supporting the pellet formation has provided the double benefits of lower viscosity and more production of FA, while formation of suspended mycelium increased the viscosity. TSC concentration of 25 g/L was considered to be the optimum one as fungal pellets and higher production FA were obtained.
Role of Production Medium (BW) pH
The BW has an original pH of 3.5 [16]. However, there is an agreement in the literature that R. oryzae grows well within the pH range of 4–9 [21]. Thus, the BW pH was adjusted from 3.5 to the required values (4, 5, 6, 7, 8, and 9) before the SmF. The effects of various pH on FA production and morphology of R. oryzae were very interesting. FA production was highest (14.16 ± 1.9 g/L) at pH 6. From pH 4–6, there was a gradual increase in FA production, but it started declining at pH 7 and continued up to pH 8 (Fig. 2a). The starting pH (4) resulted in a very lower production (3.7 ± 1.16 g/L) of FA. Beyond the pH value of 8, in fact, there was no growth of R. oryzae in the fermentation medium.
The fungus exhibited an array of distinctive morphological patterns corresponding to different pH of BW (Fig. 2b). Mycelium clumps, suspended mycelium and pellets, the common morphological forms of fungus, were manifested as a response to the variations in the pH values. However, the pellet types were not the same in their shape and size. Hairy and less aggregated pellets were dominantly formed at pH 5. The pattern changed into solid and nonaggregated type at pH 6. While reaching pH 7, formation of even more aggregated pellets was favored. The clump and suspended mycelium were formed at pH 4 and 8, respectively. The highest pH (9) value applied did not support the growth of R. oryzae in the fermentation medium although the incubation was continued for 120 h.
The production of FA and morphological behavior are highly interrelated. Many previous studies confirmed that pellet morphology was the most suitable form for FA production [13]. In the case of hairy pellets, shaving off the hairs by the hydrodynamic force (during flask shaking) reseeds into more mycelial growth and leads to damage and deactivation of both reseeded mycelia and the pellets. This initiates the aging and vacuolation of the fungal hyphae and finally leads to activity reduction of both pellet and reseeded mycelia [22]. Thus, the production of FA was influenced by the morphological forms of R. oryzae, while fermentation medium pH triggered the morphological changes under the growth conditions of 200 rpm, 30 °C, and 25 g/L of TSC. Substrate pH value of 6 supported the highest production of FA and formation of solid pellet by R. oryzae as well. Reconsidering the previously investigated best growth conditions (200 rpm, 30 °C, pH 5) for the strain R. oryzae 1526 for highest production of FA, it can be concluded that with novel substrate (BW in the present study), the optimum growth conditions for the same fungal strain varied considerably which can be mainly attributed to the medium composition [13].
Mechanical Force or Shaking Speed Effect
The optimized pH 6 (section “Role of Production Medium (BW) pH”) was further put into the investigation of mechanical force effects on the FA production and morphology of R. oryzae 1526. Different shaking speeds (100, 150, 200, 250, and 300 rpm) were tested. The other growth conditions were 30 °C and 25 g/L of TSC. The effects on FA production and R. oryzae morphology are shown in Fig. 3a, b, respectively. FA production was highest (11.8 ± 1 g/L) at 200 rpm, while at other applied shaking speed, the production level was lowered down considerably. As it can be seen from Fig. 3b, pellet morphology was reproduced at 200 rpm as obtained under same growth conditions (pH 6, 200 rpm, and 30 °C) discussed in section “Role of Production Medium (BW) pH”. The hydrodynamic effects on morphology and subsequent control on FA production were quite obvious. Suspended mycelium was formed at 100, 150, and 250 rpm shaking speeds. At the highest applied speed (300 rpm), there was no proliferation of the precultured inoculum in BW.
It is a well-known fact that in SmF, agitation is used to achieve uniform gas dispersion, homogenization, and interphase mass and heat transfer. The hydrodynamic force generated at various rpm speed can have a direct impact on the overall morphology of R. oryzae. Strong agitation can lead to fungal deactivation due to the shaving off of hyphae, while lower speed can disturb the oxygen, heat, and mass transfer [22]. Teng et al. also mentioned about the formation of distinctive morphologies as a response of shear forces [23]. It is obvious that at lower agitation speed, the precultured fungus consisting of mixed morphologies of pellets and mycelium do not become separated from each other, and their further growth leads to suspended mycelium which is actually the agglomeration. The hydrodynamic force generated out of the agitation actually dissipates specific free energy that has a control (proportional relation with an exponent of −0.25) on the fungal mean hyphal length [24]. Thus, with the increase of flask shaking speed, dissipation of specific free energy will increase exponentially and cause shorter hyphal length of loose mycelium which is less protected from the shearing forces and becomes less active [22]. Moreover, formation of pellets itself is supportive of more protection against shearing stress and less prone to deactivation. Consequently, more number of hyphae will be functional and metabolic activity will be higher. Thus, the moderate value (200) of shaking speed supported the pellet formation of R. oryzae 1526 that resulted in more production of FA compared with other applied speeds.
Variation in SmF Temperature
The fermentation experiment was also carried out at two more different temperatures, namely, 37 and 25 °C. Recently, it was reported that pellet diameter could be controlled by varying the preculture temperature for R. oryzae 1526 strain. With the increase of incubation temperature (<30 °C), pellet diameter became smaller and beyond 30 °C, the pellets with larger diameter were formed [13]. In the present investigation, the approach was not made at preculture stage but during SmF. As already discussed, in the present investigation, pellets were obtained both at preculture and fermentation stages. For SmF, the growth conditions optimized for pellet formation and higher production of FA were pH 6, 30 °C, and 200 rpm. Investigation on the possible role of SmF temperature in R. oryzae morphology and thus influencing the production level of FA was important.
In the experimental procedure, SmF was carried out at 25 and 37 °C while keeping the other growth conditions (200 rpm, pH 6, and 25 g/L of TSC) constant. After 72 h of SmF, the fungal biomass exhibited two distinct morphologies. The experiment carried out at 25 °C produced solid pellets with a reduced diameter (1 mm, Fig. 4a) compared with pellets obtained at 30 °C (3–5 mm, Figs. 2b and 3b). The other experiment designed for 37 °C resulted in suspended mycelium (Fig. 4b). The important outcome of this experiment was the marked increase in the production level of FA. The FA concentration reached 23.66 ± 2.1 g/L in 72 h of SmF. This is almost 10 g/L more as compared with the maximum concentration (14.16 ± 1.9 g/L) of FA obtained at 30 °C, 200 rpm, and pH 6. The FA concentration for 37 °C was very low (2.88 ± 1.33 g/L).
Many previous studies proved that fungal pellet formation was affected by incubation temperature. To be more specific, the approach of temperature lowering can induce pellet formation [25]. In another study by Schugerl et al., various morphological forms were observed in the incubation temperature range of 25–35 °C [26]. The conclusion made from these temperature-dependent investigations was that at higher temperature when the oxygen supply of the cells was inadequate, pellets were transformed into filamentous mycelium. Thus, the findings of the present study are in agreement with the general acceptance of temperature versus fungal morphology relationship.
Effect of Inoculum Volume
With the optimized growth conditions (pH 6, 200 rpm, 25 °C, 25 g/L TSC), SmF was also tested for the probable effects of the precultured inoculum size (% v/v) on FA production and morphology of R. oryzae 1526. Although most of the SmFs involving filamentous fungus apply 10 % (v/v) of preculture for inoculating the production medium, variation in inoculum size can generate important information on the change in FA production and morphology of R. oryzae.
Four different inoculum concentrations (2.5, 5, 10, and 20% v/v) were tested in the study. At 2.5 % (v/v) inoculum concentration, FA production was very low (2.17 g/L) and a mixed type (pellets + mycelium) of morphology were observed. The next higher concentration (5% v/v) resulted in a marked increase in the FA production (31.3 ± 2.77 g/L) and produced fungal pellets with a highly reduced diameter (Fig. 5a) which was later confirmed from SEM analysis (Fig. 6b, e). At 10 % (v/v), the results were almost repeated as obtained earlier with the growth conditions of pH 6, 200 rpm, and 25 °C. On reaching the highest concentration (20% v/v), there was a massive fungal mycelium growth (12 g/L BDW) with negligible amount (0.892 g/L) of FA production.
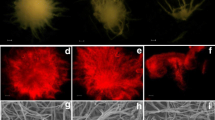
Scanning electron micrograph images of the pellets of R. oryzae 1526 obtained under different growth conditions. a, b, c The overall morphology, size, and hyphal close view of the pellets formed at pH 6, 200 rpm, 25 °C, 10 % (v/v) inoculum size, and 25 g/L TSC, respectively. d, e, f The overall morphology, size, and hyphal close view of the pellets formed at pH 6, 200 rpm, 25 °C, 5 % (v/v) inoculum size, and 25 g/L TSC, respectively
The critical role played by vegetative inoculum volume in the development of fungal morphology and relation to metabolite production in the fermentation culture was investigated previously. In one such study, fungal morphology was manipulated by means of inoculum amount, and corresponding product level was estimated [27]. The inoculum volume that supported the pellet morphology resulted in more activity of the targeted product, while with filamentous morphology, the scenario was just opposite. Although most of the studies on fungal inoculum effects are spore-type based, some of the basic concepts can also be extended for vegetative inoculum. For instance, whether it is of spore or vegetative origin, higher population (large volume of inoculum) always ends up with either big-sized fungal pellets or suspended mycelium due to agglomeration [28–30]. In the present case, 5 % (v/v) inoculum caused the formation of fungal pellets with reduced diameter and increased the FA production level to 31.3 ± 2.77 g/L.
Morphological Studies
The pellet morphology of R. oryzae 1526 was further analyzed using SEM. Many conventional measurement tools (microphotography, ruler-based scaling, and others) are applied for the measurement of fungal pellet diameter. However, accuracy and qualitative aspects are not within the scope of these techniques. A high-quality imaging system such as SEM can reveal both quantitative (diameter of pellet and hyphae) and qualitative (pellet shape, surface topology, and hyphal texture) minute details of fungal pellets. For these reasons, optimized pellet samples were subjected to SEM analysis.
The pellets formed under the growth conditions of pH 6, 200 rpm, 25 °C, and 10 % (v/v) inoculum size were observed under the high magnification and resolution states of SEM. The pellets were found to be roughly spherical and almost of uniform sizes (1 ± 0.15 mm, Fig. 6a, b). The edges were very sharp without mycelial protrusions from the pellet peripheries and thus ending in solid boundaries. The mycelium compactness was very high, and the hyphal average diameter was measured to be approximately 3.5 ± 0.5 μm (Fig. 6c).
To have a comparative account on the size and shape of the pellets obtained under all the optimized growth conditions (pH 6, 200 rpm, 25 °C, and 5 %(v/v) inoculum size), SEM analysis was also carried out for those pellets. As can be seen in the Fig. 6d, e, the pellets almost maintained uniformity in their size and shape. The pellet diameter measured was 0.440 ± 0.05 mm. The reduction (∼55 %) in pellet diameter was very significant in terms of FA production. Hyphal diameter remained almost unchanged (3 ± 0.8 μm, Fig. 6f). The structural intactness with reduced diameter (from 1 to 0.4 mm) regulated by the inoculum volume was an important finding of the present study.
From the process optimization experiments done in this study, the best growth conditions for R. oryzae 1526 could be summarized as pH 6, 200 rpm, 25 °C, 5 % (v/v) of inoculum size, and TSC of 25 g/L. With these optimized conditions, the maximum concentration of FA obtained was 31.3 ± 2.77 g/L. Considering that FA was being produced from a waste biomass without any supplementation of nutrients, the FA production level was quite high.
Conclusion
A novel combination between BW and R. oryzae 1526 was applied for the production of FA. Parameters including fermentation pH, temperature, shaking speed, inoculum size, and total solid concentration of BW were optimized for higher production of FA. The highest concentration of FA obtained in this study was 31.3 ± 2.77 g/L. A growth condition of pH 6, 25 °C, 200 rpm, 5 % (v/v) inoculum size, and 25 g/L of TSC supported for the enhanced production of FA. Spectrophotometric determination of FA was done in this study. The used substrate served the purpose of macronutrient and micronutrient for R. oryzae 1526.
Abbreviations
- BW:
-
brewery wastewater
- BDW:
-
biomass dry weight
- SEM:
-
scanning electron microscope
- FA:
-
fumaric acid
- rpm:
-
revolution per minute
- OD:
-
optical density
References
Xu, Q., Liu, L., & Chen, J. (2012). Microbial Cell Factories, 11, 24.
Yang, S. T., Zhang, K., Zhang, B., & Huang, H. (2011). Fumaric acid. In M. Bulter (Ed.), Moo-Young (pp. 163–167). The Netherlands: Comprehensive biotechnology.
Goldberg, I., Rokem, J. S., & Pines, O. (2006). Journal of Chemical Technology Biotechnology, 8, 1601–1611.
Sauer, M., Porro, D., Mattanovich, D., & Branduardi, P. (2008). Trends in Biotechnology, 26, 101–108.
Mrowietz, U., Christophers, E., & Altmeyep, R. (1999). British Journal of Dermatology, 141, 424–429.
Information Head Services. Report on chemical insight and forecasting: IHS Chemical. April 2010.
Beauchemin, K. A., & McGinn, S. M. (2006). Journal of Animal Science, 84, 1489–1496.
Temenoff, J. S., Kasper, F. K., & Mikos, A. G. (2007). Topics in tissue engineering. Ashammakhi, N., Reis, R., Chiellini, E (Eds). Fumarate-based macromers as scaffolds for tissue engineering applications (E-book).
Xu, Q., Li, S., Fu, Y., Tai, C., & Huang, H. (2010). Bioresource Technology, 101, 6262–6264.
Olajire, A. A. (2012). Journal of Cleaner Production. doi:10.1016/j.jclepro.2012.03.003.
European Commission. (2006). European Integrated Pollution Prevention and Control Bureau (EIPPCB) (Reference document on best available techniques (BAT) in the food, drink and milk industries). Seville: EIPPCB.
Brewers of Europe. (2002). Guidance note for establishing BAT in the brewing industry. Brussels: Brewers of Europe.
Zhou, Z., Du, G., Hua, Z., Zhou, J., & Chen, J. (2011). Bioresource Technology, 102, 9345–9349.
Li, Z., Shukla, V., Fordyce, A., Pedersen, A., Wenger, K., & Marten, M. (2000). Biotechnology Bioengineering, 70, 300–312.
Rodriguez Porcel, E., Casas Lopez, J., Sanchez Perez, J., Fernandez Sevilla, J., & Chisti, Y. (2005). Biochemical Engineering Journal, 26, 139–144.
Dhillon, G. S., Brar, S. K., & Verma, M. (2012). International Journal of Food Science and Technology, 47, 542–548.
Marshall, L. M., Orten, J. M., & Smith, A. H. (1949). Archives of Biochemistry, 24, 110–113.
Oda, Y., Yajima, Y., Kinoshita, M., & Ohnishi, M. (2003). Food Microbiology, 20, 371–375.
Gangl, I. C., Weigand, W. A., & Keller, F. A. (1990). Appl Biochemistry Biotechnology, 24–25, 663–677
Olander, A. (1929). Z. Physik. Chem. (Leipzig), Abt. A, 144, 49–72.
Meussen, B. J., de Graaff, L. H., Sanders, J. P. M., & Weusthuis, R. A. (2012). Applied Microbiology and Biotechnology, 94, 875–886.
Cui, Y. Q., Okkerse, W. J., van der Lans, R. G. J. M., & Luyben, K. C. A. M. (1998). Biotechnology Bioengineering, 60, 216–229.
Teng, Y., Xu, Y., & Wang, D. (2009). Bioprocess Biosystem Engineering, 32, 397–405.
Cui, Y. Q., Van der Lans, R. G. J. M., & Luyben, K. C. A. M. (1997). Biotechnology Bioengineering, 55, 715–726.
Braun, S., & Vecht-Lifshitz, S. E. (1991). Trends in Biotechnology, 9, 63–68.
Schugerl, K., Gerlach, S. R., & Siedenberg, D. (1998). Advances in Biochemical Engineering and Biotechnology, 60, 195–266.
Papagianni, M. (2004). Biotechnol Advances, 22, 189–259.
Nielsen, J., Johansen, C. L., Jacobsen, M., Krabben, P., & Villadsen, J. (1995). Biotechnology Progress, 11, 93–98.
Yanagita, T., & Kogane, F. (1963). Journal of General and Applied Microbiology, 9, 171–187.
Vecht-Lifshitz, S. E., Magdassi, S., & Braun, S. (1989). Biotechnology Bioengineering, 35, 890–896.
Acknowledgement
Financial support of the Natural Sciences and Engineering Research Council of Canada (discovery grant 355254), MAPAQ (no. 809051), and Ministère des Relations Internationales du Québec (coopération Paraná-Québec 2010–2012) is sincerely acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, R.K., Brar, S.K. Enhanced Fumaric Acid Production from Brewery Wastewater and Insight into the Morphology of Rhizopus oryzae 1526. Appl Biochem Biotechnol 172, 2974–2988 (2014). https://doi.org/10.1007/s12010-014-0739-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0739-z