Abstract
Contamination by Cd is a significant environmental problem. Therefore, we examined Cd removal from an environmental perspective. Ureolysis-driven calcium carbonate precipitation has been proposed for use in geotechnical engineering for soil remediation applications. In this study, 55 calcite-forming bacterial strains were newly isolated from various environments. Biomineralization of Cd by calcite-forming bacteria was investigated in laboratory-scale experiments. A simple method was developed to determine the effectiveness of microbially induced calcite precipitation (MICP). Using this method, we determined the effectiveness of biomineralization for retarding the flow of crystal violet through a 25-mL column. When the selected bacteria were analyzed using an inductively coupled plasma optical emission spectrometer, high removal rates (99.95 %) of Cd were observed following incubation for 48 h. Samples of solids that formed in the reaction vessels were examined using a scanning electron microscope. The CdCO3 compounds primarily showed a spherical shape. The results of this study demonstrate that MICP-based sequestration of soluble heavy metals via coprecipitation with calcite may be useful for toxic heavy metal bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most heavy metals (e.g., cadmium, copper, nickel, and zinc) exist in nature in soils, rocks, water, and biota at low concentrations that are sufficient for providing living systems with essential nutrients but are too low to cause toxicity [1]. The toxicological effects of acute Cd poisoning are manifested as a variety of symptoms, including high blood pressure, kidney damage, and red blood cell destruction. Cd is used in a wide variety of industries, including the electroplating industry, nickel–Cd batteries, pigments, plastics, pesticides, dyes, and textile operations [2]. Several methods such as precipitation, ion exchange, and adsorption are available for treating metal-contaminated effluents.
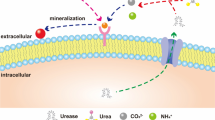
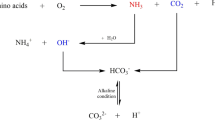
Several bacterial species have been utilized for microbially induced calcium carbonate precipitation (MICP) [3, 4]. MICP-based degradation of urea occurs through the ureolytic pathway, which produces ammonium ions as an energy source and leads to alkalinization of the surrounding environment [5]. In addition to NH4 +, carbonate ions are formed, which precipitate as calcium carbonate (CaCO3) in the presence of Ca2+ [6]. Moreover, when these reactions occur in sand, crystals form between sand particles, which hold sand particles together. Urea is hydrolyzed by urease within the cell. Urease converts urea and water into NH4 + and CO2, which are subsequently involved in various biochemical pathways. The ability to assay urease activity is essential for understanding whether urea, which is either transported into the cell [7] or produced internally through the catabolism of amino acids or purines, is assimilated into biomass. Urease activity can also be used to estimate the potential contribution of urea to total cellular nitrogen demand in phytoplankton and bacteria.
In the present study, we examined the removal of Cd from an environmental perspective and investigated the role of Lysinibacillus sphaericus CH-5, previously isolated from a mining area of Gangwondo, Korea, in the remediation of heavy metal-contaminated soil. To determine the efficiency of MICP, biomineralization products were characterized using scanning electron microscopy (SEM) and X-ray diffraction (XRD) analyses.
Materials and Methods
Isolation and Identification
Urease-producing bacteria were isolated from an abandoned expressway and abandoned mine sites in Gangwondo, Korea. Samples were collected in sterile Pyrex media bottles and stored on ice for no more than 6 h prior to processing. Samples from rivers were serially diluted and spread onto plates containing beef extract, peptone, urea (BPU) agar (3 g/L beef extract, 5 g/L peptone, 20 g/L urea, 10 g/L micro agar containing 100 μg/mL cycloheximide; pH 8.3). The plates were incubated at 30 °C for 2 days. Colonies were then transferred to urea broth for evaluation of urease production. Isolated cultures were stored at −20 °C in Miller Luria–Bertani (LB) broth (Difco, Franklin Lakes, NJ, USA) containing 20 % (v/v) glycerol. All strains were cultured at 30 °C and 200 rpm. Selected isolates were identified by sequencing of the 16S ribosomal RNA gene using universal primers (518F and 800R). PCR and sequencing were performed by Macrogen Co. (Daejeon, Korea). Sequence similarity between type strains was analyzed by nucleotide alignment using the Macrogen Alignment program, a web-based tool for identification based on 16S rRNA gene sequences [8]. Basic Local Alignment Search Tool (BLAST) analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was performed to compare the sequences with available DNA sequences registered in the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/). Sequences were aligned using the PHYDIT (http://plaza.snu.ac.kr/~jchun/phydit/) program, and the alignment was manually corrected. A phylogenetic tree was constructed by the neighbor-joining method using MEGA 5.0 software [9].
Preparation of Cadmium Stock Solutions
Cadmium stock solutions were prepared as CdCl2·5H2O (Kanto Chemical Co., Ltd., Tokyo, Japan). The 50 mM of Cd(II) stock solutions were prepared by dissolving the exact quantities of the CdCl2·5H2O in Milli-Q water and filtering through a 0.22-μm filter (Pall Co., MI, USA). Working concentrations of Cd(II) were obtained by serial dilution. The stock solutions were stored in the dark at 4 °C.
Measurement of Calcite Production and Urease Activity
One milliliter of a culture of isolated bacteria was grown overnight in BPU broth at 30 °C for 48 h with continuous aeration at 200 rpm. The bacterial suspension (500 μL) was added to 500 μL of calcium chloride dihydrate solution (350 mM). The mixture was centrifuged at 16,179×g for 5 min at 25 °C to collect the precipitate. The precipitate was dried for 24 h at 50 °C and weighed.
Urease activity was determined using the phenol-hypochlorite assay [10]. The bacterial suspension (250 μL) was added to 250 μL of sodium phosphate buffer (0.1 M) containing 500 μL of urea solution (3 M). The mixture was incubated at 37 °C for regular time intervals. Subsequently, 2 mL of phenol nitroprusside solution was added to alkaline hypochlorite solution and then incubated at 50 °C for 10 min. After incubation, absorbance was measured at 626 nm. Ammonium chloride (0–10 μM) was used as a standard. One unit of urease activity was defined as the amount of enzyme that catalyzed the hydrolysis of 1 μM urea per minute.
Impermeability Test
Cells grown in YE medium (20 g/L yeast extract, 10 g/L ammonium sulfate; pH7.0) overnight were harvested (6,000×g, 5 min), washed twice, and resuspended in 0.9 % sodium chloride solution at a final OD600 of 1.0. Cells were prepared for three treatments. Sterile silica sand (200 g, 0.45–0.7 mm, Joomoonjin Sand Co. Ltd., Korea) was mixed with 53.3 mL of urea/calcium chloride dihydrate solution. Sand slurry (50 g) was packed into a 25-mL plastic column (Corning Co. Ltd., Corning, NY, USA) (Fig. 1). Columns were run once by gravity with 10 mL of cell suspension. Ten milliliters of flow-through was reloaded onto the column. The columns were stored for 48 h to allow calcite crystal growth. Next, 2 mL of crystal violet was pipetted onto a packed sand column. The degree of impermeability was determined by measuring the migration distance of crystal violet.
Impermeability test. a A plastic pipette (25 mL) was used as a column. b Sand slurry (50 g) was packed into the individual column. c The cell suspension (10 mL) was passed through each column and flow-through was reloaded onto the column. d Crystal violet (2 mL) was dropped onto a packed sand column to measure the migration distance
Effect of Cd on Bacterial Growth
The selected bacterial strain L. sphaericus CH-5 was inoculated into BPU broth supplemented with 2 g/L CdCl2·5H2O and then incubated at 30 °C and 200 rpm for 3 days. Growth was measured by recording the optimal density (OD) at regular time intervals using a spectrophotometer (Ultrospec 2000; Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Cd Precipitation Experiments
The precipitation solution contained 1 mL inoculum, BPU and 2 g/L CdCl2·5H2O for each experiment, which was performed at 30 °C for 48 h. Bacteria showing the highest effect were used for the analysis of deposition rate of each heavy metal. Cd was measured in aqueous solution using an inductively coupled plasma optical emission spectrometer (ICP-OES) (Optima 7300DV; PerkinElmer, Waltham, MA, USA).
XRD and SEM Analyses
XRD analysis was used to construct a crystal structure model for calcium carbonate. Calcium carbonate samples prepared using different calcium salts were suspended in 100 μL of sterile water. The suspension was placed on a glass cover slip and then dried in an oven at 50 °C. The samples were then analyzed using a DMAX-2500 system (Rigaku, Tokyo, Japan). A modified SEM method was used to observe the calcium carbonate crystals produced. Previously harvested calcium carbonate crystals were suspended in water, covered with a glass plate, and then dried in an oven at 50 °C. After drying completely, the samples were coated with platinum in an ion sputter. A field emission scanning electron microscope (SEM; S-4300; Hitachi, Tokyo, Japan) was used to visualize the morphological features of the crystals.
Results and Discussion
Isolation and Identification
Fifty-five unique colony types were isolated from spread plates containing bacteria and BPU containing cycloheximide at 100 μg/mL. Four strains (CH-5, CH-11, CH-13, and CH-21) were selected by qualitatively determining high urease activity and calcite production based on the intensity of the pink color produced on urea agar media. Urea broth was used to select positive strains identified based on pink coloration resulting from the hydrolysis of urea products. Bacterial strains isolated from calcareous soils could precipitate carbonates under experimental conditions. Urea agar base was used to select urease-producing microorganisms. All isolates produced significant amounts of urease. Bacteria are known to hydrolyze urea through the action of urease in order to increase the ambient pH, utilize it as a nitrogen source, or utilize it for energy [11]. The four isolates selected from initial screening were molecularly identified based on their 16S ribosomal RNA gene sequences. These isolates were further identified using 16S rDNA sequence analysis. BLAST analysis revealed that CH-5 had 99 % similarity (100 % coverage) with L. sphaericus (Fig. 2). The 16S rRNA gene sequences determined in this study are deposited in the GenBank of NCBI under the accession numbers KF151160 (CH-13), KF151161 (CH-21), KF151163 (CH-11), and KF151164 (CH-5).
Calcite Production and Urease Activity
Among the isolates, CH-11 showed the highest urease activity (2.49 μmol/min), followed by CH-5 (2.41 μmol/min), CH-21 (0.12 μmol/min), and CH-13 (0.11 μmol/min) (Fig. 3). The highest calcite production was observed for CH-5 (9.8 mg/mL), followed by CH-11 (7.0 mg/mL), CH-13 (6.5 mg/mL), and CH-21 (4.5 mg/mL) (Fig. 3). Urease activity increased over the first 4–5 days but decreased significantly later. Carbonic anhydrase activity was also found to be correlated with urease activity. Hydrolysis of urea results in the accumulation of both HCO3 − and NH4 + in the cell, which favors the physiological and regulatory links between urea and bicarbonate metabolism [12]. Incorporation of nickel into the active site of urease depends on CO2/HCO3 − metabolism, which in turn is regulated by carbonic anhydrase [13]. Calcite precipitation is dependent on the concentrations of Ca2+ and CO3 2− in solution. An increase in CO3 2− concentration occurs under alkaline conditions when calcium (Ca2+) and carbonate (CO3 2−) ions are abundant [14].
Impermeability Test
In our study, decreased CV migration length was observed for the CH-5 strain (Fig. 4). This decrease in retention time can be attributed to the calcium carbonate crystals that were deposited between sand particles, which resulted in plugging. Improving the properties of sand has been shown to result in a 10-fold change in permeability, compressibility, shear strength, and volumetric behaviors [15]. Among the various bacteria isolated, one bacterial strain designated as CH-5 was selected based on its high calcite production, urease activity, and impermeability test results.
Effect of Cd on Bacterial Growth
Comparison of the growth of bacterial cells in Cd-free media and Cd-containing media showed a decrease in growth following Cd treatment compared to that observed for cells grown in Cd-free media (Fig. 5). L. sphaericus CH-5 grew in BPU media, although growth was relatively slow when the cells were grown in the same media containing 2 g/L Cd. L. sphaericus CH-5 produced a significant amount of urease in BPU media containing 2 g/L Cd. The urease production profile was recorded for 3 days. Maximum urease activity of L. sphaericus CH-5 was noted at 24 h, with a rate of production of 2.41 μmol/min. After 24 h, urease production was relatively low and L. sphaericus CH-5 produced 1.72 and 1.19 μmol/min of urease at 48 and 72 h, respectively. Further, calcite precipitation by ureolytic bacteria such L. sphaericus CH-5 may sequester dissolved Cd.
Cd Bioremediation
The ability of L. sphaericus CH-5 to remove Cd in BPU and BPU media containing 2 g/L Cd was investigated. Our data showed that the isolate used in this study removed 99.95 % of Cd at 2 g/L in 48 h in BPU media. Several studies have indicated that more than 90 % of heavy metals can be removed from a solution with a limestone concentration of greater than 45 g in 100 mL of heavy metal solution [16]. A study examining metal adsorption by calcite showed that heavy metals are retained on calcite according to the following sequence: Cr3+, Zn2+, and Cd2+ [17]. The Cd bioremediation efficiency of Bacillus sp. L14 in the presence of DCC or DNP did not decrease as reported for other strains, but increased from 73.6 to 93.7 % and 80.8 %, respectively [18].
SEM and XRD Analyses
Samples of solids that formed in the reaction vessels were examined using SEM. Each type of heavy metal crystal exhibited different morphological features. The precipitates produced during bacterial ureolysis were roughly rhombohedral, spherical, or needle-shaped and generally 10–50 μm in size. Figure 6 shows that the precipitated Cd was mostly spherical, with a diameter of approximately 10–40 μm. XRD analyses of the precipitates revealed the presence of Cd carbonate (Fig. 7) [19, 20]. An increased number of calcite peaks was observed when CH-5 was grown in BPU media containing Cd. Since the solubility product of otavite (CdCO3) is much lower than that of calcite (CaCO3), recrystallization processes taking place at the calcite surface (Ostwald ripening) can, from a thermodynamic perspective, lead to the formation of a Ca–Cd solid solution at the surface.
Conclusion
In this study, we demonstrated that heavy metal contaminants, such as Cd, can be precipitated by bacterial urease. The ureolysis capabilities of various bacterial strains isolated from soil were analyzed and utilized to mediate efficient in situ precipitation of different heavy metal contaminants. Our impermeability test can be applied for measuring the bioremediation properties of various ureolytic bacteria. Further, the effectiveness of MICP for the bioremediation of Cd has been demonstrated. The introduction of this indigenous bacterium provides a potential bioremediation process for environments with high Cd contamination.
References
Li, M., Cheng, X., & Guo, H. (2013). International Biodeterioration and Biodegradation, 76, 81–85.
Yavuz, Ö., Guzel, R., Aydin, F., Tegin, I., & Ziyadanogullari, R. (2007). Polish Journal of Environmental Studies, 16, 467–471.
Boquet, E., Boronat, A., & Cormenzana, A. R. (1973). Nature, 246, 527–529.
Rivadeneyra, M. A., Delgado, R., Moral, A. D., Ferrer, M. R., & Cormenzana, A. R. (1993). FEMS Microbiology Ecology, 13, 197–204.
Whiffin, V. S., Paassen, L. A., & Harkes, M. P. (2007). Geomicrobiology Journal, 24, 417–423.
Hammes, F., & Verstraete, W. (2002). Reviews in Environmental Science and Biotechnology, 1, 3–7.
Stocks-Fischer, S., Galinat, J. K., & Bang, S. S. (1999). Soil Biology & Biochemistry, 31, 1563–1571.
Chun, J., Lee, J. H., Jung, Y., Kim, M., Kim, S., & Kim, B. K. (2007). International Journal of Systematic and Evolutionary Microbiology, 57, 2259–2261.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). Molecular and Biological Evolution, 28, 2731–2739.
Natarajan, K. R. (1995). Journal of Chemical Education, 73, 556–557.
Hammes, F., Boon, N., De Villiers, J., Verstraete, W., & Siciliano, S. D. (2003). Applied and Environmental Microbiology, 69, 4901–4909.
Stahler, M. F., Ganter, L., Katherin, L., Manfred, K., & Stephen, B. (2005). FEMS Immunology and Medical Microbiology, 44, 183–189.
Park, I. S., & Hausinger, R. P. (1995). Science, 267, 1156–1158.
Qian, C., Wang, R., Cheng, L., & Wang, J. (2010). Chinese Journal of Chemistry, 28, 847–857.
DeJong, J. T., Mortensen, B. M., Martinez, B. C., & Nelson, D. C. (2010). Ecological Engineering, 36, 197–210.
Hamidi, A. A., Mohd, N. N., & Kamar, S. A. (2008). Bioresource Technology, 99, 1578–1583.
Sanchez, A. G., & Ayuso, E. A. (2002). Minerals Engineering, 15, 539–547.
Luo, S., Xiao, X., Xi, Q., Wan, Y., Chen, L., Zeng, G., et al. (2011). Journal of Hazardous Materials, 190, 1079–1082.
Cheung, K. H., Lai, H. Y., & Gu, J. D. (2006). Journal of Microbiology and Biotechnology, 16, 855–862.
Cheung, K. H., & Gu, J. D. (2007). International Biodeterioration & Biodegradation, 59, 8–15.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2011-0025229).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, CH., Han, SH., Shin, Y. et al. Bioremediation of Cd by Microbially Induced Calcite Precipitation. Appl Biochem Biotechnol 172, 1929–1937 (2014). https://doi.org/10.1007/s12010-013-0626-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0626-z