Abstract
In this paper, the production of rhamsan gum from Sphingomonas sp. CGMCC 6833 at different pH values was investigated. Based on kinetic analysis, a two-stage strategy for pH control was proposed. During the first 10 h, pH was controlled at 7.5 to maintain high specific cell growth rate and specific glucose consumption rate. After 10 h, pH decreased naturally to 7.0; this value was retained to maintain high specific rhamsan gum formation rate. Using this method, the maximum concentration and productivity of rhamsan gum reached 18.56 ± 1.68 g/L and 0.290 ± 0.026 g/L/h, which are 12.83 and 12.84 % higher than the optimum results obtained at natural pH, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
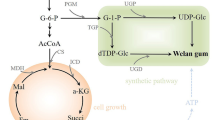
The gellan family is composed of water-soluble exopolysaccharides (EPS) produced by Sphingomonas species; examples of these EPS include gellan gum, welan gum, rhamsan gum, S-657, and S-88 [1]. In general, the backbone structure of these EPS is a repeating linear tetrasaccharide of d-glucose, d-glucuronic acid, and l-rhamnose at a ratio of 2:1:1. Members of the gellan family are widely used as thickening, stabilizing, and suspending agents in various industries, including food [2, 3], pharmaceutical [4], oil field [5, 6], and concrete [7, 8], because of their high viscosity and high stability.
Rhamsan gum has the same backbone structure, and each d-glucosyl residue next to the l-rhamnosyl residue is substituted at O-6 by α-d-glucosyl-(1 → 6)-β-d-glucosyl disaccharide side chains. Although rhamsan gum is non-gel forming, it gives a thermostable, highly viscous solution even at temperatures greater than 100 °C. Compared with other gellan family members, rhamsan gum has broader applications in the food industry because it can tolerate high concentrations of phosphate and sodium chloride [9]. Its use as a food additive was approved by the Japanese Ministry of Health and Welfare in 1996 [10]. As a good anionic water-soluble EPS, rhamsan gum is also safe to use in plastic surgeries [11]. Rhamsan gum is now produced commercially by CP-Kelco Company.
Fermentation can be significantly influenced by various physical and chemical parameters; among which pH is one of the most important because it can affect the duration and rate of fermentation as well as the production of fermentation metabolites [12, 13]. Previous reports revealed that gellan gum should be produced at a neutral pH [1, 14]. Our previous research and pilot scale fermentation showed that broth pH decreases below 6.0 as fermentation time is prolonged, causing a reduction in the synthetic rate of rhamsan gum. Acidic conditions might not be beneficial for cell growth, substrate consumption, and polysaccharide formation, hence, influencing rhamsan gum production. pH is a possible limiting environmental factor in rhamsan gum production.
To achieve maximum rhamsan gum production with efficient substrate conversion and increased rhamsan gum formation, the optimization of rhamsan gum production under various pH conditions needs to be studied. In this work, the effect of pH on the production of rhamsan gum from Sphingomonas sp. CGMCC 6833 was systematically studied. A pH control strategy was proposed to enhance the production of rhamsan gum in a 7.5 L fermenter. A two-stage pH control strategy was established and successfully applied to improve rhamsan gum production.
Materials and Methods
Microorganism
Sphingomonas sp. RH-1 (CGMCC 6833), a rhamsan gum-producing strain, was originally isolated from a soil sample collected from Laoshan National Forest Park of Nanjing (Nanjing, China) [15].
Media and Culture Methods
The seed medium contained 20 g/L glucose, 1 g/L yeast extract, 3 g/L peptone, 2 g/L K2HPO4, and 0.1 g/L MgSO4 at pH 7.2 to 7.4. The batch fermentation medium contained 40 g/L glucose, 5.38 g/L yeast extract, 5.71 g/L K2HPO4, and 0.32 g/L MnSO4.
Sphingomonas sp. CGMCC 6833 was inoculated into 100 mL of fresh seed medium in 500 mL flasks and then aerobically incubated for 16 h with shaking at 200 r/min and 30 °C. The seed culture (5 %, v/v) was inoculated into the fermentation medium. Batch fermentation was carried out in a 7.5-L stirred fermenter (Rushton impeller, BioFlo110, New Brunswick Scientific, USA) with the following features: diameter, 6 cm; bioreactor internal diameter, 17.8 cm; height, 32.1 cm; and working volume, 4.5 L. All cultivations were carried out at 30 °C. The aeration rate and agitation speed were controlled at 1.0 L/L/min and 600 rpm in the batch fermentation, respectively.
Effect of pH on Rhamsan Gum Production in Batch Fermentation
To investigate the effect of pH on rhamsan gum production, Sphingomonas sp. CGMCC 6833 was cultivated in the fermentation medium in a 7.5-L stirred fermenter at different pH values (natural, 6.5; 7.0; 7.5; and 8.0). Except for the natural pH fermentation, the other fermentation processes were maintained at their respective pH by adding 6 mol/L NaOH or 6 mol/L HCl to the culture broth until the end of cultivation.
Two-Stage pH Control Strategy for Rhamsan Gum Production in Batch Fermentation
Based on the specific cell growth rate and specific rhamsan gum formation rate, a two-stage pH control strategy was proposed as follows. The initial pH was maintained at 7.5 for 10 h of cultivation. Subsequently, pH was decreased to 7.0; this value was maintained to promote further rhamsan gum synthesis in later cultivation.
Analytical Methods
The biomass was determined for at least three 10 mL cell suspensions that were harvested by centrifugation, washed with distilled water, and then dried at 60 °C for 24 h to a constant weight. Glucose concentration was analyzed using a biosensor equipped with glucose oxidase electrode (SBA-40C, Shandong Academy of Sciences, China) [16]. The viscosity of fermentation broth was measured by a rotational viscometer (NDJ-1, Shanghai Hengping Scientific Instrument Co. Ltd., China) using rotor no. 3 at 60 r/min. pH was measured using a precision pH meter (Shanghai Leici Instrument Co., Ltd.). The concentration of rhamsan gum was measured as follows. A certain volume of fermentation broth was heated in a water bath at 70 to 80 °C for 15 min. After cooling, twice volumes of anhydrous ethanol were added with stirring until a flocculent precipitate appeared. The solution was placed in a refrigerator at 4 °C for 12 h. After centrifugation, the supernatant solution was removed. The process was then repeated with anhydrous ethanol. The precipitate was dried in a 60-°C oven to constant weight.
Calculation of Kinetic Parameters
The specific cell growth rate (μ, h−1), specific glucose consumption rate (q s , h−1), and specific rhamsan gum formation rate (q p , h−1) were estimated from the experimental or fitted data of cell growth (C x , g/L), residual glucose concentration (Cs, g/L), and rhamsan gum production (Cp, g/L) using Eqs. 1 to 3, respectively. The fitted data were obtained by interposing the experimental data of cell growth, residual glucose concentration, or rhamsan gum production at a definite time (dt = 0.1 h) with the approximation method of cubic spline interpolation in Grapher software (Version 4, Golden Software, Inc., USA)
Results and Discussion
Time Profiles of Rhamsan Gum Fermentation Without pH Control
The typical fermentation of Sphingomonas sp. CGMCC 6833 without pH control is shown in Fig. 1. The pH of the fermentation broth decreased from its initial value of 7.45 ± 0.10 to 6.13 ± 0.27 within 72 h of fermentation. Before the pH decreased to 6.67 ± 0.28, the cell growth was rapid. Subsequently, the cell growth reached the platform phase. After 56 h, the biomass decreased. This result may be attributed to the low pH. The rhamsan gum accumulation started at the beginning of the fermentation and reached the maximum of 16.45 ± 1.23 g/L at 64 h. However, rhamsan gum accumulation stopped afterward, indicating that no rhamsan gum production occurred after prolonged cultivation. The concentration of glucose decreased from its initial concentration of 40 ± 0.50 to 21.75 ± 1.50 g/L within 56 h and was consumed slowly afterward. The residual glucose concentration remained at 20.75 ± 1.25 g/L after 72 h of cultivation. The above results suggest that high pH can increase rhamsan gum production and that the pH in the culture broth is critical in cell growth and rhamsan gum production. Thus, the cell growth may be prolonged and the rhamsan gum production may be enhanced by properly controlling the pH in the culture broth.
Effects of pH on Rhamsan Gum Production in Batch Fermentation
Figure 2 shows the effects of pH on the fermentation of rhamsan gum from Sphingomonas sp. CGMCC 6833. During the cultivation, pH was maintained at pH 6.5, 7.0, 7.5, and 8.0. At pH 6.5 (Fig. 2a), rhamsan gum and the viscosity of the fermentation were both low. The glucose concentration at 72 h was the highest among the four pH values, indicating that cell growth and product accumulation did not consume too much glucose. As shown in Fig. 2b, cell growth increased when pH was increased from 6.5 to 7.0. At pH 7.5, biomass (7.02 ± 0.65 g/L) reached its maximum values at 56 h, rhamsan gum production (17.43 ± 1.78 g/L), and fermentation batch viscosity (3.56 ± 0.30 g/L) all reached their maximum values at 64 h (Fig. 2c). Glucose concentration (17.50 ± 1.25 g/L) was the lowest at 72 h. Biomass, rhamsan gum production, and fermentation batch viscosity at pH 8.0 decreased compared with those at pH 7.5. Glucose concentration at 72 h was increased than that at pH 7.5. This result suggested that the production of rhamsan gum from Sphingomonas sp. CGMCC 6833 was affected by pH and that either low or high pH was not beneficial for rhamsan gum production. Consistent with the increase in cell growth, the consumption of glucose increased from pH 6.5 to 7.5. In addition, the increase in cell growth increased rhamsan gum production at higher pH. However, pH values higher than 8.0 were not beneficial for rhamsan gum production.
Kinetic Analysis of Rhamsan Gum Fermentation at Different pH Values
To analyze the kinetic characteristics of the effect of pH on cell growth and rhamsan gum production, μ, q s , and q p were calculated based on the data of pH 7.0 and 7.5 in Fig. 2. As shown in Fig. 3, the profiles of μ, q s , and q p exhibited similar tendencies and the maximum values appeared at approximately 6 h. The result showed that μ and q s were higher at pH 7.5 than at pH 7.0 at the beginning of rhamsan gum fermentation (approximately 10 h). Thus, pH 7.5 was better for cell growth and glucose consumption at the beginning of rhamsan gum fermentation. After 10 h, however, pH 7.0 was beneficial for rhamsan gum formation with a high value of q p .
Based on the analysis of μ, q s , and q p , a two-stage pH control strategy was proposed as follows. pH was controlled at 7.5 in the first 10 h to maintain high μ and q s . After 10 h, pH decreased naturally to 7.0; this pH value was retained to maintain high q p with Sphingomonas sp. CGMCC 6833.
Rhamsan Gum Production with Two-Stage pH Control Strategy
The time course of the proposed strategy for rhamsan gum fermentation is shown in Fig. 4. Table 1 lists the results of constant and two-stage pH control strategy for controlling experiments. The two-stage pH control strategy not only considerably improved rhamsan gum production but also increased rhamsan gum productivity. The maximum concentration and productivity of rhamsan gum reached 18.56 ± 1.68 g/L and 0.290 ± 0.026 g/L/h, which are 12.83 and 12.84 % higher than the optimum results obtained at natural pH, respectively. The results showed that the two-stage pH control strategy remarkably improved the productivity of rhamsan gum from Sphingomonas sp. CGMCC 6833. Moreover, the two-stage pH control strategy was beneficial for cell growth. Up to 7.23 ± 0.65 g/L of biomass was obtained after fermentation with the two-stage pH control strategy, whereas only 6.46 ± 0.42 g/L was obtained after fermentation at natural pH.
Conclusion
This paper developed a novel two-stage pH control strategy based on the kinetic analysis of efficient rhamsan gum fermentation using Sphingomonas sp. CGMCC 6833. This method was proven effective for the enhancement of rhamsan gum concentration and productivity. By applying this pH control strategy, rhamsan gum concentration and productivity were increased by 12.83 and 12.84 %, respectively, compared with the optimum results of natural pH process.
References
Banik, R. M., Kanari, B., & Upadhyay, S. N. (2000). World Journal of Microbiology & Biotechnology, 16, 407–414.
Morrison, N. A., Clark, R. C., Chen, Y. L., Talashek, T., & Sworn, G. (1999). Progress in Colloid and Polymer Science, 114, 127–131.
Bajaj, I., & Singhal, R. (2007). Food Chemistry, 104, 1472–1477.
Coviello, T., Dentini, M., Rambone, G., Desideri, P., Carafa, M., Murtas, E., et al. (1998). Journal of Controlled Release, 55, 57–66.
Dennis, H. H., Thomas, O. M., & Paul, S. (1991). Oil reservoir permeability profile control with crosslinked welan gum biopolymers. US Patent No: 4981520
Vercaemer, C. J., Davies, S. N., Pafitis, D. G., Maintland, G. C., & Poyet, J. P. (1997). Selective zonal isolation of oil wells. US Patent No: 5697441
Plank, J. (2004). Applied Microbiology and Biotechnology, 66, 1–9.
Allen, F. L., Best, G. H., & Linfroth, T. A. (1991). Welan gum in cement compositions. US Patent No: 5004506
Kang, K. S., & Pettitt, D. J. (1993). Xanthan, Gellan, Welan, and Rhamsan. In J. N. BeMiller & R. L. Whistler (Eds.), Industrial gums: polysaccharides and their derivatives (pp. 341–397). San Diego: Academic.
Hagiwara, A., Imai, N., Doi, Y., Sano, M., Tamano, S., Omoto, T., et al. (2010). The Journal of Toxicological Sciences, 35, 493–501.
Paula, M. D., Goissis, G., & Martins, V. C. A. (2007). Journal of Materials Science: Materials in Medicine, 18, 1683–1690.
Serafim, V., Sneghana, R., Konstantsa, P., Margarita, K., Ivan, P., & Svoboda, D. (2013). Applied Microbiology and Biotechnology, 97, 5303–5313.
Joo, J. H., Lim, J. M., Kim, H. O., Kim, S. W., Hwang, H. J., Choi, J. W., et al. (2004). World Journal of Microbiology & Biotechnology, 20, 767–773.
West, T. P., & Fullenkamp, N. A. (2001). Microbios, 105, 133–140.
Xu, H., Xu, X. Y., Dong, S. H., Li, S., Liang ,J. F. (2012) A kind of Sphingomonas sp. and its application in the production of rhamsan gum. China Patent Application No: 201210504461.3
Xu, H., Jiang, M., Li, H., Lu, D. Q., & Ouyang, P. K. (2005). Process Biochemistry, 40, 519–523.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21006050 and 21106062) and the National Key Technology R&D Program (No. 2011BAD23B04)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ying, X.X., Ping, Z., Sha, L. et al. Production of Rhamsan Gum Using a Two-Stage pH Control Strategy by Sphingomonas sp. CGMCC 6833. Appl Biochem Biotechnol 172, 168–175 (2014). https://doi.org/10.1007/s12010-013-0492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0492-8