Abstract
Keeping in view the vast potential of endophytic fungi to produce bioactive molecules, this study aimed at isolating and screening endophytes for the production of acetylcholinesterase inhibitors. Fifty-four endophytic fungi were isolated from Ricinus communis and screened for their AChE inhibitory activity using Ellman’s colorimetric assay method. Six isolates were found to possess AChE inhibitory activity with maximum inhibition of 78 % being evinced by culture Cas1 which was identified to be Alternaria sp. on the basis of molecular as well as microscopic methods. Optimization of inhibitor production was carried out using one factor at a time approach. Maximum production of inhibitor was obtained on potato dextrose broth after 10 days incubation. The IC50 of the chloroform extract was observed to be 40 μg/ml. The extract was purified on silica gel and eluted stepwise with a gradient of chloroform/methanol. The insecticidal potential of the extract was evaluated by feeding the larvae of Spodoptera litura on diet containing varying concentrations of the extract. It was observed that with increase in the concentration of the extract, mortality of the larvae increased. The culture has the potential of being exploited in medicine as well as a biocontrol agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease is a progressive, degenerative disease characterized by memory loss, language deterioration, poor judgment, impaired visuospatial skills, etc. The pathogenesis of Alzheimer’s disease (AD) is related to deficiency in the brain neurotransmitter acetylcholine (ACh) [1]. The ACh which is released from the nerve endings has a very short half-life due to the presence of large amounts of AChE, an enzyme which hydrolyses the ester bond in the molecule, thus leading to loss of stimulatory activity. Reversible inhibition of this enzyme leads to an increase in neurotransmitter concentration within the synaptic cleft, which positively affects Alzheimer’s disease patients [2]. This makes enzyme AChE an attractive target for rational drug design and the discovery of mechanism-based inhibitors for the treatment of Alzheimer’s disease. The inhibitors of AChE promote an increase in concentration and duration of action of ACh [3]. AChE inhibitors are the most effective approach to treat the cognitive symptoms of Alzheimer disease [4, 5] and other possible therapeutic applications in the treatment of Parkinson’s disease, senile dementia, and ataxia [6]. AChE inhibitors such as eserine, tacrine, donepezil, rivastigmine, and galanthamine are the drugs currently approved for the treatment of AD; however, these drugs are known to have limitations for clinical use due to their low bioavailability, short half-lives, and/or unfavorable side effects like hepatotoxicity [7]. Therefore the search for novel acetyl cholinesterase inhibitors with better properties is necessary.
Another major use of AChEIs is in agriculture where they are used for the control of insects and some other arthropod pests. This use extends into pharmacy since some AChEIs are used as insecticides for treating infections of headlice. Insecticidal activity is based on the overstimulation of the cholinergic system in the insect. This property of the inhibitor has been exploited to develop newer insecticides against a wide range of insect pests [8].
AChE inhibitors have been reported to be produced by some fungi; the fungi Aspergillus terreus [9, 10], Penicillium sp. [11, 12] Chrysosporium sp. [13], and Xylaria sp. [14] have been reported to produce AChE inhibitors. But traditionally, most fungi have been isolated from soil samples. Although soil fungi have provided a broad spectrum of secondary metabolites with diverse chemical structures, the most exciting recent discoveries have come from exploration of fungi living in unusual ecological niches such as endophytic fungi [15, 16]. Endophytes are microorganisms that reside asymptomatically in the tissues of higher plants and are a promising source of novel organic natural metabolites exhibiting a variety of biological activities. Endophytes provide a broad variety of bioactive secondary metabolites with unique structure, including alkaloids, benzopyranones, chinones, flavonoids, phenolic acids, quinones, steroids, terpenoids, tetralones, xanthones, and others [17]. Such bioactive metabolites find wide-ranging application as agrochemicals, antibiotics, immunosuppressants, antiparasitics, antioxidants, and anticancer agents [18]. Endophytic fungi, in spite of their immense potential as sources of novel and important bioactive molecules, however, have not been screened and characterized for AChE inhibitors. Very few reports of screening of endophytic fungi for AChE inhibitors are available [19].
Thus, keeping in view the vast potential of endophytic fungi to produce bioactive molecules, this study aimed at isolating and screening the endophytes for the production of cholinesterase inhibitors from Ricinus communis and screening them for their insecticidal potential against Spodoptera litura (Fab). S. litura is one of the most destructive pest of crops like cruciferous vegetables, cucurbits, groundnut, cotton, maize, potato, soybean, tobacco, and some pulses [20]. Pesticides belonging to different groups have been recommended for the control of this pest; however, S. litura has developed physiological resistance against commonly used insecticides.
Materials and Methods
Chemicals
Acetylthiocholine iodide (ATCI), 5, 5′- dithiobis [2-nitrobenzoic acid] (DTNB), acetylcholinesterase from electric eel (type VI-S lyophilized powder, 425.94 U mg−1 solid, 500 U mg−1 protein; Sigma, USA), galanthamine (Sigma, USA), bovine serum albumin (BSA), sodium dihydrogen orthophosphate (NaH2PO4·2H2O), sodium chloride (NaCl), and magnesium chloride (MgCl2) [Himedia Laboratories, Mumbai.]
Isolation of Endophytic Fungi
Fifty-four cultures were isolated from R. communis. Different samples of stems and leaves from 15 healthy plants of R. communis were collected from Guru Nanak Dev University campus of Amritsar (India) for the isolation of endophytic fungi. The material was thoroughly washed using distilled water, followed by treatment with 70 % ethanol for 2 min and 4 % sodium hypochlorite for 3 min to accomplish surface sterilization. It was again rinsed in sterile distilled water prior to plating. The water obtained after the last wash was plated on PDA to ensure complete surface sterilization. The samples were cut into 5–6 pieces (2–6 mm size) and placed on water agar plates supplemented with chloramphenicol (200 μg/ml) and incubated at 30 °C for 3–4 days to few weeks till the growth initiated. The hyphal tips that emerged from the plant parts were picked, purified, and maintained on PDA plates for further studies.
Production of Cholinesterase Inhibitor
For primary screening, Erlenmeyer flasks (250 ml) containing 50 ml of liquid production medium (malt extract) were inoculated with three agar plugs (8 mm diameter) taken from the periphery of growing cultures of endophytic isolates purified on PDA plates. The flasks were incubated at 250 rpm on a rotary shaker at 30 °C for 10 days. Thereafter, 50 ml of ethyl acetate was added to each of the flask and extraction was carried at 120 rpm and 40 °C for 1.5 h. The organic phase thus obtained was separated and concentrated on rotary evaporator (BUCHI). The concentrated samples were then re-suspended in HPLC grade water for further assay.
Identification of Culture
The culture exhibiting maximum production was identified by both conventional methods (morphological and microscopic methods) and molecular analysis (DNA isolation, amplification). Slide culturing was done to determine the microscopic features for morphological characterization according to standard taxonomic key [21].
Molecular Characterization
The culture was identified by using primers primer pairs ITS1 and ITS4 for amplification of region covering ITS I-rDNA-ITS II involving the following steps:
-
(i)
Isolation of DNA
For DNA extraction, the fungus was grown in glucose broth for 72 h at 30 °C under shaking conditions (120 rpm) and the resultant mycelium was harvested by vacuum filtration and stored at −20 °C. The chilled mycelia (200 mg) was ground in 550 μl of extraction buffer (50 mmol l−1 Tris–Cl, pH 8.0; 700 mmol l−1 NaCl; 10 mmol l−1 EDTA, 1 % (v/v) β-mercaptoethanol and 10 % (w/v) SDS) and then 300 μl of equilibrated phenol and extraction buffer was added. The contents were homogenized and incubated for 15 min at 65 °C. The DNA in the aqueous phase was purified with repeated extractions using equal volumes of saturated phenol, chloroform, and isoamyl alcohol (PCI) mixture (25:24:1). The DNA was precipitated with 9 parts of ice cold isopropyl alcohol and 1 part of sodium acetate (3 mol l−1, pH 8.0) and kept at −20 °C for 2 h, followed by centrifugation for 15 min at 8,000×g. The DNA pellet was rinsed with 70 % (v/v) ethanol, air dried, suspended in 50 μl of sterilized double distilled water, and stored at 4 °C.
-
(ii)
PCR Amplification
DNA coding for internal transcribed spacers (ITS I and ITS II) and the intervening 5.8S rDNA region was amplified using universal primers, ITS1 (5′ TCCGTAGGTGAACCTGCGG 3′) and ITS4 (5′ TCCTCCGCTTATTGATATGC 3′). The PCR amplification was carried out in 0.2 ml PCR tubes, using Master cycler personal (Eppendorf). The PCR reaction mixture (50 μl) contained 25 μl of PCR master mix (Genei, Bangalore, India), 2.5 μl of DMSO, 1 pmol l−1 of each primer, and 100 ng of template DNA. Thermal cycling conditions were as follows: initial denaturation (4 min at 95 °C), followed by 30 cycles of denaturation (94 °C for 50 s), annealing (51 °C for 1 min), and primer extension (72 °C for 1 min), followed by final extension step for 10 min at 72 °C. Amplification products were electrophoretically resolved on 1.4 % (w/v) agarose gel containing ethidium bromide, using 1× TAE buffer at 70 eV [22].
Phylogenetic Analysis
The purified amplified internal transcribed spacer (ITS) region was sequenced by SPA services (Genei, Bangalore, India). The ITS sequence was aligned to sequences retrieved from NCBI databases, using multiple sequence alignment software (CLUSTAL W). Phylogenetic analysis was carried out using MEGA software ver. 5.0 [23]. The neighbor-joining (NJ) method was used to infer the evolutionary history of the isolates and the bootstrapping was carried out using 1,000 replications. Aspergillus fumigatus was taken as out group. The ITS sequence was deposited with NCBI GenBank.
Acetylcholinesterase Inhibition Assay
Acetylcholinesterase inhibition assay (in vitro) was carried out by modified Ellman’s method [24]. Buffer A (50 mM Tris–Cl) of pH 8.0 was prepared for the preparation of buffer B and buffer C. The reaction mixture contained 140 μl of buffer B, 10 μl of dithiobisnitrobenzoate (DTNB 3 mM in buffer C), 20 μl of test compound solution, and 20 μl of AChE solution (0.2 U/ml) which were mixed and incubated for 10 min at 25 °C (where buffer B was prepared by adding 0.1 % bovine serum albumin in buffer A and buffer C was prepared by adding 0.1 M NaCl and 0.02 M MgCl2 in buffer A) [25]. The reaction was then initiated with the addition of 10 μl acetylthiocholine iodide (15 mM in buffer C). After the incubation, hydrolysis of acetylthiocholine iodide was monitored at a wavelength of 415 nm by the formation of a yellow colored 5-thio-2-nitrobenzoate anion as a result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine iodide. All the reactions were performed in triplicate in 96-well micro-plate. Galanthamine, a known acetylcholinesterase inhibitor, was used as a positive control. The percent inhibition was calculated using the formula:
False positive results were eliminated according to method described by Rhee et al. [26].
Estimation of the IC50 Value
The concentrations of the extract that inhibited the hydrolysis of substrate acetylthiocholine by 50 % (IC50) was determined by monitoring the effect of increasing concentrations of these compounds in the assays on the inhibition values [27].
TLC for AChE Inhibition
Acetylcholinesterase inhibitory activity was also determined using TLC assay method and staining with Ellman’s reagent (DTNB), following the technique described by Rhee et al. [26]. Ten microliters (40 μg/ml) of test sample (dissolved in methanol) was spotted on silica gel TLC plate. Ten microliters (100 μg/ml) of galanthamine solution in methanol was also spotted as a reference. Solvent system used contained chloroform, methanol, and water in the ratio of 7:3:1. After developing the TLC plate, enzyme inhibitory activity was detected by spraying it with the substrate and dye solution, followed by enzyme solution spray after proper drying. The presence of cholinesterase inhibitory activity was determined by the formation of well-defined white spots made visible by spraying with enzyme, which gives a yellow background.
Optimization of Inhibitor Production
The culture showing maximum inhibitory activity was selected for further optimization studies. The effect of media types was studied by growing the culture in different media viz. Malt yeast extract broth (MYEB), Czapek–Dox broth (CDB), Sabouroud dextrose broth (SB), potato dextrose broth (PDB), lactose based medium broth (LPM), dextrose peptone broth (DPM), and glucose broth (GB). Effect of incubation time on the production of metabolite was studied for 14 days. Inhibitory activity was measured at regular intervals of 24 h. Extraction was carried out in different solvents (diethyl ether, butanol, ethyl acetate, chloroform, and hexane) to determine the best solvent for extraction. Inoculum level was optimized by inoculating the flasks with different number of plugs [1–4] of 8 mm diameter taken from the periphery of an actively growing colony.
Partial Purification
The concentrated extract (1 ml) obtained from 1 l of fermented broth was chromatographed on silica gel column (60–120) and eluted with a CHCl3–MeOH gradient (from 9:1 to 1:9). Fractions of 20 ml each were collected and analyzed for inhibitory activity after concentration.
Evaluation of Insecticidal Activity
Insect Rearing
Initial stocks for the culture of S. litura were obtained from the cauliflower fields around Amritsar (Punjab), India and subsequent generations were reared under controlled temperature and humidity conditions of 25 °C and 70 %, respectively. The rearing was carried out in battery jars (15 × 10 cm) on castor leaves with daily change of diet. The pupae were transferred to pupation jars containing 2–3 cm layer of moist sterilized sand covered with filter paper. The adults on emergence were shifted to oviposition jars similar to pupation jars except for a cotton swab soaked with honey solution (1:4 honey/water) as food, hanging from the muslin cloth covering the jar. The oviposition jars were lined with filter paper to facilitate egg laying. The newly hatched larvae were transferred to fresh castor leaves.
Bioassay Studies
The artificial diet of S. litura was prepared as recommended by Koul et al. [28]. To evaluate the insecticidal effect, the diet was supplemented with different concentrations of extract of Cas1 (5, 10, 15, and 20 μl/ml) to study the effect on growth and development of S. litura, the 2nd instar larvae were reared on amended as well as unamended diets. The effect of partially purified extract on survival of S. litura was also evaluated by supplementing the diet at a concentration of 20 μl/ml. Each experiment was replicated six times with five larvae per replication. To avoid cannibalism, the larvae were kept individually in plastic containers (4 × 6 cm) and diet was changed regularly. The observations on larval mortality were recorded daily.
Statistical Analysis
The values on percent larval mortality were represented as their mean ± SE. To compare difference in means, one-way analysis of variance (ANOVA) with Tukey’s test at p ≤ 0.05 was performed. SPSS software for windows version 16.0 (SPSS Inc, Chicago) and Microsoft office Excel 2003 (Microsoft Corp., USA) were used to perform the statistical analysis. The difference between the mean value of treated and untreated (control) concentrations was estimated for significance by applying Student’s t test.
Results and Discussion
In the present study, 54 endophytic fungal isolates were isolated from R. communis and screened for their potential to produce acetylcholinesterase inhibitors. Forty-two isolates evinced negligible activity whereas activity in the range of 50–70 % was observed in three isolates. Maximum inhibition was observed in culture Cas1 (78 %). The fungus was identified according to standard taxonomic key including colony diameter, color, and the morphology of hyphae and conidia. Colonies were fast growing, black to olivaceous-black or grayish, felt like and had an average diameter of 5 cm when incubated on PDA plates at 30 °C for 7 days (Fig. 1a). The reverse side was brown to black due to pigment production. The hyphae were septate and dark. Multicelled conidia with short beaks were produced in chains (Fig. 1b). On the basis of the observed morphological characteristics, the strain Cas1 could be positioned in the genus Alternaria. The genetic relationships of strain Cas1 were determined by amplification of the ITS region including 5.8S rDNA by PCR. The size of the amplified sequence was 645 bps. After sequencing, the newly identified sequence was submitted to and deposited into GenBank under accession number JX177676. The sequence of strain Cas1 was found to share 99 % similarity with Alternaria sp. KL-2010. After alignment with homologous nucleotide sequences, phylogenetic relationship revealed the strain Cas1 to be closest to the genus Alternaria (Fig. 2).
Phylogenetic tree showing the position of Cas1 on the basis of ITS1-5.8 rDNA-ITS2 gene sequence analysis. The evolutionary history was inferred using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The evolutionary distances were computed using the Kimura two-parameter method and were in the units of the number of base substitutions per site. The analysis involved 14 nucleotide sequences. All ambiguous positions were removed for each sequence pair. There were a total of 1,800 positions in the final dataset. Evolutionary analyses were conducted in MEGA5
The culture Cas1 was selected for further investigations as it was found to be potent producer of AChE inhibitor. Soil fungi like A. terreus [9, 10] and Penicillium sp. [11, 12], Chrysosporium sp. [13], and Xylaria sp.[14] have been reported to produce AChE inhibitors. AChE inhibitory activity has also been reported in yeast Yarrowia lipolytica [29]. But inspite of the tremendous potential of endophytes as a source of bioactive molecules, there are very few reports on their ability to produce AChE inhibitors. Extracts of culture filtrates of fungal taxa belonging to Chaetomium sp., Guignardia mangiferae, Pestalotiopsis guepinii, Phomopsis sp., Physalospora sp., Xylaria sp., and sterile mycelium isolated as endophytes from leaves of members of the plant families Anacardiaceae, Apocynaceae, Leguminosae, and Palmae are reported to possess inhibitory activity towards AChE [19]. Endophytic fungi Shiraia sp. and Cladosporium cladosporoides isolated from the leaves of Huperzia serrata have been shown to produce an AChE inhibitor, huperzine [30, 31]. But there are no reports of production of AChE inhibitor by any endophytic Alternaria sp.
Optimization of various parameters was undertaken to enhance the production of the inhibitor using one variable at a time approach. The inhibitory activity was analyzed after growing the culture on seven different media. Maximum inhibition was obtained on potato dextrose broth (75 %) followed by LPM (70 %) whereas on Czapek–Dox negligible activity was obtained (Fig. 3). The best solvent for extraction was determined to be chloroform (Fig. 4). Effect of incubation time on the production of the metabolite was studied for 14 days. Inhibitory activity was measured at regular intervals of 24 h. Maximum activity was obtained after 10 days of incubation. The effect of inoculum size on the production was determined by varying the number of plugs used for inoculation. No significant difference in inhibitory activity was observed when number of plugs was increased from one to four. An effort was made to relate the production of the compound with the biomass produced. As evident from the growth curve, the stationary phase was attained after 6 days of incubation whereas the activity appeared on the 5th day reaching its maximum level after 10 days indicating that the inhibitor is a product of secondary metabolism (Fig. 5). The IC50 value of extract which was obtained from intersection point of % activity and % inhibition versus extract concentration curve equaled to 40 μg/ml (Fig. 6).
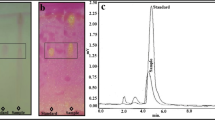
The crude extract was partially purified on silica (60–120) using gradient of chloroform: methanol. The inhibitory activity was obtained in 9th fraction when eluted with 9:1 (chloroform/methanol). TLC-based bioassay for the inhibitor was carried out for Cas1 using the method described by Rhee et al. [26]. Both the crude and partially purified extracts were subjected to TLC. On comparison of the plates visualized under UV (254 nm; Fig. 7a) and subjected to bioautography, it could be concluded that the inhibitor had an Rf value of 0.881 when the solvent system used was chloroform/methanol/water, 7:3:1 (Fig. 7b). The absorption maxima of partially purified extract was found to be at 240 nm ( ; Fig. 8).
; Fig. 8).
The crude as well as partially purified extracts were evaluated for insecticidal activity. All the concentrations of crude extract induced significantly higher mortality in S. litura larvae as compared to control. The concentrations ranging from 10 to 20 μl/ml were significantly more effective than the lowest concentration (Fig. 9). The partially purified extract at a concentration of 20 μl/ml induced significantly higher larval mortality than the same concentration of crude extract (t = 0.54, p ≤ 0.05; Fig. 10). Species of the genus Alternaria are known to produce substances active against insects. Podova et al. [32] isolated a potent insecticidal substance from stationary cultures of an unidentified species of Alternaria which inhibited the development of Drosophila melanogaster. It has been also reported as a pathogen of onion thrips, Thrips tabaci, and cereal leaf beetle, Oulema gallaeciana larvae [33]. Alternaria sp. has also been isolated from 3rd and 4th instar larvae of species of Aedes, Anopheles, and Culex [34]. Christias et al. [35] have also reported the potential the Alternaria alternata for the biological control of aphids.
Percent mortality of S. litura larvae by crude and partially purified extract of Cas1. (Paired sample t test at p ≤ 0.05 applied to compare mortality in larvae fed on diet containing 20 μl/ml concentration of partially purified and crude extract of Cas1. Single asterisk indicates significant differences between the treatments). Values are represented as mean ± standard error (n = 6)
It is evident from these studies that the AChE inhibitor produced by Alternaria sp. can be a good candidate for the biocontrol of S. litura. Further work on the effect of the fungus on the development and physiology of the insect is in progress. The compound after purification and characterization can have potential in the field of medicine as well as agriculture.
References
Bartus, R. T., & Dean, R. L. (1982). Science, 30, 408–414.
Darvesh, S., & Hopkins, D. A. (2003). The Journal of Comparative Neurology, 463, 25–43.
Rollinger, J. M., Hornick, A., Langer, T., Stuppner, H., & Prast, H. (2004). Journal of Medicinal Chemistry, 47, 6248–6254.
Holden, M., & Kelly, C. (2002). Advance Psychology Treatment, 8, 89–96.
Choudhary, M. I., Yousuf, S., Nawas, S. A., Ahmed, S., & Rahman, A. U. (2004). Chemical and Pharmaceutical Bulletin, 52, 1358–1361.
Ahmad, W., Ahmad, B., Ahmad, M., Iqbal, Z., Nisar, M., & Ahmad, M. (2003). Journal of Biological Sciences, 3, 1046–1049.
Melzer, D. (1998). BMJ, 316, 762–764.
Houghton, P. J., Ren, Y., & Howes, M. J. (2006). Natural Product Reports, 23, 181–199.
Ling, K. H., Yang, C. K., & Peng, F. T. (1979). Applied and Environmental Microbiology, 37, 355–357.
Kim, W.-G., Cho, K.-M., Lee, C. K., & Yoo, I. D. (2003). The Journal of Antibiotics, 56, 351–357.
Kuno, F., Otoguro, K., Shiomi, K., Iwai, Y., & Omura, S. (1996). The Journal of Antibiotics, 49, 742–747.
Otoguro, K., Shiomi, K., Yamaguchi, Y., Arai, N., Sunazuka, T., & Masuma, R. (2000). The Journal of Antibiotics, 53, 50–57.
Sekhar, K. C., Divakar, S., Karanth, N. G., & Sattur, A. P. (2001). The Journal of Antibiotics, 54, 848–849.
Lin, Y., Wu, X., Feng, S., Jiang, G., Luo, J., & Zhou, S. (2001). The Journal of Organic Chemistry, 66, 6252–6256.
Schulz, B., Boyle, C., Draeger, S., & Krohn, K. (2002). Mycological Research, 106, 996–1004.
Strobel, G. A. (2003). Journal Microbiologica Information, 5, 535–544.
Tan, R. X., & Zou, W. X. (2001). Natural Product Reports, 18, 448–459.
Jalgaonwala, R. E., Mohite, B. H., & Mahajan, R. T. (2011). Journal Microbiology Biotechnology Research, 1, 21–32.
Rodrigues, K. F., Sieber, T. N., Grünig, C. R., & Holdenrieder, O. (2004). Mycological Research, 108, 45–52.
Xue, M., Pang, Y. H., Wang, H. T., Li, Q. L., & Liu, T. N. (2009). Journal of Insect Science, 10, 186–194.
Larone, D.H. (2002). Medically important fungi: a guide to identification. Washington, DC: ASM press.
Sharma, M., Chadha, B. S., Kaur, M., Ghatora, S. K., & Saini, H. S. (2008). Letters in Applied Microbiology, 46, 526–535.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). Molecular Biology and Evolution, 28, 2731–2739.
Ellman, G. L., Courtney, K. D., Andres, V., & Featherstone, R. M. (1961). Biochemical Pharmacology, 7, 88–95.
Pagliosa, L. B., Monteiro, S. C., Silva, K. B., deAndrade, J. P., Dutilh, J., Bastida, J., et al. (2009). Journal Phytomedicine, 17, 698–701.
Rhee, I. K., Meent, M. V., Ingkaninan, K., & Verpoorte, R. (2001). Journal of Chromatography, 915, 217–223.
Wetiwitayaklung, P., Limmatvapirat, C., Phaechmud, T., & Koeokitichi, S. (2007). Silpakorn University Science and Technology Journal, 1, 20–28.
Koul, O., Shankar, J. S., Mehta, N., Taneja, S. C., Tripathi, A. K., & Dhar, K. L. (1997). Journal Applied Entomology, 121, 245–248.
Lee, D. H., Lee, J. S., Yi, S. H., & Lee, J. S. (2008). Korean Society Mycologie, 36, 102–105.
Zhu, D., Wang, J., Zeng, Q., Zhang, Z., & Yan, R. (2010). Journal Applied Microbiology, 109, 1479–1486.
Zhang, Z. B., Zeng, Q. G., Yan, R. M., Wang, Y., Zou, Z. R., & Zhu, D. (2011). World Journal of Microbiology Biotechnology, 27, 479–486.
Podova, M., Dobias, J., & Nemec, P. (1977). Biologia (Bratislava), 32, 657–662.
Machowicz-Stefaniak, Z., & Miczulski, B. (1985). Roczniki Nauk Rolniczych, 15, 151–157 (in Polish).
Rybalchenko, V. M., & Gopkalo, E. L. (1980). Mikrobiologicheskii Zhurnal (Kiev), 42, 446–452. in Russian.
Christias, C. H., Hatzipapas, P., Dara, A., Kaliafas, A., & Chrisanthis, G. (2001). BioControl, 46, 105–124.
Acknowledgments
The authors are grateful to Council of Scientific and Industrial Research (CSIR), New Delhi for providing financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, B., Thakur, A., Kaur, S. et al. Acetylcholinesterase Inhibitory Potential and Insecticidal Activity of an Endophytic Alternaria sp. from Ricinus communis . Appl Biochem Biotechnol 168, 991–1002 (2012). https://doi.org/10.1007/s12010-012-9835-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9835-0










 )
)
