Abstract
Supermacroporous poly(2-hydroxyethyl methacrylate) [poly(HEMA)] monolithic cryogel was prepared by radical cryocopolymerization of HEMA with N,N′-methylene bisacrylamide as crosslinker. Reactive Green 5 dye was immobilized to the cryogel with nucleophilic substitution reaction, and this dye attached cryogel column was used for affinity purification of papain from Carica papaya latex. Reactive Green 5-immobilized poly(HEMA) cryogel was characterized by swelling studies, Fourier transform infrared spectroscopy, scanning electron microscopy, and energy dispersive X-ray analysis. Maximum papain adsorption capacity was found to be 68.5 mg/g polymer while nonspecific papain adsorption onto plain cryogel was negligible (3.07 mg/g polymer). Papain from C. papaya was purified 42-fold in single step with dye attached cryogel, and purity of papain was shown by silver-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteases are enzymes that catalyze hydrolytic reactions in which protein molecules are degraded to peptides and amino acids. These constitute a very large and complex group of enzymes, which differ in properties such as substrate specificity, active site and catalytic mechanism, pH and temperature optima, and stability profile [1]. Papain, a highly stable enzyme, is one of the sulfhydryl proteases of Carica papaya latex. Papain is a carbohydrate-free, basic, single chain protein. Papain has molecular weight of 23,350 Da and consists of 212-amino acid residues (methionine absent; pI 8.75) with four disulfide bridges and catalytically important cysteine (position 25) and histidine residues (position 158) [2]. Papain presents anti-inflammatory, antibacterial, and antioxidant properties and can be used in the treatment of large skin lesions [3]. In the food industry, papain is used to tenderize meat and related derivatives, to produce protein hydrolysate, to clarify juice and bear in the brewing industry, for cheese production in dairy industry, in baking industry, and for the extraction of flavor and color compounds from plants [4]. Papain has been used in cell isolation and in the separation of biomolecules in large-scale industry processes. Besides, papain is widely used in the leather, cosmetic, textiles, detergents, food, and pharmaceutical industries [5].
Purification of papain from C. papaya latex has traditionally been achieved by precipitation methods [6–9]. However, the purified enzyme still remains contaminated with other proteases. An alternative purification strategy has involved the use of various chromatographic techniques including ion exchange, covalent, or affinity column [10–12].
A number of textile dyes, known as reactive dyes, have been used for protein separation in dye ligand affinity systems, since they bind a variety of proteins in a selective and reversible manner. Most of the reactive dyes used in dye-affinity systems consist of a chromophore (either azo dyes, anthraquinone, or phthalocyanine), linked to a reactive group (often a mono- or dichlorotriazine ring). They also have sulfonic acid groups to provide the desired solubility of the molecule in aqueous media. Dye ligands are commercially available and inexpensive. They can easily be immobilized, especially on matrices bearing hydroxyl groups. The interaction between the dye ligand and proteins can be by complex combination of electrostatic, hydrophobic, and hydrogen bonding [13–17]. Reactive Green 5, also named Procion Green H-4G, is a monochlorotriazine dye (Fig. 1), which contains seven acidic sulfonate groups and three basic secondary amino groups. It also possesses a phthalocyanine moiety containing Cu(II) ion [18].
Increasing demands for pure biologically active substances (low molecular weight compounds, proteins, DNA, etc.) require development of polymeric materials used in bioseparation [19]. One of the new polymeric materials with considerable potential in biotechnology is cryogels. Cryogels are prepared as a result of cryogenic treatment (freezing, storage in the frozen state for a definite time, and defrosting) of precursors which capable of gelling [20]. The main applications of cryogels are found in biocatalysis with immobilized cells and enzymes, in bioseparations for purification of biological compounds, chromatography of cells and its organelles, and in biomedical applications as 3D matrixes for culture of cells [21]. In our research group, cryogels with different functionalities were used for purification and separation of different kinds of proteins [15, 22–27].
The aim of this study was to investigate the affinity of the Reactive Green 5 for C. papaya papain. So far, only a few dye-affinity adsorbents have been reported for the adsorption of papain [5, 28–30]. For this, poly(2-hydroxyethyl methacrylate) [poly(HEMA)] cryogel was prepared by radical cryocopolymerization of HEMA and N-N′-methylene bisacrylamide (MBAAm) as a crosslinker directly in a plastic syringe. Then, Reactive Green 5 dye was immobilized to the cryogel with nucleophilic substitution reaction. The Reactive Green 5-immobilized cryogel was characterized by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), energy dispersive X-ray (EDX), and swelling test. Papain adsorption studies on the dye-immobilized cryogel were carried out in a series of aqueous solutions containing different amounts of papain at different buffers and pHs, and ionic strengths. Desorption of the papain and the reusability of the dye-immobilized cryogel were also investigated. Papain from C. papaya latex was purified by dye attached cryogel, and purity was demonstrated with silver-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE).
Materials and Methods
Materials
Papain (from C. papaya, EC 3.4.22.2), casein, HEMA, MBAAm, N,N,N′,N′-tetramethylene diamine (TEMED), ammonium persulfate (APS), and Reactive Green 5 (Procion Green H-4G) were supplied by Sigma (St. Louis, MO, USA). All other reagents were of analytical grade and used without further purification. All solutions were prepared with deionized ultrapure Millipore Simplicity® (18.2 MΩ cm) water.
Preparation of Poly(HEMA) Cryogel
Preparation of the poly(HEMA) cryogel is described below. HEMA (1.3 mL) were dissolved in 5.0 mL deionized water. MBAAm (0.283 g) was dissolved in 10 mL deionized water. Second solution was mixed with previous one. The cryogel was then produced by free radical polymerization initiated by TEMED and APS. After adding 20 mg of APS (1 % (w/v) of total monomers), the solution was cooled in an ice bath for 2 to 3 min. Then 25 μL of TEMED (1 % (w/v) of total monomers) was added, and the reaction mixture was poured into a plastic syringe (5 mL, i.d. 0.8 cm) with closed outlet at the bottom. The polymerization solution in the syringe was frozen at −12 °C for 24 h and then thawed at room temperature. Extensive cleaning procedure for removal of unconverted monomers and initiator was performed. Briefly, washing solutions (i.e., a dilute HCl solution and water–ethanol mixture) were recirculated through the monolithic cryogel column, until to be assured that the cryogel column is clean [31].
Reactive Green 5 Immobilization to Poly(HEMA) Cryogel
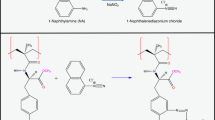
Reactive Green 5 immobilization studies were performed in a recirculating system equipped with a water jacket temperature control. The poly(HEMA) cryogel was washed with 30 mL of deionized water. Then, 100 mL of Reactive Green 5 solution (5 mg/mL) containing NaOH (5 g) was pumped through the glass column under recirculation at 80 °C for 2 h. A nucleophilic substitution reaction takes place between the chloride of Reactive Green 5’s triazine ring and the hydroxyl group of the HEMA, with the elimination of NaCl, resulting in the coupling of Reactive Green 5 to the poly(HEMA) cryogel under alkaline conditions.
Characterization of Dye-Immobilized Cryogel
The FTIR spectrum of the Reactive Green 5 and Reactive Green 5-immobilized cryogel was analyzed by using a FTIR spectrophotometer (Varian FTS 7000, USA). For this, dry cryogel (0.1 g) was mixed with KBr (0.1 g) and pressed into a pellet form. The FTIR spectrum was recorded. The morphology of a cross section of the dried cryogel was investigated by scanning electron microscopy. The sample was fixed in 2.5 % glutaraldehyde solution for overnight. Then the sample was dehydrated at −50 °C in freeze dryer (Freezone 6, Model 77520; Labconco Co. Kansas City, MO, USA). Finally, it was coated with gold and examined using a scanning electron microscope (Philips XL-30S FEG, the Netherland). Reactive Green 5 loading to poly(HEMA) cryogel was determined by EDX analysis instrument (LEO, EVO 40, Carl Zeiss NTS, Peabody, MA, USA). The amount of Reactive Green 5 loading on the poly(HEMA) cryogel was calculated from these data considering the sulfur stoichiometry. The swelling degree of cryogel (S) was determined as follows: Cryogel was washed with water until washing water was clear. Then it was sucked dry and then transferred to previously weighed vial and weighed (m wet gel). After drying to constant mass in the oven at 60 °C, the mass of dried sample was determined (m dry gel). The swelling degree was calculated as:
Papain Adsorption–Elution Studies from Aqueous Solutions
Papain adsorption studies were carried out in a column system equipped with a water jacket for temperature control. The cryogel was washed with 50 mL of water and then equilibrated with 20 mM Tris–HCl buffer (pH 8.0). Then, the prepared papain solution (10 mL of papain solution) was pumped through the column for 2 h. The adsorption was determined by Bradford method at 595 [32]. The effects of pH and buffer type, initial papain concentration, and ionic strength on adsorption capacity were investigated. To determine the effects of pH and buffer type on the adsorption 3-(N-morpholino)propanesulfonic acid (MOPS; pH 6.0–7.5), 2-(N-morpholino)ethanesulfonic acid (MES; pH 5.5–6.5), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.0–8.0), Tris–HCl (pH 7.0–8.5), acetate (pH 5.0–6.0), and phosphate (pH 6.0–7.5), buffers were used. To observe the effects of the initial concentration of papain on adsorption, it was changed between 0.5 and 4.0 mg/mL. To observe the effects of ionic strength, NaCI solution was used at ionic strength values of 0.01 and 1.0. Papain elution from cryogel was performed with 1.0 M NaCl. In order to show the reusability of cryogel, adsorption–elution cycle of papain was repeated 40 times by using the same cryogel.
Assay of Papain Activity
Papain activity was measured at room temperature in 50 mM phosphate buffer (pH 7.5) containing 38 mM EDTA and 34 mM cysteine chloride, using casein as substrate, by the modified method of Kembhavi et al. [33]. For this, 400 μL of 1 % (w/v) aqueous solution of casein was added to 50 μL of enzyme solution diluted in 350 μL of buffer, and the reaction mixture was incubated at room temperature for 10 min. After incubation casein, proteolysis was stopped by addition 800 μL of 10 % trichloroacetic acid solution. The mixture was incubated at room temperature for 30 min, centrifuged at 12,000×g for 10 min (Universal 32R, Hettich, Tuttlingen, Germany), and the absorbance of the supernatant measured at 280 nm. One unit of enzyme was taken as the amount of enzyme that hydrolyzes casein to produce equivalent absorbance to 1 μmol of tyrosine/min with tyrosine as standard. Total protein concentrations were measured by the Bradford method.
Purification of Papain from C. papaya Latex
Fresh latex was obtained from developing green fruits directly picked from trees in the vicinity of Alanya Province of Southwest Anatolia, Turkey in August. Three or four vertical incisions were made in the fruits with a sharp stainless steel instruments to a depth of 2 to 3 mm. The latex that surfaces after incision lasts only 1 to 2 min and then rapidly coagulates and can be collected into a glass container. After extraction, the latex was immediately used for the purification of papain in its native state or stored at −8 °C. Then 1 mL of latex was mixed with 10 mL of 50 mM phosphate buffer, pH 7.5 containing 38 mM EDTA and 34 mM cysteine chloride. The mixture was incubated at 4 °C for 1 h. After centrifugation for 10 min at 5,000×g, 6 mL of supernatant was pumped through the Reactive Green 5-immobilized cryogel column at a flow rate of 0.5 mL/min for 2 h. The elution of papain from the cryogel was carried out with 10 mL of 1.0 M NaCl solution. Eluated protein amount was determined and the activity of papain was determined spectrophotometrically. The purity of papain in the purified samples was analyzed using SDS–PAGE (Bio-Rad, Mini Protean 3) using 10 % (w/v) acrylamide in gels as described by Laemmli [34]. Protein bands were visualized by Coomassie Brilliant Blue R-250 and silver staining.
Results and Discussion
Characterization of Dye-Immobilized Cryogel
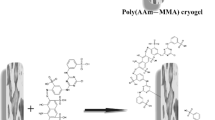
Poly(HEMA) cryogel was synthesized by copolymerization in the frozen state of HEMA with MBAAm in the presence of APS/TEMED. Then, the hydroxyl groups on the poly(HEMA) allowed modification with Reactive Green 5. The chemical structure of dye-immobilized cryogel was demonstrated in Fig. 1. Figure 2 shows the FTIR spectra of poly(HEMA) cryogel and Reactive Green 5-immobilized poly(HEMA) cryogel. The FTIR spectrum of Reactive Green 5-immobilized poly(HEMA) cryogel has characteristic stretching vibration band of hydrogen bonded alcohol, O–H, around 3,500 cm−1. Those are at 3,400, 1,550, and 1,260 cm−1 characteristic N–H stretching, conjugated C=N, and aromatic C–N vibration. The bands at 1,078 and 1,161 cm−1 represent symmetric stretching of S=O and asymmetric stretching of S=O, respectively. The SEM image of the internal structure of the dye-immobilized cryogel is shown in Fig. 3. The dye-immobilized cryogel has porous and thin polymer walls, large continuous interconnected pores (10–20 μm in diameter) that provide channels for the mobile phase flow through. Pore size of the cryogel is much larger than the size of papain, allowing them to enter easily through the pores. As a result of the convective flow, the mass transfer resistance is practically negligible. The equilibrium swelling degree of the dye-immobilized cryogel was 7.72 g H2O/g cryogel. This cryogel is sponge-like, elastic, and green as a result of dye immobilization. The elemental analysis of the dye-immobilized cryogel was performed using the EDX on the SEM. Figure 4 shows the EDX spectrum of the dye-immobilized cryogel. The spectra obtained during EDX studies were used for carrying out the quantitative analysis. The incorporation of dye was found to be 4.02 μmol/g cryogel using sulfur stoichiometry. This sulfur amount determined by EDX comes from only incorporated Reactive Green 5 groups into the cryogel. The Reactive Green 5-immobilized cryogel was extensively washed with methanol until to ensure that there is no dye leakage from any of the dye-immobilized cryogel and in any media used at adsorption–desorption steps.
Papain Adsorption–Elution Studies from Aqueous Solutions
Papain adsorption onto Reactive Green 5-immobilized cryogel was investigated depending the buffer system and pH. For this purpose, adsorption studies were performed using MOPS (pH 6.0–7.5), MES (pH 5.5–6.5), HEPES (pH 7.0–8.0), Tris–HCl (pH 7.0–8.5), acetate (pH 5.0–6.0), and phosphate (pH 6.0–7.5) buffers. Figure 5 shows papain adsorption capacity in these buffer systems at different pH values. The highest papain adsorption capacity was observed at pH 8.0 for Tris–HCl buffer and 55.47 mg/g polymer. The isoelectric point of C. papaya papain is 8.75, and thus, protein would be cationic at pH values below 8.75. Reactive Green 5 molecule has seven acidic sulfonate, three secondary amino groups, and a Cu(II) ion containing phthalocyanine group. The pKa value of sulfonate groups of the Reactive Green 5 molecules is around 0.8, and dye ligand tends to be negatively charged under given experimental conditions. At pH 8.0, papain has positive charges and it could interact with the negatively charged dye ligand at this pH value. It should also be noted that most of the papain variants are isolated using a strong cation exchanger [35, 36]. Additional interactions (hydrophobic, electrostatic, and hydrogen bonding) could be present between papain and Reactive Green 5 molecules at pH 8.0 that may result from both the ionization state of several groups on both the dye ligand molecule (i.e., sulfonate, secondary amine, and hydrophobic groups) and amino acid side chains of papain.
The adsorption capacities of the Reactive Green 5-immobilized cryogel was determined by changing the initial concentration of papain between 0.5 and 4.0 mg/mL. Figure 6 shows the effect of equilibrium concentration of papain on the papain adsorption capacity onto dye-immobilized cryogel and plain cryogel [poly(HEMA)]. The adsorption values increased with increasing papain concentration, and the saturation value was achieved at 3.0 mg/mL of papain concentration which represents saturation of the active adsorption sites on the dye-immobilized cryogel. Maximum adsorption capacity was found to be 68.5 mg/g polymer. The nonspecific interaction between plain cryogel and papain molecules should be minimum to consider the interaction as specific. As seen in Fig. 6, nonspecific papain adsorption onto plain cryogel was negligible (3.07 mg/g polymer).
Adsorption isotherms were used to evaluate the properties of papain adsorption onto Reactive Green 5-immobilized cryogel. The Langmuir model is based on the assumption of surface homogeneity such as equally available adsorption sites, monolayer surface coverage, and no interaction between adsorbed species. The Freundlich isotherm is applicable to heterogeneous systems and reversible adsorption [37]. Langmuir and Freundlich isotherms are represented, respectively, as follows:
where b is the Langmuir isotherm constant, K f the Freundlich constant, and n is the Freundlich exponent. 1/n is a measure of the surface heterogeneity ranging between 0 and 1, becoming more heterogeneous as its value gets closer to zero. The ratio of q e gives the theoretical monolayer saturation capacity of dye-immobilized cryogel [38]. Some model parameters were determined by nonlinear regression with commercially available software as shown in Table 1. Comparison of all theoretical approaches used in this study shows that the Langmuir equation fits the experimental data best.
Figure 7 shows the effect of ionic strength on papain adsorption. As seen in the figure, the adsorption capacity of the Reactive Green 5-immobilized cryogel decreased with increasing ionic strength. The adsorption of papain decreased by about 92.4 % as the NaCl concentration changed from 0.01 to 1.0 M. This decrease could be attributed to the repulsive electrostatic interactions between the dye-immobilized cryogel and papain molecules. When the ionic strength increased, this could lead to neutralization of the deprotonated sulfonic acid groups of the dye with sodium ions of the salt (NaCl), which lead to low protein adsorption [14].
In order to show the stability and reusability of dye-immobilized cryogel, adsorption–desorption cycle was repeated 20 times using same cryogel. Up to 95.0 % of the adsorbed papain was desorbed by using 1.0 M NaCl as elution agent. For sterilization after one adsorption–desorption cycle, the cryogel was washed with 50 mM NaOH solution for 30 min. After the procedure, cryogel was washed with distilled water for 30 min, then equilibrated with the Tris–HCl buffer at pH 8.0 for the next adsorption and desorption cycle. Cryogels were very stable and maintained their adsorption capacity around 92.0 % at the end of 20 cycles.
Purification of Papain from C. papaya Latex
The latex of species C. papaya is well-known for being a rich source of the four cysteine endopeptidases: papain, chymopapain, glycyl endopeptidase, and caricain. Many methods were presented to purify papain from the other components present in the crude proteolytic mixture refined from latex. These methods are salt precipitation using (NH4)2SO4 [6] and NaCl [8], precipitation followed by affinity chromatography [39], hydrophobic and ionic exchange chromatography [40], precipitation at low temperature [36], aqueous two phase extraction using PEG and (NH4)2SO4 [41], and by adsorption onto membranes [12, 42]. In this study, single-step papain purification was carried out. It is easier-handling than that classical purification method, including several steps such as precipitation, centrifugation, and adsorption. The purity of desorbed papain was shown by silver-stained SDS–PAGE (Fig. 8). The calculated purification effectiveness and recovery are listed in Table 2. It was founded that the papain from C. papaya latex was purified by 42-fold. The specific activity of the purified papain with Reactive Green 5-immobilized cryogel was 3.45 U/mg, and this value was comparable to commercial papain (11.1 U/mg).
Conclusions
Dye ligand affinity chromatography has played an important role in the separation, purification, and recovery of proteins; because many inexpensive, stable, and group-specific dyes are available, they can be used for the separation of a large number of proteins and enzymes. Cryogels are a very good alternative to protein purification with many advantages. Several advantages of cryogels are large pores, short diffusion path, low pressure drop, and very short residence time for both adsorption and elution. This work reports on the purification of papain from C. papaya latex by dye ligand affinity chromatography with a cryogel column. Papain was purified 42-fold in single step. Purity of papain was shown by silver-stained SDS–PAGE. The Reactive Green 5 affinity cryogel provided a good method to purify papain, showing a high binding capacity and a high selectivity for papain.
References
Sumantha, A., Larroche, C., & Pandey, A. (2006). Food Technology and Biotechnology, 44, 211–220.
Ghosh, S. (2005). Colloids and Surfaces A, 264, 6–16.
Vasconcellos, F. C., Goulart, G. A. S., & Beppu, M. M. (2011). Powder Technology, 205, 65–70.
Homaei, A. A., Sajedi, R. H., Sariri, R., Seyfzadeh, S., & Stevanato, R. (2010). Amino Acids, 38, 937–942.
Chen, T. X., Nie, H. L., Li, S. B., White, C. B., Su, S. N., & Zhu, L. M. (2009). Colloids and Surfaces B, 72, 25–31.
Kimmel, J. R., & Smith, E. L. (1954). Journal of Biological Chemistry, 207, 515–531.
Finkle, B. J., & Smith, E. L. (1958). Journal of Biological Chemistry, 230, 669–690.
Baines, B. S., & Brocklehurst, K. (1979). Biochemical Journal, 177, 541–548.
Nitsawang, S., Kaul, R. H., & Kanasawud, P. (2006). Enzyme and Microbial Technology, 39, 1103–1107.
Tombaccini, D., Mocali, A., Weber, E., & Paoletti, F. (2001). Analytical Biochemistry, 289, 231–238.
Govrin, E., & Levine, A. (1999). Protein Expression and Purification, 15, 247–250.
Nie, H. L., Chen, T. X., & Zhu, L. M. (2007). Separation and Purification Technology, 57, 121–125.
Yavuz, H., Duru, E., Genç, Ö., & Denizli, A. (2003). Colloids and Surfaces A, 223, 185–193.
Yavuz, H., Akgöl, S., Say, R., & Denizli, A. (2006). International Journal of Biological Macromolecules, 39, 303–309.
Demiryas, N., Tüzmen, N., Galaev, I. Y., Pişkin, E., & Denzili, A. (2007). Journal of Applied Polymer Science, 105, 1808–1816.
Akgöl, S., Yavuz, H., Şenel, S., & Denizli, A. (2003). Reactive and Functional Polymers, 55, 45–51.
Altıntaş, E. B., & Denizli, A. (2006). International Journal of Biological Macromolecules, 38, 99–106.
Wongchuphan, R., Tey, B. T., Tan, W. S., Taip, F. S., Kamal, S. M. M., & Ling, T. C. (2009). Biochemical Engineering Journal, 45, 232–238.
Lozinsky, V. I., Plieva, F. M., Galaev, I. Y., & Mattiasson, B. (2002). Bioseparation, 10, 163–188.
Lozinsky, V. I., Galaev, I. Y., Plieva, F. M., Savina, I. N., Jungvid, H., & Mattiasson, B. (2003). Trends in Biotechnology, 21, 445–451.
Plieva, F. M., Galaev, I. Y., & Mattiasson, B. (2007). Journal of Separation Science, 30, 1657–1671.
Avcıbaşı, N., Uygun, M., Çorman, M. E., Akgöl, S., & Denizli, A. (2010). Applied Biochemistry and Biotechnology, 162, 2232–2243.
Derazshamshir, A., Baydemir, G., Andaç, M., Say, R., Galaev, I. Y., & Denizli, A. (2010). Macromolecular Chemistry and Physics, 6, 657–668.
Alkan, H., Bereli, N., Baysal, Z., & Denizli, A. (2009). Biochemical Engineering Journal, 45, 201–208.
Bereli, N., Andaç, M., Baydemir, G., Say, R., Galaev, I. Y., & Denizli, A. (2008). Journal of Chromatography. A, 1190, 18–26.
Yılmaz, F., Bereli, N., Yavuz, H., & Denizli, A. (2009). Biochemical Engineering Journal, 43, 272–279.
Bereli, N., Şener, G., Altındaş, E. B., Yavuz, H., & Denizli, A. (2010). Materials Science and Engineering: C-Mater, 30, 323–329.
Nie, H. L., & Zhu, L. M. (2007). International Journal of Biological Macromolecules, 40, 261–267.
Nie, H. L., Chen, T. X., & Zhu, L. M. (2007). Separation and Purification Technology, 57, 121–125.
Su, S. N., Nie, H. L., Zhu, L. M., & Chen, T. X. (2009). Bioresource Technology, 100, 2336–2340.
Perçin, I., Sağlar, E., Yavuz, H., Aksöz, E., & Denizli, A. (2011). International Journal of Biological Macromolecules, 48, 577–582.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Kembhavi, A. A., Kulkarni, A., & Pant, A. (1993). Applied Biochemistry and Biotechnology, 38, 83–92.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Suster, C. R. L., Priolo, N. S., & Morcelle, S. R. (2011). Preparative Biochemistry and Biotechnology, 41, 107–121.
Monti, R., Basilio, C. A., Trevisan, H. C., & Contiero, J. (2000). Brazilian Archives of Biology and Technology, 43, 501–507.
Rauf, M. A., Bukallah, S. B., Hamour, F. A., & Nasir, A. S. (2008). Chemical Engineering Journal, 137, 238–243.
Karataş, M., Akgöl, S., Yavuz, H., Say, R., & Denizli, A. (2007). International Journal of Biological Macromolecules, 40, 254–260.
Burke, D. E., Lewis, S. D., & Shafer, J. A. (1974). Archives of Biochemistry and Biophysics, 164, 30–36.
Azarkan, M., El Moussaoui, A., van Wuytswinkel, D., Dehon, G., & Looze, Y. (2003). Journal of Chromatography B, 790, 229–238.
Nitsawang, S., Hatti-Kaul, R., & Kanasawud, P. (2006). Enzyme and Microbial Technology, 39, 1103–1107.
Llerena-Suster, C. R., Priolo, N. S., & Morcelle, S. R. (2011). Preparative Biochemistry and Biotechnology, 41, 107–121.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uygun, D.A., Akduman, B., Uygun, M. et al. Purification of Papain Using Reactive Green 5 Attached Supermacroporous Monolithic Cryogel. Appl Biochem Biotechnol 167, 552–563 (2012). https://doi.org/10.1007/s12010-012-9707-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9707-7