Abstract
Candida rugosa lipase was encapsulated within a chemically inert sol–gel support prepared by polycondensation with tetraethoxysilane and octyltriethoxysilane in the presence of β-cyclodextrin-based polymer. The catalytic activity of the encapsulated lipases was evaluated both in the hydrolysis of p-nitrophenylpalmitate and the enantioselective hydrolysis of racemic Naproxen methyl ester. It has been observed that the percent activity yield of the encapsulated lipase was 65 U/g, which is 7.5 times higher than that of the covalently immobilized lipase. The β-cyclodextrin-based encapsulated lipases had higher conversion and enantioselectivity compared with covalently immobilized lipase. The study confirms an excellent enantioselectivity (E >300) for the encapsulated lipase with an enantiomeric excess value of 98% for S-naproxen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs), another class of compounds with a macrocyclic structure, have been successfully used to improve enzymes activity and to increase the reaction rate and E in enzyme-catalyzed reactions in organic solvents [1, 2]. They are a family of chiral cyclic α-(1–4)-linked d-glucose oligomers with six, seven, or eight glucose units, corresponding to α-, β-, and γ-homologues, possessing toroidal conformation in the solid state and in solution. The internal hydrophobic cavity and the external hydrophilic rim of chemically modified cyclodextrins render them ideal for modeling enzyme substrate binding [3].
Lipase (triacyl glycerol ester hydrolase, EC 3.1.1.3) is an enzyme that catalyzes the hydrolysis of triacylglycerols to fatty acids, mono- and di-acylglycerols, and glycerol [4]. It also catalyzes various other reactions, such as esterification, interesterification, and transesterification. Lipase has been immobilized on various supports either by physical adsorption, covalent binding, entrapment, and encapsulation [5–8]. Sol–gel encapsulation has proven to be a particularly easy and effective way to immobilize enzymes [9]. Lipases are interfacial-active enzymes with hydrophobic domains [10–17]. Lipophilic interactions with substrate molecules induce conformational changes in the lipase. In certain types of lipases it is the movement of a short a-helical loop which uncovers the active site. Reetz and co-worker [18] speculated that pure SiO2 is not the ideal matrix for lipases, and that hydrophobic matrices could provide a better microenvironment [19–21]. Thus, monomers of the type RSi(OCH3)3 or mixtures of RSi(OCH3)4 and Si(OCH3)4 were used in the sol–gel process. These heterogeneous biocatalysts are now commercially available [22] and can be employed in regio- and enantioselective acylations [23] and even in hydrolysis reactions [23].
Lipase is a versatile enzyme with many potential industrial applications; it has been used for the modification of fats and oils and the synthesis of flavor esters and food additives. Because lipase is also enantioselective, it can be used for the resolution of chiral compounds and the synthesis of high-value pharmaceutical intermediates [24].
Naproxen, (S)-(+)-2-(6-methoxy-2-naphthyl) propionic acid is a kind of an important group of medicines called non-steroidal anti-inflammatory drug with analgesic and anti-pyretic properties, which is widely used in the treament of rheumtic and other inflammatory and for the relief of mild to moderate pain [25]. It has one stereogenic center which gives rise to two optical isomers in which pharmacological activity resides in the (S)-enantiomer, while the (R)-enantiomer causes some unwanted side effects [26]. The physiological activity of the S form of naproxen is 28-fold that of the R form [27–31]. Therefore, separation racemic naproxen is necessary for assuring good quality in pharmaceutical production of naproxen and in other naproxen-related scientific research work as well. In the case of naproxen, lipase has been used to prepare optically pure naproxen by enantioselective hydrolysis of its racemic esters [32–35].
Previously in our study, in order to see the influence of two different activating agents (hexamethylene diisocyanate and glutaraldehyde) on the lipase activitiy and stability, we have reported prepare of cyclodextrin-based polymers and lipase immobilization as covalently [31] In this study, we used to these β-cyclodextrin-based polymers on sol–gel encapsulation procedure as additive materials and observed to effects of the cyclodextrin-based polymers in the enantioselective hydrolysis reaction of (R/S)-naproxen methyl ester.
Materials and Methods
Materials
Candida rugosa lipase (CRL) was a commercial enzyme obtained from Sigma-chemical Co., (St. Louis, MO) used in the immobilization. β-cyclodextrin (β-CD), hexamethylene diisocyanate, Bradford reagent, bovine serum albumin 99% (BSA), and p-nitrophenylpalmitate (p-NPP) were purchased from Sigma-chemical Co., (St. Louis, MO). Acetone and ethanol were provided by Merck (Darmstadt, Germany). All aqueous solutions were prepared with deionized water that had been passed through a Millipore Milli-Q Plus water purification system. All other chemicals (Merck, Darmstadt, Germany) were of analytical grade and used without further purification.
Instrumentation
The surface morphology of samples was examined by scanning electron microscope (SEM; Jeol, JSM 5310, Japan). Ultraviolet–visible (UV–vis) spectra were obtained with a Shimadzu 1700 UV–vis recording spectrophotometer. High-performance liquid chromatography (HPLC) Agilent 1200 Series was carried out using a 1200 model quaternary pump, a G1315B model Diode Array and Multiple Wavelength UV–vis detector, a 1200 model standard and preparative auto sampler, a G1316A model thermostated column compartment, a 1200 model vacuum degasser, and an Agilent Chemstation B.02.01-SR2 Tatch data processor.
The enantiomeric excess determination was performed with HPLC (Agilent 1200 Series) by using a Chiralcel OD-H column at the temperature of 25 °C with n-hexane/2-propanol/trifluoroacetic acid (100/1/0.1, v/v/v). The flow rate of 1 mL/min; the UV detector was fixed at 254 nm.
Preparation of β-CD-Based Polymer
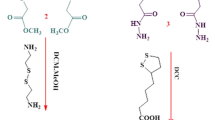
The cross-linked polymer has been prepared in one step by β-CD using only hexamethylene diisocyanate (HMDI). In our previous work, β-CD-based polymer (β-CD-HMDI) was synthesized by procedures published in the literature [31]. Two grams of β-CD (1.76 mmol) was dissolved in 15 mL of dry DMF in a 100-mL round bottom flask at room temperature. Then 17.6 mmol of hexamethylene diisocyanate in 5 mL of dry DMF for polymer was added dropwise. The mixture of polymer was stirred at 70 °C for 3 h. The precipitate was filtered and washed with acetone several times. The support (β-CD-HMDI) was dried in vacuum for 24 h.
General Procedure for Sol–Gel Encapsulation of Lipases (Fig. 1)
Sol–gel encapsulated lipases were prepared according to a modified method of Reetz et al. [36]. A commercial lipase powder (lyophilizate) such as CRL type VII (60 mg) was placed in a 50-mL Falcon tube (Corning) together with phosphate buffer (390 μL; 50 mM; pH 7.0) and the mixture was vigorously shaken with a Vortex-Mixer at 25 °C for 10 min.. The β-CD-HMDI polymer (0.05 g) was included. Then 100 μL of aqueous polyvinyl alcohol (4%, w/v), aqueous sodium fluoride (50 μL of 1 M solution) and isopropyl alcohol (100 μL) were added, and the mixture homogenized using a Vortex-Mixer at 25 °C for 15 min. Then the alkylsilane (2.5 mmol) and tetraethoxysilane (TEOS; 0.5 mmol; 74 μL; 76 mg) were added and the mixture agitated once more for 5 min. Gelation was usually observed within seconds or minutes while gently shaking the reaction vessel. Following drying overnight in the opened Falcon tube at 4 °C, isopropyl alcohol (10 ± 15 mL) was added in order to facilitate removal of the white solid material (filtration). The gel was successively washed with distilled water (10 mL) and isopropyl alcohol (10 mL). The resulting encapsulated lipases (β-CD-HMDI-E) were lyophilized and stored at 4 °C prior to use.
Enzyme Activity Assay
Activity of the free or immobilized lipase was assayed using 0.5% (w/v) p-nitrophenyl palmitate in 2-propanol as substrate. To the reaction mixture consisting 1 mL of 0.05 M phosphate buffer (pH 7.0) and 25 mg of immobilized lipase (or 0.1 mL free lipase) was added 1 mL of substrate that was mixed for 5 min at room temperature [37] for initiation of the reaction. The termination of the reaction has been achieved by adding 2 mL of 0.5 N Na2CO3 followed by centrifuging at 4,000 rpm for 10 min. The increase in the absorbance measured at 410 nm by a Shimadzu UV-1700 (Japan) spectrophotometer is caused by releasing p-nitrophenol in the enzymatic hydrolysis of p-NPP. A molar extinction coefficient (ε = 410) of 15.000 M−1 cm−1 for p-nitrophenol was used in Beer’s law. One unit (U) of lipase activity was defined as the amount of enzyme necessary to hydrolyze 1 μmol/min of p-NPP under the conditions of assay [38].
Protein Determination
The total protein originally taken for immobilization and protein present in the supernatants after immobilization were estimated by the method of Bradford [39] using BSA as a standard. The amount of protein bound on the support was found by subtracting the unbound protein from total protein.
Effect of pH and Temperature on Activity
The effect of pH on activity of free and immobilized lipases was assayed in the phosphate buffer (50 mM) of pH ranging from 4 to 10 by using the standard activity assay procedure mentioned above.
The rates of thermal inactivation of the free and immobilized lipases were studied in the temperature range 25–60 °C. Both forms of enzyme were incubated in PBS (50 mM, pH 7.0) for 20 min. at different temperatures and, after cooling, the remaining activity was assayed and measured under the standard conditions.
Thermal and Storage Stability
Free and immobilized lipase preparations were stored in the phosphate buffer solutions (50 mM, pH 7.0) at 60 °C for 2 h, respectively. Samples were periodically withdrawn for activity assay. The residual activities were determined as above.
Free and immobilized enzymes were stored at 4 °C in 50 mM phosphate buffer (pH 7.0). The storage stability of enzymes was determined by the measurement of the activity of samples taken at regular time intervals and compared.
Hydrolysis of Racemic Naproxen Methyl Ester
Racemic Naproxen was produced in the laboratory by the racemization of optically pure S-naproxen as described by Wu and Liu [40].
Hydrolysis reactions were carried out in an aqueous phase-organic solvent batch reaction system consisted of 2 mL isooctane as solvent dissolving racemic Naproxen methyl ester (20 mM) and 2 mL buffer solution (pH 7.0, 50 mM phosphate buffer solution) including encapsulated lipases (5 to 50 mg depending on the activity). The reactions were carried out in a horizontal shaker at 150 rpm at 30 °C and samples drawn from isooctane phase at 24 h were analyzed by HPLC to calculate the conversion and enantioselectivity.
The enantioselectivity was expressed as the enantiomeric ratio (E) calculated from the conversion (x) and the enantiomeric excess of the substrate (ees) and the product (eep) using the equation of Chen et al. [41].
Where
where E, ees, eep, x, C R, and C S denote enantiomeric ratio for irreversible reactions, enantiomeric excess of substrate, enantiomeric excess of product, racemate conversion, concentration of R-enantiomer, and concentration of S-enantiomer, respectively.
Results and Discussions
The main focus of this work is to examine the effect of cyclodextrin-based polymer using as addivites on the enantioselective hydrolysis reaction of racemic Naproxen methyl ester. For this reason, we used the cyclodextrin-based polymers synthesized previously, [31] our study on sol–gel encapsulation prosedure and compared with covalently immobilized lipases in point of the enantioselectivity and conversion.
Previous studies by Reinhoudt and co-workers [42], Griebenow and co-workers [43], Khmelnitsky and co-workers [44] and Liu and co-workers [45], respectively, had shown that lipases show higher activities and occasionally enhanced stereoselectivities when used in the presence of 18-crown-6, or cyclodextrin derivatives.
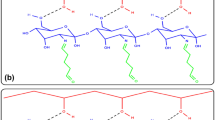
Cyclodextrin-based materials are as hydrophylic and hydrophobic properties. In fact, the cyclodextrin building blocks of CD-HMDI is characterized by internal hydrophobic cavities and external hydrophylic surfaces that give rise to an intrinsically amphiphilic structure [46]. Based on this peculiar property, a number of studies on the interaction of CDs and enzymes have been carried out [47–49].
The main problem one has to confront with during the employment of lipases for industrial purposes is interfacial activation that relates with the presence of a mobile protein domain (lid) that protects the substrate binding site: in order to get an active enzyme, the lid must lie in an “open” conformation [46].
The cross-linked polymer has been prepared in one step by β-CD using only HMDI. In our previous work, β-CD-HMDI was synthesized and characterized by procedures published in the literature [31].
It has been reported that sol–gel encapsulation has proven to be a particularly easy and effective way to immobilize enzymes and the sol–gel lipase immobilizates were excellent catalysts in the kinetic resolution of chiral alcohols and amines. Thus, in this work the CRL was encapsulated within a chemically inert sol–gel support prepared by polycondensation by TEOS and octyltriethoxysilane (OTES) in the presence of cyclodextrin-based polymer as additive [36] (Fig. 1).
SEM allowed the verification of morphological differences between the β-CD-HMDI polymer, and lipase encapsulated on β-CD-HMDI (Fig. 2a, b). For the support, microcapsule has a large internal cavity. After encapsulation, the surface cavity of the β-CD-HMDI was filled with lipase and the other materials. Figure 2b shows the rounded structure, which is presumably protein aggregate.
Table 1 shows the activity of the encapsulated lipases under optimum reaction conditions. In comparison with non-β-CD-HMDI encapsulated lipase was found to give 95 U/g of support while β-CD-HMDI-encapsulated lipase was found to give 65 U/g of support.
In our previous work [31], the activity of covalently immobilized lipase (β-CD-HMDI) was found to give 8.82 U/g which is approximately 7.5 times less than that of the encapsulated lipase with β-CD-HMDI.
According to the results obtained, however, the conformational structure of the encapsulated lipase does not suffer too much as compared with the covalently immobilized lipase as its activity was decreased too much. Therefore, the sol–gel immobilization method onto cyclodextrin polymer is superior to the covalent binding method used in this paper.
From the activity results of the sol–gel encapsulated lipases (see Table 1), it was concluded that the encapsulated lipase without β-CD-HMDI was higher activity than encapsulated lipase with β-CD-HMDI. This is not surprising result owing to the free –NCO groups of β-CD-HMDI. It is well known that these groups containing compounds have highly effective deactivating agents, which means –NCO groups interact with active center of lipase.
The ability of CD cavities to host aromatic and hydrophobic side chains of amino acid residues sitting on the protein surface [50], the presence of a complex network of tertiary contacts between the protein residues, the OH groups exposed on the exterior of CD moieties.
Effect of pH and Temperature on the Activity of Encapsulated Lipase
The pH is one of the important parameters capable of altering enzymatic activities in aqueous solution. Immobilization is likely to result in a conformational change of the enzyme, which leads to inactivity of the enzyme. By studying the variation of relative activity of the encapsulated lipases at different pH values as shown in Fig. 3, it shows that their optimum pH values are 5.0 and 7.0, by encapsulated lipase and encapsulated lipase without support, respectively. Upon immobilization on β-CD-HMDI, the optimum pH for reactions catalyzed by free lipase was slightly shifted towards acidic values. The shift depends on as well as the structure and charge of the matrix [51, 52]. Generally, an acidic shift in the pH optimum is expected when enzymes are immobilized onto polycationic supports [52]. Immobilized lipase showed better pH stability and resistance to acidic environments than encapsulated lipase without support. A similar observation with the lipase encapsulated on supports was reported [53, 54].
In our previous work, it was observed that the optimum pH of lipase immobilized on β-CD-HMDI polymer by covalently was 7.0 [31].
In our recent study, it has been noticed that lipase (C. rugosa) could be stabilized when encapsulated in the presence of sporopollenin in the pH range of 4.0–10.0 with optimum pH 5.0 [35].
The effect of temperature on encapsulated lipases is given in Fig. 3b. The effect of temperature on the activity of encapsulated lipases for p-NPP hydrolysis at pH 7.0 in the temperature range of 25–60 °C is shown in Fig. 3b. It was found that the optimum temperature for the encapsulated lipase without support was approximately 35 °C while it shifted nearly to 40 °C for β-CD-HMDI-E. Furthermore, the temperature profile of the immobilized lipase is broader than those of the encap-lipase without support, which means that the immobilization methods preserved the enzyme activity over a wider temperature range.
One of the main reasons for enzyme immobilization is the anticipated increase in stability toward various deactivating forces, due to restricted conformational mobility of the molecules following immobilization [55–57]. This was either due to the creation of conformational limitation on the enzyme movement as a result of electrostatic interaction and hydrogen bond formation between the enzyme and the support or a low restriction in the diffusion of the substrate at high temperature. Thus, the immobilized enzymes showed their catalytic activities at a higher reaction temperature [54, 58]. In our previous work, it was found that the optimum temperature for immobilized lipase by covalently on cyclodextrin-based polymer was 45 °C, respectively [31].
Thermal and Storage Stability on the Activity of Encapsulated Lipase
In this work, thermal stabilities of the encapsulated lipases were evaluated as shown in Fig. 4a. Encapsulated lipases were incubated for 2 h at 60 °C and the enzyme activity was measured at various time intervals. It can be observed that the encapsulated lipase without support loses its initial activity within 100 min at 60 °C while the β-CD-HMDI-E retains their initial activities of about 38% after 120 min of heat treatment at 60 °C. Improvement in thermal stability of immobilized enzymes typically reflects the interaction between the enzyme and support preventing conformational transitions of the enzyme at high temperatures. The observed enhancement in thermostability of the immobilized enzyme is consistent with the work of Yilmaz and co-workers [35] and has been attributed to the tight confinement of the lipase in the sol–gel matrix. Interactions between the lipase and sol–gel matrix, such as hydrophobic interaction and hydrogen bonding are also believed to be responsible for the enhanced activity of the immobilized lipase.
The presence of a complex network of tertiary contacts between the protein residues, the OH groups exposed on the exterior of CD moieties and the urethane or the carbamate moieties, that may stabilize the enzyme via entropy changes [46, 59].
Storage stability is one of the most important criteria for the application of an enzyme on the commercial scale. Generally, if an enzyme is in solution, it is not stable during storage, and the activity is gradually reduced. The encapsulated lipases were stored in PBS at 4 °C and activities were measured periodically over duration of 45 days. The residual activity of the encapsulated enzymes at different time intervals was estimated and results are given in Fig. 4b. The encapsulated lipase without β-CD-HMDI rapidly loses its activity with a residual value of 15% after 12 days, the decrease in activity occurs more slowly with the encapsulated lipases, and about 90% of their initial activity were recovered after the same period. The storage stability of the encapsulated enzyme (β-CD-HMDI-E) was clearly better than the lipase-enc. The retention in activity is usually observed after enzyme immobilization. This could be explained by the modification in three-dimensional structure of the enzyme, which leads to conformation change of the active center. The presence of matrix hinders the accessibility of substrate to the enzyme active site, and limitation of mass transfer of substrate and product to or from the active site of the enzyme may also be responsible. This explanation is in agreement with the results reported [60–62].
Enantioselective Hydrolysis of Racemic Naproxen Methyl Ester with the Immobilized Lipases
Table 2 shows that the conversion (x), enantiomeric excess (ee) and enantiomeric ratio (E) results in the course of (R,S)-naproxen methyl ester hydrolysis by the sol–gel encapsulated lipases and immobilized lipase as covalently. The enantioselective hydrolysis reactions of racemic Naproxen methyl ester were studied in aqueous buffer solution/isooctane reaction system. From the results, it has been revealed that the sol–gel encapsulated lipases have a highly enantioselectivity (E) and conversion (x) compared with the covalently immobilized lipase.
The resolution reaction with encapsulated lipase was terminated after 24 h, obtaining Naproxen methylate (unreacted R ester) and corresponding acid (eep) 98% at conversion of 46% and the enantioselectivity being very high (E >400). Whereas the resolution reactions with covalently immobilized lipase gave an unreacted Naproxen methylate (R)-ester and corresponding acid (eep) 98% at conversion of 14% and the E being 135. Consequently, the sol–gel encapsulation of lipase led to high enantioselectivity, high conversion, and fast recovery of product compared with covalently immobilized lipase.
In our previous work [54], it was found that excellent E (382) has been noticed for most lipase preparations with an ee value of >98% for S-naproxen. The results indicate that in particular cyclodextrin-based encapsulated lipases have higher conversion and enantioselectivity compared with the sol–gel lipase without support.
The result was not surprising because in the literature Tsai and co-workers [63] used lipase MY from C. rugosa and catalyzed hydrolysis of racemic Naproxen esters in water-saturated isooctane as the model system. They found the E value as 510. In addition, in our previous work [35], CRL we used enantioselective hydrolysis of racemic Naproxen methyl ester with sol–gel encapsulated lipase in the presence of sporopollenin and observed excellent enantioselectivity (E >400). In our other study [53], CRL was immobilized on glutaraldehyde-activated aminopropyl glass beads by using covalent binding method or sol–gel encapsulation technique. Enhanced enantioselectivity of enzyme with an E value of 400 was obtained after encapsulation (E = 135 for the covalent form). In their former studies, Takac and Mutlu [64] showing that C. rugosa lipase immobilized on Amberlite XAD 7 exhibited the best enantioselectivity (E = 174.2) and conversion (49%) in the hydrolysis of racemic Naproxen methyl ester in aqueous phase/isooctane biphasic medium.
In chiral resolution using enzyme as a catalyst, it has been reported that variation of pH might influence chiral selectivity since the conformation of an enzyme depends on its ionization state [65]. The effects of pH on enantioselectivity of immobilized lipase were determined by incubating immobilized lipase in the presence of β-CD-HMDI polymers at different pH (i.e., pH 5.0 and 7.0) and, at 35 °C for 24 h. At the end of the incubation time the rate of enzyme reaction and ee were determined using HPLC (Agilent 1200 Series) equipped with Chiralcel OD-H column at the temperature of 25 °C. The optimum pH values were determined from the graph of pH plotted against the percentage of conversion (x) (Fig. 5a). The optimum pH values were found to be 5.0 for immobilized lipases. In our recent study [54] pH 5.0 was the value where the highest conversion and enantioselectivity for Fe3O4-Spo-E were obtained in the hydrolysis reaction.
The temperature dependence of the percentage of conversion (x) and enantiomeric ratio (E) of the hydrolysis reaction catalyzed by immobilized lipases was studied in the interval from 35 to 50 °C and the results are shown in Fig. 5b. It was observed that the optimum temperature value was found to be 45 °C for immobilized lipases. This is not surprising, because higher reaction temperature means better diffusion of substrates into the active site of the enzyme and of the products away from the enzyme, resulting in higher reaction rate. Uyanik and co-workers [66] were showed that 45 °C was the hydrolysis temperature where the highest conversion and enantioselectivity were obtained.
The reusability of immobilized lipases is also important for economical use of the enzyme. Figure 6 shows that the immobilized lipases were still retained 26 and 2% of their conversion ratios for β-CD-HMDI-E enc and β-CD-HMDI-E cov after the 5th reuse, respectively. These results are due to the inactivation of the enzyme denaturation of protein and the leakage of protein from the supports upon use.
Conclusions
In this study, CRL was immobilized by sol–gel encapsulation technique within a chemically inert sol–gel support prepared by polycondensation with TEOS and OTES in the presence and absence of β-CD-HMDI polymers as additive. The catalytic activity of the immobilized lipases was evaluated into model reactions, i.e. the hydrolysis of p-NPP, and the enantioselective hydrolysis of racemic Naproxen methyl ester. It was observed that the activity of the encapsulated lipase (β-CD-HMDI-E enc) was 65 U/g, which is 7.5 times higher than that of the covalently immobilized lipase (β-CD-HMDI-E cov). Enhanced enantioselectivity of enzyme with an E value of 382 was obtained after encapsulation (E = 135 for the covalent form). On the basis of these results, we recommend immobilized lipases as a prospective preparation for continuous industrial applications. The sol–gel method was worthy of further investigations to achieve higher activity and enantioselectivity of enzymes compared with conventional immobilization method.
References
Harper, J. B., Easton, C. J., & Lincoln, S. F. (2000). Current Organic Chemistry, 4, 429–454.
Griebenow, K., Laureano, Y. D., Santos, A. M., Clemente, I. M., Rodriguez, L., Vidal, M. W., et al. (1999). Journal of the American Chemical Society, 121, 8157–8163.
D’Souza, V. T., & Bender, M. L. (1987). Miniature organic models of enzymes. Accounts of Chemical Research, 20, 146–152.
Murty, V. R., Bhat, J., & Muniswaran, P. K. A. (2002). Biotechnology and Bioprocess Engineering, 7, 57–66.
Rahman, M. B. A., Tajudin, S. M., Hussein, M. Z., Rahman, R. A., Salleh, A. B., & Basri, M. (2005). Applied Clay Science, 29, 111–116.
Yujun, W., Jian, X., Guangsheng, L., & Youyuan, D. (2007). Bioresource Technology, 99, 2299–2303.
Noureddini, H., Gao, X., & Philkana, R. S. (2005). Bioresource Technology, 96, 769–777.
Tutar, H., Yilmaz, E., Pehlivan, E., & Yilmaz, M. (2009). International Journal of Biological Macromolecules, 45, 315–320.
Avnir, D., Braun, S., Lev, O., & Ottolenghi, M. (1994). Chemistry of Materials, 6, 1605–1614.
Brady, L., Brzozowski, A. M., Derewenda, Z. S., Dodson, E., Dodson, G., Tolley, S., et al. (1990). Nature, 343, 767–770.
Brzozowski, A. M., Derewenda, U., Derewenda, Z. S., Dodson, G. G., Lawson, D. M., Turkenburg, J. P., et al. (1991). Nature, 351, 491–494.
Ollis, D. L., Cheah, E., Cygler, M., Dijkstra, B., Frolow, F., Franken, S. M., et al. (1992). Protein Engineering, 5, 197–211.
Jaeger, K. E., Ransac, S., Koch, H. B., Ferrato, F., & Dijkstra, B. W. (1993). FEBS Letters, 332, 143–149.
Cygler, M., Grochulski, P., Kazlauskas, R. J., Schrag, J. D., Bouthillier, F., Rubin, B., et al. (1994). Journal of the American Chemical Society, 116, 3180–3186.
Kazlauskas, R. J. (1994). Trends in Biotechnology, 12, 464–472.
Schrag, J. D., Li, Y., Cygler, M., Lang, D., Burgdorf, T., Hecht, H. J., et al. (1997). Structure, 5, 187–202.
Kim, K. K., Song, H. K., Shin, D. H., Hwang, K. Y., & Suh, S. W. (1997). Structure, 5, 173–185.
Reetz, M. T., Zonta, A., Vijayakrishnan, V., & Schimossek, K. (1998). Journal of Molecular Catalysis A-Chemical, 134, 251–258.
Reetz, M. T., Simpelkamp, J., & Zonta, A. (1994). Patent DE 4408152A1.
Reetz, M. T., Zonta, A., & Simpelkamp, J. (1995). Angewandte Chemie, 107, 373–376.
Reetz, M. T., Zonta, A., & Simpelkamp, J. (1996). Biotechnology and Bioengineering, 49, 527–534.
Information sheet from Fluka, Switzerland, Reagent of the Year (1997).
Reetz, M. T. (1997). Advanced Materials, 9, 943–954.
Lee, D. H., Park, C. H., Yeo, J. M., & Kim, S. W. (2006). Journal of Industrial and Engineering Chemistry, 12, 777–782.
Campiglio, A. (1998). Analyst, 123, 1571–1574.
Tang, K. W., Chen, Y. Y., Huang, K. L., & Liu, J. J. (2007). Tetrahedron-Asymmetry, 18, 2399–2408.
Margolin, A. L. (1993). Enzyme and Microbial Technology, 15, 266–279.
Tsai, S. W., Lin, S. F., & Chang, C. S. (1999). Journal of Chemical Technology and Biotechnology, 74, 751–758.
Lin, H. Y., & Tsai, S. W. (2003). Journal of Molecular Catalysis B: Enzymatic, 24, 111–120.
Lee, E. G., & Chung, B. H. (2000). Korean Journal of Biotechnology and Bioengineering, 15, 415–422.
Ozmen, E. Y., Sezgin, M., & Yilmaz, M. (2009). Journal of Molecular Catalysis B: Enzymatic, 57, 109–114.
Giordano, C., Castaldi, G., Cavicchioli, S., & Villa, M. (1990). Tetrahedron, 45, 4243–4252.
Cui, Y. M., Wei, D. Z., & Yu, J. T. (1997). Biotechnology Letters, 19, 865–868.
Xu, Y., Chen, J., Xin, J., Li, S., Xia, C., & Cui, J. (2001). Biotechnology Letters, 23, 1975–1979.
Yilmaz, E., Sezgin, M., & Yilmaz, M. (2010). Journal of Molecular Catalysis B: Enzymatic, 62, 162–168.
Reetz, M. T., Tielmann, P., Wisenhofer, W., Konen, W., & Zonta, A. (2003). Advanced Synthesis and Catalysis, 345, 717–728.
Chiou, S. H., & Wu, W. T. (2004). Biomaterials, 25, 197–204.
Johri, S., Verma, V., Parshad, R., Koul, S., Taneja, S. C., & Qazi, G. N. (2001). Bioorganic Medical Chemistry, 9, 269–273.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Wu, J. Y., & Liu, S. W. (2000). Enzyme and Microbial Technology, 26, 124–130.
Chen, C. S., Fujimoto, Y., Girdaukas, G., & Sih, C. J. (1982). Journal of the American Chemical Society, 104, 7294–7299.
Reinhoudt, D. N., Eendebak, A. M., Nijenhuis, W. F., Verboom, W., Kloosterman, M., & Schoemaker, H. E. J. (1989). Journal of the Chemical Society, Chemical Communications, 399–400.
Griebenow, K., Laureano, Y. D., Santos, A. M., Clemente, I. M., Rodriguez, L., Vidal, M. W., et al. (1999). Journal of the American Chemical Society, 121, 8157–8163.
Khmelnitsky, Y. L., Welch, S. H., Clark, D. S., & Dordick, J. S. (1994). Journal of the American Chemical Society, 116, 2647–2648.
Liu, Y. Y., Xu, J. H., & Hu, Y. (2000). Journal of Molecular Catalysis B: Enzymatic, 10, 523–529.
Boscolo, B., Trotta, F., & Ghibaudi, E. (2010). Journal of Molecular Catalysis B: Enzymatic, 62, 155–161.
Wang, Y., & Mei, L. (2007). Journal of Bioscience and Bioengineering, 103, 345–349.
Avila-Gonzalez, R., Perez-Gilabert, M., & Garcia-Carmona, F. (2005). Journal of Bioscience and Bioengineering, 100, 423–428.
Ghanem, A. (2003). Organic and Biomolecular Chemistry, 1, 1282–1291.
Aachmann, F., Otzen, D., Larsen, K., & Wimmer, R. (2003). Protein Engineering, 16, 905–912.
Liu, X., Guan, Y., Shen, R., & Liu, H. (2005). Journal of Chromatography B, 822, 91–97.
Faber, K., Ottolina, G., & Riva, S. (1993). Biocatalysis, 8, 91–132.
Yilmaz, E., Can, K., Sezgin, M., & Yilmaz, M. (2011). Bioresource Technology, 102, 499–506.
Yilmaz, E., Sezgin, M., & Yilmaz, M. (2011). Journal of Molecular Catalysis B: Enzymatic, 69, 35–41.
Arica, M. Y. (2000). Journal of Applied Polymer Science, 77, 2000–2008.
Arica, M. Y., & Bayramoglu, G. (2004). Journal of Molecular Catalysis B: Enzymatic, 27, 255–265.
Phadtare, S. D., Britto, V., Pundle, A., Prabhune, A., & Sastry, M. (2004). Biotechnology Progress, 20, 156–161.
Tang, Z. X., Qian, J. Q., & Shi, L. E. (2007). Materials Letters, 61, 37–40.
Hanefeld, U., Gardossi, L., & Magner, E. (2009). Chemical Society Reviews, 38, 453–468.
Kartal, F., Akaya, A., & Kilinc, A. (2009). Journal of Molecular Catalysis B: Enzymatic, 57, 55–61.
Dincer, A., & Telefoncu, A. (2007). Journal of Molecular Catalysis B: Enzymatic, 45, 10–14.
Li, S. F., Chen, J. P., & Wu, W. T. (2007). Journal of Molecular Catalysis B: Enzymatic, 47, 117–127.
Tsai, S. W., Chen, C. C., Yang, H. S., Ng, I. S., & Chen, T. L. (2006). Biochimica Et Biophysica Acta-Proteins And Proteomics, 1764, 1424–1428.
Takac, S., & Bakkal, M. (2007). Process Biochemistry, 42, 1021–1027.
Pereira, E. B., Castro, H. F., Moraes, F. F., & Zanin, G. M. (2001). Applied Biochemistry and Biotechnology, 91, 739–752.
Uyanik, A., Sen, N., & Yilmaz, M. (2011). Bioresource Technology, 102, 4313–4318.
Acknowledgment
The authors thank the Scientific Research Projects Foundation of Selcuk University (SUBAP grant number 08101024) for financial support of this work produced from a part of E. Yilmaz’s Ph.D. thesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yilmaz, E., Sezgin, M. Enhancement of the Activity and Enantioselectivity of Lipase by Sol–Gel Encapsulation Immobilization onto β-cyclodextrin-Based Polymer. Appl Biochem Biotechnol 166, 1927–1940 (2012). https://doi.org/10.1007/s12010-012-9621-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9621-z