Abstract
The effects of exogenous CO2 on the growth and lipid accumulation of a local screened facultative heterotrophic microalgae strain Auxenochlorella protothecoides (UMN280) as well as nutrient removal from concentrated municipal wastewater stream (centrate) were examined in this study. A 12-day batch experiment was conducted with CO2 aeration at three levels, namely, 0%, 1%, and 5% (v/v) CO2 mixed with air, under light intensity of 60 μmol/(m2 @@s). A two-stage growth pattern was observed. The first stage (first–fifth day) was dominated by heterotrophic growth in which organic carbon was the main carbon source. The second stage (6th–12th day) was dominated by autotrophic growth in which exogenous CO2 had a positive effect on algal biomass accumulation. The addition of 5% CO2 was better than that of 1% CO2 on the biomass and lipid production. The uptakes of nutrients were similar between injection and no injection of CO2, except on phosphorus removal which was affected by the acidification of CO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae-based biofuel production systems offer advantages over other systems. Progresses made so far through research on algae’s ability to fix CO2, treat wastewater, and provide renewable energy feedstock have helped shape microalgae into a leading contender in offering solutions to growing energy security and environmental concerns. Cultivation of microalgae has been tested on different wastewater streams, including municipal wastewater [1–3], undigested animal manure [4], digested animal manure [4, 5], and agricultural runoff [6]. However, significant obstacles must be overcome before large-scale algae production systems can be commercialized. Significant improvements in algal growth rate, nutrient uptake rates, integration of cultivation systems with flue gas, wastewater and water reclamation systems, design of novel bioreactors for cost reduction, and efficiency enhancement in light utilization, gas transfer, and mixing must be achieved [7]. The goal of the present study was to improve algal biomass production and CO2 fixation rate by growing mixotrophic microalgae on concentrated municipal wastewater with exogenous CO2 aeration.
Mixotrophy is a metabolic mode in which microorganisms exhibit the capability of utilizing both organic and inorganic carbon sources. Inorganic carbon is fixed during photosynthesis which is influenced by light intensity, while organic compounds are assimilated during the heterotrophic route which is influenced by the availability of organic carbon [8]. It was reported that the specific growth rate of microalgae in mixotrophic mode was approximately the sum of autotrophic and heterotrophic growth rates representing the fastest way of growing algae biomass [9, 10]. Several studies showed that higher specific growth rate for certain algal strains was obtained when they were grown mixotrophically than when they were grown either photoautotrophically or heterotrophically alone [11, 12].
Centrate, an internal recycle liquid stream in municipal wastewater treatment plant after sludge centrifugation, was used in this study. Different from influent stream which is low in carbon (C), nitrogen (N), and phosphorus (P) concentrations, centrate is essentially an organic-carbon-enriched culture medium with high levels of N and P. Therefore, algae growth in a centrate-based cultivation system has the potential to be in the mixotrophic mode.
In general, exogenous CO2 stimulates photosynthesis in photoautotrophic cultivation. Previous research shows that higher microalgal biomass accumulation was obtained when CO2-enriched air was injected into the culture media compared with no CO2 injection [13–15]. However, the effect of CO2 on algal growth in an organic-carbon-enriched medium remains unclear. Ingram et al. indicated that algae Nostoc sp., strain Mac, preferred CO2 at a high light intensity, while the presence of organic carbon sources had no positive effect on the overall biomass yield [16]. Other studies showed that the preference of algae for different carbon sources varied under different conditions [17, 18]. However, there has been little conclusive evidence to suggest that the addition of exogenous CO2 to the centrate-based algae system does not provide additional benefits to the system.
Most large wastewater treatment plants generate CO2-enriched flue gas from sludge burning. If exogenous CO2 is proven to be beneficial to algal growth, utilization of such flue gas for algae cultivation will improve not only algae productivity but also CO2 mitigation. Thus, the objective of this study was to examine the role of exogenous CO2 on algal biomass and lipid accumulation as well as nutrient removal in the organic-carbon-rich cultivation system. Results of this study will provide essential information for the development of a mass algal production system with the dual purposes: wastewater treatment and biofuel production.
Material and Methods
Wastewater Source
The centrate, a recycled stream obtained from the Metropolitan Wastewater Treatment Plant (Metro Plant) in Saint Paul, Minnesota, was used throughout the experiments. Since centrate was generated through dewatering sludge from primary and secondary treatment, it was usually characterized by high levels of chemical oxygen demand (COD), ammonia, and phosphorus.
Due to the high turbidity and indigenous bacteria, the centrate was filtered using Wypall X70 wipers (Kimberly-Clark Corp. USA), autoclaved at 121°C for 15 min, and kept overnight for solid settlement. The upper clear solution was used as the medium. The characteristics of the autoclaved centrate are listed in Table 1.
Algae Strain and Seed Culture Condition
In the study, algae strain UMN280, isolated from local freshwater, was used. UMN280 was identified as Auxenochlorella protothecoides, a Chlorella-like alga which shares numerous features with Chlorella protothecoides var. acidicola, such as the inability to grow on nitrate, tolerance to high concentrations of NaCl, the same upper limit of temperature for growth, etc. [19, 20]. The UMN280 strain was able to grow both on the classic BG-11 medium in the light condition and on the modified BG-11 medium in which Na2CO3, the inorganic carbon component, was replaced with glucose in the dark condition, which suggested that UMN280 could grow both photoautotrophically and heterotrophically and had the potential for mixotrophic growth.
The seeds were cultivated in BG-11 medium containing the following chemicals: K2HPO4·3H2O 0.04 g/L, MgSO4·7H2O 0.075 g/L, CaCl2·2H2O 0.036 g/L, citric acid 0.006 g/L, ferric ammonium citrate 0.006 g/L, EDTA 0.001 g/L, NaNO3 1.5 g/L, Na2CO3 0.02 g/L, and trace metal mix A5 1.0 mL. Trace metal mix A5 solution consisted of H3BO3 2.86 g/L, MnCl2·4H2O 1.81 g/L, ZnSO4·7H2O 0.222 g/L, NaMoO4·2H2O 0.39 g/L, CuSO4·5H2O 0.079 g/L, and CoCl2·6H2O 0.05 g/L [21].
The enriched seeds were inoculated at 10% (υ inoculation/υ medium) on 700-mL liquid medium in 2-L Erlenmeyer flasks placed on a magnetic mixer (VWR International, LLC., USA) with a magnetic stir bar working at 100 rpm. The culture was kept at 25 ± 2°C under a continuous cool-white fluorescent light illumination at 100 μmol/(m2 s).
Experiment Design
To determine the effects of CO2 on the growth of UMN280 in centrate, batch experiments were carried out with CO2-enriched air aeration at three levels, namely, 0%, 1%, and 5% CO2 at 0.5 vvm. As shown in Fig. 1, the experiments, which were performed in 1-L Roux culture bottles (Corning Inc., USA), were started with a mixture of 500-mL wastewater and 80-mL seed microalgae solution, providing an initial cell concentration of approx 1 × 106 cells/mL. A magnetic stir bar with 100 rpm was placed at the bottom of the bioreactor to mix the solution. An aeration device composed of a CO2 tank, an air compressor, and flow meters was constructed to supply ambient air or CO2-enriched air to the bioreactors. All cultures were cultivated at a temperature of 25 ± 2°C with 60-μmol/(m2·s) continuous cool-white fluorescent illumination above the bioreactors. Each experiment was performed in triplicate.
Analytical Methods
Sampling and Algal Growth Determination
Seven-milliliter samples were taken from the well-mixed culture broth in the bioreactors every 24 h for algal growth and wastewater nutrient content analyses. The pH values of the cultures were measured daily. Five milliliters of the algae samples was used for the measurement of the total volatile suspended solid (TVSS) by using the standard method [22], and the cell density based on a blood cell counter. TVSS and cell density were used to indicate growth rate.
Nutrient Analysis
The remaining 2 mL of the samples was first centrifuged at 7,000 rpm for 10 min, and then, the concentrations of chemical oxygen demand (COD), NH3-N, total nitrogen, and PO4-P in the supernatants were determined by following the instructions for nutrient analysis in the Hach DR5000 spectrophotometer manual [23]. Nutrient removal efficiencies were calculated by dividing the nutrient concentration differences between the first day and final day by the initial nutrient concentrations.
where R i is the removal efficiency for substrate i, S i1 is the initial concentration of i in the batch culture systems, and S i12 is the final concentration of i in the culture system after 12-day batch cultivation.
Total Lipid Content Analysis
After the 12-day cultivation, algae cells were harvested by centrifugation and then dried with a freeze dryer (Savant Instruments Inc., USA) for total lipid analysis. The lipids were extracted based on a one-step extraction method adapted from Folch [24]. Triplicate 0.1-g dried algae samples were shaken vigorously with a 10-mL 2:1 chloroform/methanol (v/v) mixture for 30 min at 30°C. Every extract was purified to remove water-soluble components by biphasic partitioning with 0.9% NaCl solution. The lower phase was collected, and the volatile solvent was removed under N-EVAP Analytical Nitrogen Evaporator (Organomation Associates, Inc., USA) to get the dried lipid extracts. The total lipid content (%) is expressed as the percentage of total lipids on TVSS basis.
The total lipid production (g/L), another index for the measurement of total lipids which means the lipid yield per liter algal culture at the 12th day, was calculated by Eq. 2:
Statistical analysis was conducted using one-way analysis of variance (ANOVA) with the JMP8.0 software package (SAS Institute Inc., NC). Any treatment with a p-value less than 0.05 was considered significant.
Results and Discussion
The Effect of CO2 on Algal Biomass Growth
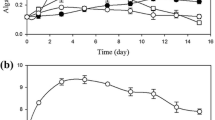
Algal growth in terms of cell density and TVSS at the three levels of CO2 aerations is plotted against time (Fig. 2). The growth of UMN280 in centrate did not show any obvious lag phase in any of the three curves, indicating that the UMN280 strain adapted well in centrate wastewater. The average cell densities on the 12th day were 0.167 × 108, 1.10 × 108, and 1.15 × 108 cells/mL for cultures with 0%, 1%, and 5% CO2-enriched air aeration, respectively, and the maximal TVSS values were 0.975, 2.01, and 2.51 g/L, respectively. The algal biomass concentration of 2.51 g/L was higher than that in the autotrophic cultivation mode (normally about 1.0 – 2.0 g/L [25, 26]). Chen and Zhang found that a higher biomass concentration (2.4 g/L) could be obtained in mixotrophic cultivation of Spirulina platensis in Zarouk medium containing 2.0-g/L glucose than that in the photoautotrophic cultivation mode (2.0 g/L), which indicated that mixotrophy is superior to photoautrophy for algal biomass cultivation [26]. Similar cell density and TVSS growth patterns in the first 5 days were observed for the cultures grown under the three CO2 levels. In the following 7 days, algae grown under pure air bubbling stayed in the stationary phase, while the cultures under CO2-enriched air aeration showed continued growth, which reflected that the addition of CO2 had a positive effect on algal growth (p < 0.05). These results revealed that the entire mixotrophic cultivation period with the exogenous CO2 aeration could be defined as a “two-stage” mode. The first stage (first–fifth day) was dominated by heterotrophic cultivation due to high content of initial organic carbon in centrate. According to Fig. 2b, there was no significant difference in TVSS curves, which indicated that CO2 had no significant effect on algal growth in the phase dominated by the heterotrophic growth. When the organic carbon was depleted to a certain level, the algae began to focus on the assimilation of CO2, the onset of the second stage (6th–12th days) dominated by photoautotrophic activity. Since exogenous CO2 stimulated photosynthesis, TVSS with 5% CO2 aeration had the highest biomass content.
Algal growth curves under 5% (v/v) CO2-enriched air (diamonds), 1% (v/v) CO2-enriched air (squares), and pure air (triangles). a Time curves of cell density for 12-day culture. b Time curves of the total volatile suspended solids for 12-day culture. Each point represents the average of three replications
An inconsistence between the cell density and TVSS content was found after a comparison of Fig. 2a, b. Generally, cell density and TVSS have positive relationship. However, in Fig. 2a, 1% CO2 aeration supported the highest algal cell density, while in Fig. 2b, 5% CO2 aeration provided the highest biomass concentration based on dry weight. This inconsistence could be explained by the results of a previous study in which algal cell density has a strong negative relationship with cell volume overall after the analysis of 59 different algal species in 121 cultures covering a wide range of cell volumes and densities [27]. In this study, the algae cells with 1% CO2 aeration were smaller than those with 5% CO2 aeration. Therefore, it is reasonable to observe that the TVSS in the algal culture with 5% CO2 was higher than that with 1% CO2 aeration. One possible explanation for the interesting phenomenon that the relationship between cell density and CO2 concentration was not markedly positive since the third day (Fig. 2a) is that 5% CO2 was over the CO2 limitation required for the photosynthetic activity during the heterotrophy-dominated stage so that the algal population growth in culture with 5% CO2 aeration was inhibited. But, the cell densities of culture with 1% and 5% CO2 injection were both higher than the one with air injection since algal population growth could be enhanced by CO2 [28].
The Effect of CO2 on Wastewater Nutrient Removal
COD Removal
COD test is commonly used to indirectly measure the amount of organic compounds in wastewater [29]. Over the course of the experiments, COD removal patterns were similar among the three levels of CO2 aeration. The removal of COD mainly occurred in the first 4 days and remained at a stationary level in the next 8 days (Fig. 3a).
As an indicator of organic carbon concentration, the decrease in COD to a stationary level in the first 4 days meant that the usable organic carbon was assimilated by algae, thanks to the heterotrophic growth. There was no further COD reduction in the last 8 days, while TVSS still climbed, which suggested that a photoautotrophic growth was dominated with the exogenous CO2 being the sole carbon source. The COD reduction curve well supported the two-stage growth theory discussed in the earlier section. The results suggested that a two-stage designation for a future scale-up system with CO2 addition in the second stage might be appropriate.
In the first three days, the COD reduction rates were inversely related to the CO2 concentrations (P < 0.05). Amblard et al. similarly found that the values for the autotrophic/heterotrophic activity index increased regularly with the decreasing effluent concentration of dissolved organic carbon in the experiment of the cultivation of periphytica algae in a pulp and paper mill effluent with increasing concentration (0.5%, 2.5%, and 10% v/v) [30]. Heterotrophy does not permit a net carbon fixation because the substrates for heterotrophic reactions, such as phosphoenol pyruvate and pyruvate, are regenerated by decarboxylation of the four carbon acids in the Krebs cycle, evolving CO2 [31]. This led the authors to consider that maybe the addition of CO2 suppresses the heterotrophic metabolic pathway so that fewer organic carbon substrates could be taken up through the heterotrophic pathway.
Phosphorus Removal
Phosphorus is an important part of many structural and biochemically functional components, such as phospholipids and NTPs, for algal cellular energetics and cell growth, and it has been identified as the primary limiting factor for algal growth in fresh water [32]. It was reported that there are two major ways to remove phosphorus from wastewater: (1) direct cellular absorption under aerobic conditions and (2) sedimentation [33].
Phosphate becomes unavailable at high-pH medium because it would be in the sediment in the forms of calcium, iron, or aluminum complex salts and organic species [34]. Boyd observed that with a Ca2+ concentration of 20 mg/L, more than 10 mg/L of orthophosphate can exist in solution at pH of 8, but at pH of 10, the orthophosphate concentration in water would not exceed 0.25 mg/L due to the formation of insoluble calcium phosphate Ca3(PO4)2 [35].
In this study, up to 75.05% of phosphorus removal was observed in the culture with air aeration, which was largely due to the sedimentation caused by pH elevation during the cultivation. However, only 26.90% and 25.40% phosphorus were removed with 1% and 5% CO2-enriched air aeration, respectively (Table 2). The pH curves of the three cultures are illustrated in Fig. 4. The pH value of the air-aerated culture went up to 9, which was high enough to cause the P to precipitate. The addition of CO2 would decrease the pH value, and less P would be removed through sedimentation. Therefore, a reversed relationship was found between the pH (Fig. 4) and the phosphorus concentration (Fig. 3b). The higher concentration of phosphorus in the solution with 1% and 5% CO2-enriched air aeration would stimulate the algal growth, which, in turn, confirmed the algal growth charts (Fig. 2).
Ammonia and Total Nitrogen Removal
Nitrogen is a significant contributor to oxygen depletion and eutrophication in receiving waters. Treatment for its removal or oxidation to the nitrate–nitrogen form is practiced at many treatment facilities [36].
In the study, ammonia, the inorganic nitrogen form in centrate, was significantly removed. The efficiencies of NH3-N removal for cultures with air and 1% and 5% CO2-enriched air aeration were 72.97%, 52.06%, and 69.31%, respectively (Table 2). This study showed that the UMN280 strain could use ammonia, one of the primary nitrogen sources for many organisms. The removal efficiency of ammonia by algae in culture with air aeration in our study was higher than those with CO2-enriched air aeration (Fig. 3c). This could be explained by pH differences. Ammonia nitrogen (NH3-N) in a fluid exists in two forms: ammonium ion (NH +4 ) and dissolved ammonia gas (NH3). It has been reported that the proportion of volatile NH3 of total NH3-N is a function of pH and temperature [37]. Depending on the equation of weak acids and weak bases, the ammonium equilibrium constant K a could be calculated from the following equation: pK a = 10.05 − 0.032 × T (T = temperature (°C)) [36]. Therefore, at 25°C and a pH of 9.25, the concentration of NH3-N and NH +4 -N existing as the two forms of ammonia nitrogen in a solution are equal. Above pH 9.25, NH3 is greater; below pH 9.25, NH +4 dominates. The culture system with air aeration raised the pH over 9 after the fourth day, thus improving the ammonia-stripping phenomena (Fig. 4). Therefore, it is proposed that the depletion of NH3-N was not only attributed to the N uptake by algae, but also ammonia volatilization.
The total nitrogen removal efficiencies with air and 1% and 5% CO2-enriched air aeration were 73.63%, 64.51%, and 68.78%, respectively (Table 2). Similar to the results shown on the ammonia removal chart, the algae culture with air aeration had the highest TN removal efficiency (Fig. 3c, d). It has been noted that the total nitrogen removal was mainly composed of inorganic nitrogen and was attributed to two ways: (1) the uptake of inorganic nitrogen by algae and (2) the volatilization of ammonia gas from wastewater surface [38].
It is known that carbon and nitrogen metabolisms are associated with each other in plants, including algae, since they must share energy and the organic carbon supplied directly from photosynthetic electron transport and CO2 fixation or from respiration of fixed carbon by glycolysis [39]. If algal cells are provided with a metabolizable source of organic carbon, nitrogen assimilation is independent of the photosynthates [40]. If the supply of carbon to amino acid synthesis depends on photosynthesis, withholding either light or CO2 inhibits nitrogen assimilation [40, 41]. Inhibitors of carbon fixation and photosynthetic electron transport, e.g., DL-glyceraldehyde or 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), mimic this effect [40, 41]. Thacker and Syrett proved that illuminated Chlamydomonas reinhardi cells in which CO2 assimilation was prevented by DCMU did not assimilate either ammonium or nitrate [41]. The comparison of the nitrogen removal in the culture with 1% and 5% CO2-enriched air aeration which had similar pH situations (Fig. 4a) show that in either ammonium nitrogen removal or TN removal, algae in the culture with 5% CO2-enriched air aeration was more effective than that with 1% CO2-enriched air aeration, especially after the sixth culture day, suggesting a higher nitrogen metabolism efficiency in algae grown in the culture with 5% CO2 bubbling (Fig. 3d). Therefore, the aforementioned phenomenon indicated that 5% CO2 addition stimulated the algal autotrophic metabolism better than 1% CO2 addition, which coincided with the results of algal cell density and dry weight (Fig. 2).
The Effect of CO2 on Lipid Production
Lipids are substances related biosynthetically or functionally to fatty acids and their derivatives [42]. Microalgal lipids exist in cells in the forms of membrane and energy storage bodies.
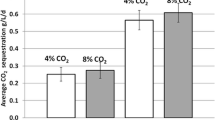
The results of the algal lipid content were showed in Fig. 5. The lipid contents from the algal biomass obtained from the culture with 0%, 1%, and 5% CO2-enriched air aeration were 18.66%, 20.82%, and 20.58%, respectively. However, statistical analysis showed that there were no significant differences in the total lipid contents (% of TVSS) among the algae cultivated under the three CO2 concentrations (p > 0.5). Because the algal biomass concentrations of the three cultures varied from each other, the total lipid productions (g/L) were different. The algal lipid production in cultures with pure air and 1% and 5% CO2-enriched air aeration was 0.182, 0.418, and 0.516 g/L, respectively (Fig. 5), indicating that the lipid production increased with increasing CO2 concentration. However, the data in the study are not enough for the determination of the optimal CO2 concentration for maximizing biomass and total lipid production.
Lipid content of algae cultivation broth with different CO2 concentrations. Filled columns indicate the total lipid content (%); white columns indicate the total lipid production in the culture (g/L). Total lipid production was estimated by multiplying total lipid content with TVSS (g/L) on the 12th day
In the commercial production of algal lipids, higher cell density cultivation is desirable in order to reduce down-stream process cost. The lipid and fatty acid contents and productivity of microalgae vary with culture conditions. Supplement of soluble carbon substrates to the culture media in a sterilized bioreactor may provide a feasible means for the accumulation of high-value products, such as algal oil and photosynthetic accessory pigments in algal cells. Ogbonna et al. reported that they could achieve a high Chlorella biomass concentration with high cellular photosynthetic products by using the method of sequential heterotrophic/autotrophic cultivation [43]. Centrate has high organic carbon concentration, with COD around 2,242.5 mg/L, and is a good medium for an initial quick multiplication of algal cells. The aeration with CO2-enriched air offered carbon source for the photosynthetic growth especially when the organic carbon substances were limited. So, algae could continuously grow, producing fatty acids, lipids, and normal proteins both in heterotrophic and autotrophic pathways and accumulating photosynthetic proteins, chlorophylls, etc., in photosynthesis.
Conclusion
UMN280 cultivated in centrate with CO2 aeration resulted in a two-phase growth pattern in this study. In the first 4 ~ 5 days, heterotrophic growth dominated, while after 4 ~ 5 days, autotrophic growth dominated when organic-carbon nutrients became limited. With the continuous light intensity of 60 μmol/(m2 s), the addition of CO2 was found to be positive for the continuous growth of UMN280 in centrate. Increasing CO2 concentration favored biomass production after 4 ~ 5 days. For nutrient removal, though the algae in culture under air aeration had the highest nutrient removal efficiencies on COD, NH3-N, TN, and PO4-P, the growth rates of algae under 5% CO2 aeration were close to the highest ones, except for PO4-P removal, which had close relationship with alkaline sedimentation. This study opens up the possibility of using flue gas emitted from industry for algal biomass production and algae-based wastewater remediation.
References
Lau, P. S., Tam, N. F. Y., & Wong, Y. S. (1996). Wastewater nutrients removal by Chlorella vulgaris: optimization through acclimation. Environmental Technology, 17(2), 183–189.
Hernandez, J. P., de-Bashan, L. E., & Bashan, Y. (2005). Starvation enhances phosphorus removal from wastewater by the microalga Chlorella spp. Co-immobilized with Azospirillum brasilense. Enzyme and Microbial Technology, 38(2006), 190–198.
Li, Y., Chen, Y. F., Chen, P., Min, M., Zhou, W., Martinez, B., et al. (2011). Characterization of a microalgae Chlorella sp. well adapted to highly concentrated municipal wastewater in nutrient removal and biodiesel production. Bioresource Technology, 102(8), 5138–5144.
Wang, L., Wang, Y., Chen, P., & Ruan, R. (2010). Semi-continuous cultivation of Chlorella vulgaris for treating undigested and digested dairy manure. Applied Biochemistry and Biotechnology, 162(8), 2324–2332.
Mulbry, W. W., & Wilkie, A. C. (2001). Growth of benthic freshwater algae on dairy manure. Journal of Applied Phycology, 13, 301–306.
Spatharis, S., Danielidis, D. B., & Tsirtsis, G. T. (2007). Recurrent Pseudo-nitzschia calliantha (Bacillariophyceae) and Alexandrium insuetum (Dinophyceae) winter blooms induced by agricultural runoff. Harmful Algae, 6(6), 811–822.
Kumar, A., Ergas, S., Yuan, X., Sahu, A., Zhang, Q., Dewulf, J., et al. (2010). Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends in Biotechnology, 28, 371–380.
Andrade, M. R., & Costa, J. A. V. (2007). Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture, 264(1–4), 130–134.
Lee, H. Y., Lee, S. Y., & Park, B. K. (1989). The estimation of algal yield parameters associated with mixotrophic and photoheterotrophic growth under batch cultivation. Biomass, 18(2), 153–160.
Xu, F., Hu, H., Cong, W., Cai, Z., & Ouyang, F. (2004). Growth characteristics and eicosapentaenoic acid production by Nannochloropsis sp. in mixotrophic conditions. Biotechnology Letters, 26(1), 51–53.
Martínez, F., & OrÚs, M. I. (1991). Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM101. Plant Physiology, 95, 1150–1155.
Kobayashi, M., Kakizono, T., Yamagichi, K., Nishio, N., & Nagai, S. (1992). Growth and astaxanthin formation of Haematococcus pluvialls in heterotrophic and mixotrophic conditions. Journal of Fermentation and Bioengineering, 74(1), 17–20.
Chiu, S., Kao, C., Chen, C., Kuan, T., Ong, S., & Lin, C. (2008). Reduction of CO2 by a high-density culture of Chlorella sp. in semicontinuous photobioreactor. Bioresource Technology, 99(9), 3389–3396.
Yang, Y., & Gao, K. (2003). Effects of CO2 concentrations on the freshwater microalgae Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). Journal of Applied Phycology, 15, 379–389.
Kodama, M., Ikemoto, H., & Miyachi, S. (1993). A new species of highly CO2-tolerant fast-growing marine microalga suitable for high-density culture. Journal of Marine Biotechnology, 1(1), 21–25.
Ingram, L. O., Calder, J. A., Van Baalen, C., Plucker, F. E., & Parker, P. L. (1973). Role of reduced exogenous organic compounds in the physiology of the blue-green bacteria (algae): photoheterotrophic growth of a “heterotrophic” blue-green bacterium. Journal of Bacteriology, 114(2), 695–700.
Baalan, C. V., Pulich, W. M., & Brandeis, M. G. (1973). Heterotrophic growth of the microalgae. CRC Critical Reviews in Microbiology, 2(2), 229–254.
Darley, W. M., Wimpee, B. B., & Ohlman, C. T. (1981). Heterotrophic and photoheterotrophic utilization of lactate by the diatom, Cylindrotheca fusiformis. British Phycological Journal, 16, 423–428.
Zhou, W., Li, Y., Min, M., Hu, B., Chen, P., & Ruan, R. (2011). Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresource Technology. doi:10.1016/j.biortech.2011.04.038.
Huss, V. A., Ciniglia, C., Cennamo, P., Cozzolino, S., Pinto, G., & Pollio, A. (2002). Phylogenetic relationships and taxonomic position of Chlorella-like isolates from low pH environments (pH < 3.0). BMC Evolutionary Biology, 2, 13.
Rippka, R., Deruelles, J., Waterbury, J., Herdman, M., & Stanier, R. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology, 111, 1–61.
APHA, AWWA, WEF. (1995). Standard methods for the examination of water and wastewater (19th ed.). Washington: American Public Health Association.
Hach. Procedure manual. (2008) Hach, Loveland, CO.
Folch, J., Lees, M., & Sloane Stanley, G. H. (1956) A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 497–509.
Tredici, M. R., Papuzzo, T., & Tomasell, L. (1986). Outdoor mass culture of Spirulina platensis in sea-water. Applied Microbiology and Biotechnology, 24(1), 47–50.
Chen, F., & Zhang, Y. (1997). High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzyme and Microbial Technology, 20(3), 21–224.
Agusti, S., Duarte, C. M., & Kalff, J. (1987). Algal cell size and the maximum density and biomass of phytoplankton. Limnology and Oceanography, 32(4), 983–986.
James, C. M., Al-Khars, A. M., & Chorbani, P. (1988). pH dependent growth of Chlorella in a continuous culture system. Journal of the World Aquaculture Society, 19, 27–35.
Pitter, P. (1976). Determination of biological degradability of organic substrances. Water Research, 10(3), 231–235.
Amblard, C., Couture, P., & Bourdier, G. (1990). Effects of a pulp and paper mill effluent on the structure and metabolism of perphytic algae in experimental streams. Aquatic Toxicology, 18(3), 137–161.
Morris, I., Yentsch, C. M., & Yentsch, C. S. (1971). Relationship between light carbon dioxide fixation and dark carbon dioxide fixation by marine algae. Limnology and Oceanography, 16(6), 854–858.
OECD: Eutrophication of waters. (1982). Monitoring, assessment and control. Pairs: OECD Publications.
González, L. E., Cañizares, R. O., & Baena, S. (1997). Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresource Technology, 60, 259–262.
Zhou, A., Tang, H., & Wang, D. (2005). Phosphorus adsorption on natural sediments: modeling and effects of pH and sediment composition. Water Research, 39(7), 1245–1254.
Boyd, C. E. (1982). Water quality management for pond fish culture. Amsterdam: Elsevier Scientific.
Ferrara, R. A., & Avci, C. B. (1982). Nitrogen dynamics in waste stabilization ponds. Journal of the Water Pollution Control Federation, 54(4), 361–369.
Srinath, E. G., & Loehr, R. C. (1974). Ammonia desorption by diffusion aeration. Journal of the Water Pollution Control Federation, 46(8), 1939–1957.
Meron, A. (1971). Kinetics of algal systems in waste treatment field studies. University of California, Berkeley, FWQA, USDOI, PB 206812.
Huppe, H. C., & Turpin, D. H. (1994). Integration of carbon and nitrogen metabolism in plant and algal cells. Annual Review of Plant Physiology and Plant Molecular Biology, 45, 577–607.
Amory, A. M., Vanlerberghe, G. C., & Turpin, D. H. (1991). Demonstration of both a photosynthetic and nonphotosynthetic CO2 requirement for NH +4 assimilation in the green alga Selenastrum minutum. Plant Physiology, 95, 192–96.
Thacker, A., & Syrctt, P. J. (1972). The assimilation of nitrate and ammonium by Chlamydomonas reinhardtii. The New Phytologist, 71, 423–433.
Christie, W. (2003). Lipid analysis, isolation, separation, identification and structural analysis of lipids (3rd ed.). Dundee: MRS Lipids Analysis Unit, Scottish Crop Research Institute, Invergowrie.
Ogbonna, J. C., Masui, H., & Tanaka, H. (1997). Sequentical heterotrophic/autotrophic cultivation—an efficient method of producing Chlorella biomass for health food and animal feed. Journal of Applied Physics, 9(4), 359–366.
Acknowledgement
The study was supported by grants from the University of Minnesota Initiative for Renewable Energy and the Environment (IREE) and Metropolitan Council Environmental Services (MCES), as well as the Legislative-Citizen Commission on Minnesota Resource (LCCMR). The authors are also grateful to Robert C. Polta and Adam Sealock of Saint Paul MCES Wastewater Treatment Plant for helping with the sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, B., Min, M., Zhou, W. et al. Influence of Exogenous CO2 on Biomass and Lipid Accumulation of Microalgae Auxenochlorella protothecoides Cultivated in Concentrated Municipal Wastewater. Appl Biochem Biotechnol 166, 1661–1673 (2012). https://doi.org/10.1007/s12010-012-9566-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9566-2