Abstract
The chemical modification of developed ethyl cellulose-based membrane was carried out to make it suitable for bioseparation. The different reagents were used for the modification of membrane to couple protein A (PA) to study the purification of immunoglobulin G (IgG) from blood. The chemical modification was carried out using relatively simple and mild reaction conditions. The attenuated total reflectance Fourier transform infrared analysis of chemically modified membrane showed new peak at 1,596.06 and 1,716.49 cm−1. The scanning electron microscopy of PA-coupled membrane, which was used for IgG purification showed open pores and 950 ± 21.5 LMH (L m−2 h−1) operational flux at 0.5-bar out pressure. The flux of unmodified membrane was 1,746 ± 18.5 LMH at 0.5-bar out pressure. The equilibrium adsorption concentration (318.5 ± 5.9 μg cm−2) was obtained at 3 h. The adsorption character of PA-coupled membrane was consistent with the Langmuir adsorption model and the non-specific binding was 67.08 ± 1.3 μg cm−2. The sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis showed similar purification pattern for purified IgG from human serum and commercial preparation of IgG. All the results have suggested a high potential of PA-coupled ethyl cellulose-based membrane for large-scale purification of IgG.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Downstream processing is a very cost-intensive operation, which can contribute up to 80 % of the total production costs of a biopharmaceutical [1, 2]. Thus, it is highly essential to develop a cost-effective method for large-scale purification of therapeutically useful biomolecules. Conventionally, this has been carried out using column chromatography containing beads or particles coupled with chemically or biologically active material [3, 4]. However, this type of technique often requires lengthy procedures, which can lead to the degradation of sensitive biomolecules [5]. Secondly, a high-pressure drop across the column and dependence on intra-particle diffusion for the transport of solute molecules to their binding sites within the pores are the major obstacles [2, 5].
To overcome all these problems, membrane chromatography has been recently emerged as an alternative to column chromatography. The high flow rate at low-pressure drop can be obtained by using membrane. This can result in improvements of washing, elution, and regeneration process. Subsequently, this helps in decreasing the possibility of biomolecule deactivation by shortening their exposure to an unfavorable medium. Secondly, membrane chromatography has more advantages such as higher surface area, better scalability, and lower costs of production [2]. The natural polymers such as cellulose acetate, chitosan- and chitin-based polymers, and synthetic polymers such as polyethylene, polysulfone, nylon and polyvinyl alcohol, and composites have been used largely for membrane chromatography [2]. Conventionally, cellulose always remains a first choice as a matrix for bioseparation. Cellulose is environmentally friendly and one of the most abundant renewable natural polymers with relatively low cost and it has also good compatibility with biological compounds [6]. However, cellulose and its derivatives have been widely used in conventional form such as beads in bioseparation [7, 8]. There are some reports regarding the use of cellulose-based membranes in immunoglobulin G (IgG) purification [9–11]. The use of cellulose-based composite membranes in IgG purification has been also reported [12–14]. The composite membranes were developed to achieve the desired chemical as well as mechanical properties that required in bioseparation [12–14]. However, the major drawback of membrane is the fouling, which drastically reduces the operating flux and enhance the overall operating and maintenance cost. Therefore, most of the affinity membranes have been developed using microporous/microfiltration membranes to achieve high flux and high throughput [15]. Thus, the pore size of membrane is of high importance in membrane chromatography. Membrane has to undergo different chemical modification to couple respective ligand that requires for selective bioseparation. This can result in the reduction of pore size, which significantly affects the operating flux and throughput. It was reported that the commercially available cellulose derivative membranes have relatively small pores and are unsuitable for use as an affinity matrix [15]. Therefore, modified synthetic membranes have been widely used in affinity bioseparation [1, 16]. Recently, the preparation of affinity membrane from regenerated cellulose using environment friendly chemicals has been reported [2]. However, it fails to achieve high flux that generally required in such operations.
To overcome all these problems, we have developed ethyl cellulose-based highly porous membrane [17]. Comparatively, ethyl cellulose has a better chemical stability than the other cellulose derivatives. Therefore, we have selected it as a base polymer for the development of membrane. Secondly, ethyl cellulose-based membrane has been also widely used in operations such as pervaporation [18] and controlled release [19, 20]. However, ethyl cellulose-based membrane has not been used in affinity bioseparation so far. In the present paper, we have carried out different studies to evaluate the utility of developed ethyl cellulose-based highly porous membrane in affinity bioseparation. The chemical modification was carried out using relatively simple and mild reaction conditions to make it suitable for the purification of IgG from blood. The chemical modification and bioseparation was verified using analytical techniques such as attenuated total reflectance Fourier transform infrared (ATR/FT-IR) and electrophoresis.
Materials and Methods
Materials
Epichlorohydrin, glutaraldehyde, sodium borohydride (Loba, India); polyethylenimine (MW ∼25,000), bisoxiranes, ethyl cellulose (48 % ethoxyl; viscosity–300 cP, 5 % in toluene/ethanol 80:20 [lit.]), acrylamide, (Aldrich Chemicals); human IgG, protein A (PA), human serum albumin (HSA), low range molecular weight markers (Sigma Chemicals) were used as received. All other chemicals used were of ACS grade.
Preparation of PA-Coupled Membrane
The membrane was prepared as per the procedure described elsewhere [17]. The base polymer of membrane was ethyl cellulose and the buffer flux (50 mM sodium phosphate buffer pH 7.0) of membrane was 1,746 ± 18.5 LMH at 0.5-bar out pressure. Firstly, the membrane was treated with 2 M HCl at 60 °C for 1 h. Then, after complete washing with double distilled water (DDW), it was treated with 1 % polyethylenimine at room temperature for 24 h. The washing of membrane was carried out until the pH and conductivity of wash become equal to DDW. Thereafter, it was treated with glutaraldehyde (1 %, v/v) at 37 °C for 24 h after complete washing. The modified membrane was washed with DDW and then it was fixed in an Amicon flow cell having 13.4-cm2 surface area. The membrane was equilibrated with 50 mM sodium phosphate buffer pH 7.0 prior to PA adsorption. The commercially available PA was reconstituted in 25 mM phosphate buffer pH 7.0 as 1 mg mL−1. The 5 mL PA was loaded on the membrane fixed in an Amicon flow cell after closing the permeate outlet and treated as a feed. It was allowed to react for 2 h at room temperature under mild stirring. Then it was collected after applying 0.5-bar out pressure and treated as wash I. The further washing was carried out two times sequentially by loading 5 mL 25 mM phosphate buffer pH 7.0. Each time buffer was allowed to stir for 5 min and then collected by applying 0.5-bar pressure and treated as wash II and wash III. The adsorption of PA was calculated from the difference in concentration of protein present in total feed and total wash (I + II + III). The protein concentration was estimated using Folin–Ciocalteu reagent and HSA as a standard [21].
IgG Adsorption/Desorption
The PA-coupled membrane was washed with 25 mM phosphate buffer pH 7.0 and then 5 mL 2 % glycine prepared in a 25 mM phosphate buffer pH 7.0 was loaded. It was allowed to react for 15 min under mild stirring at room temperature. After glycine treatment, membrane was treated similarly with 5 mL 0.2 % sodium borohydride prepared in 25 mM phosphate buffer pH 7.0. Then the membrane was washed and equilibrated with 25 mM phosphate buffer pH 7.0 containing 150 mM NaCl. Thereafter, 5 mL of commercial human IgG reconstituted in 25 mM phosphate buffer pH 7.0 containing 150 mM NaCl as 1 mg ml−1 was loaded on the membrane. It was allowed to react for 1 h at room temperature using mild stirring. After 1 h, washing was carried out with 25 mM phosphate buffer pH 7.0 containing 150 mM NaCl and samples of wash I, II, and III were collected. The elution of adsorbed IgG was carried out sequentially using 2 mL of 0.1 M sodium acetate buffer at pH 4.5 and 4.0 followed by 0.1 M glycine HCl buffer pH 3.0 and 2.3 as eluent. After collection of all the samples, membrane was washed with 25 mM phosphate buffer pH 7.0 and stored in 0.02 % sodium azide prepared in same buffer at 4 °C. The calculation of IgG adsorption and desorption was carried out by measuring protein concentration in collected samples.
Human Serum Adsorption/Desorption
The human serum was obtained from a healthy donor and used as a feed for adsorption/desorption study after diluting it 40 times with 25 mM phosphate buffer pH 7.0. The adsorption/desorption studies were carried out using similar method used for commercial human IgG as mentioned above. Except, the adsorption and washing was carried out using 25 mM phosphate buffer pH 7.0 without containing NaCl.
SDS-PAGE
The purity of separated commercial human IgG and human serum using PA-coupled membrane was checked using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The SDS-PAGE was carried out as per the procedure described by Laemmli [22] using a 3.9 % stacking gel and 8 % resolving gel and gels were stained by Coomassie brilliant blue G-250.
Membrane Characterization
The membrane was characterized using techniques such as ATR/FT-IR spectrometry and SEM. ATR/FT-IR was carried out using Perkin Elmer FT-IR system, Spectrum GX and standard procedures were used for preparation of samples. SEM was obtained by using Leo/Leica Cambridge (UK) Model–Stereoscan 440 and standard procedures were used for preparation of samples.
Adsorption Isotherm of IgG
Adsorption isotherm for PA-coupled membrane was carried out using commercial human IgG as a feed. Similar procedure was used as mentioned in the “IgG adsorption/desorption” section except IgG concentration. The commercial human IgG was reconstituted in 5 mL 150 mM NaCl prepared in 25 mM phosphate buffer pH 7.0 and different volume of this was made to 5 mL with same buffer and loaded on the membrane. The exact concentration of IgG applied on the membrane was 0.266, 0.532, 0.798, 1.064, 1.845, 2.214, 2.583, 2.952, 3.321, and 3.690 mg ml−1. It was allowed to react with the membrane up to 3 h to determine the equilibrium adsorption capacity. The adsorption of IgG was calculated from the difference in concentration of protein present in total feed and total wash (I + II + III).
Analysis of the Equilibrium Adsorption Data
The theoretical Langmuir isotherm as reported earlier [23] is often used to describe sorption of a solute from a liquid solution as Eq. (1):
The constants q m and K a are characteristics of the Langmuir equation and can be determined from a linearized form of Eq. (1), represented by Eq. (2):
It will have a straight line with a slope of 1/(Ka qm) and an intercept of 1/q m when 1/q is plotted against 1/c, where c is the equilibrium concentration (in milligram per square centimeter), q equilibrium concentration of IgG bound to the PA-coupled membrane (in milligram per square centimeter), q m maximum binding capacity of the PA-coupled membrane (in milligram per square centimeter), and K a is the sorption equilibrium constant (in milligram per square centimeter).
Non-Specific Binding
The procedure which was adapted for the study of human serum adsorption was used to study the non-specific binding of PA-coupled membrane. The commercial HSA was reconstituted in 5 ml in 25 mM phosphate buffer pH 7.0 which corresponds to 1 mg ml−1. Then it was loaded (5 mL) on the membrane and allowed to react for 3 h at room temperature under mild stirring. The elution was carried out sequentially with 5 mL of 0.1 M sodium acetate buffer pH 4.0 and 0.1 M glycine HCl buffer pH 2.3. The adsorption of HSA was calculated from the difference in concentration of protein present in total feed and total wash (I + II + III).
Treatment of Experimental Data
Every experimental point reported in the figures represents the average value of three experiments performed under the same conditions. The experimental errors did not exceed 5 %.
Results and Discussion
Effect of Membrane Activation on PA Adsorption
An ideal membrane for successful chromatographic application supposed to be microporous, hydrophilic, contain functional groups to allow activation, chemically stable, and readily available at low cost to facilitate industrial applications [15]. The membrane developed by us has most of the properties that required to make it suitable for chromatographic applications [17]. However, to make a membrane suitable for an affinity bioseparation need a ligand that can be covalently coupled to membrane. As aim of the study was to make a developed ethyl cellulose-based membrane suitable for the purification IgG from blood therefore, PA was selected as a ligand. PA has been widely used for the purification of IgG from biologically derived fluids [1, 15, 16]. In general, the preparation of an affinity matrix is a two-step reaction, which consists of activation of the matrix and coupling of the ligand to this modified matrix [15]. The reagents such as bisoxiranes, epichlorohydrin, glutaraldehyde, and polyethylenimine were used for the activation of membrane in the present study. The reagent such as bisoxiranes and epichlorohydrin has been used for the activation of matrix having hydroxyl functions and glutaraldehyde has been used for the activation of matrix having amine functions for the immobilization of an enzyme [24, 25]. Similarly, the polyethylenimine was reported to be used for the activation of diethylaminoethyl cellulose for the immobilization of an enzyme [26].
In the present study, it was observed that the adsorption of PA to the modified membrane varies according to the nature of activation regent. The membrane activated with bisoxiranes, glutaraldehyde, and epichlorohydrin showed 85.09 ± 2.9, 73.66 ± 2.3, and 92.71 ± 3.3 μg cm−2 PA adsorption, respectively. Whereas, membrane activated using polyethylenimine showed 129.54 ± 4.8 μg cm−2 PA adsorption. This was almost 6.8-fold higher than the unmodified membrane (19.05 ± 0.6 μg cm−2). Therefore, it was decided to use polyethylenimine as an activation agent in the further study. The higher adsorption obtained with polyethylenimine can be attributed to the availability of more groups because of polymeric nature of polyethylenimine. However, the use of polymeric material is not new in bioseparation. The polymeric material such as dextran and polyvinylalcohol has been also used to achieve higher density of affinity ligands on the membranes [27]. Secondly, polyethylenimine is an organic polymer, which has a high density of amino groups and these amino groups can be protonated by varying pH. It was reported that the polyethylenimine can act as a polycation at physiological pH and it can be used to bind deoxyribonucleic acid [28]. This suggests that the membrane coupled with polyethylenimine can be used as an ion exchanger. To verify this, further study was carried out to understand the adsorption/desorption behavior of polyethylenimine-coupled membrane.
Effect of Polyethylenimine on Adsorption/Desorption
The adsorption/desorption behavior of polyethylenimine-coupled membrane was studied using human serum as a feed. The adsorption of human serum on the membrane was carried using adsorption buffer of pH 7.0. The significant difference in adsorption/desorption behavior of human serum proteins was observed on modified and unmodified membrane. The unmodified membrane showed 21.2 ± 0.5 μg cm−2 adsorption of human serum proteins. On the contrary, 184.8 ± 4.5 μg cm−2 adsorption of human serum proteins was obtained with the modified membrane. This indicates that the coupling of polyethylenimine to the membrane has enhanced its adsorption capacity significantly. Similarly, the desorption profile obtained for adsorbed human serum proteins on modified and unmodified membrane was also significantly different. After sufficient washing with adsorption buffer, desorption was carried sequentially using buffer of pH 4.5, 2 M NaCl prepared in buffer pH 4.5 and 0.1 N NaOH as eluent. The unmodified membrane showed 96.5 ± 2.5 % desorption only with 0.1 N NaOH. However, split desorption profile was obtained with the modified membrane. The modified membrane showed 54.2 ± 1.3 % desorption at pH 4.5, whereas 15.5 ± 0.35 and 26.2 ± 0.75 % desorption was obtained with NaCl and NaOH, respectively. This indicates that the blood proteins which have acquired negative charge at pH 7.0 were adsorbed on the membrane. Similarly, blood proteins which have acquired positive charge at pH 4.5 was desorbed from the membrane. The maximum desorption was obtained at pH 4.5 without NaCl. This indicates that the proteins which were tightly bound to the membrane got eluted in the presence of NaCl. As well as those proteins which were comparatively more tightly bound, got eluted with NaOH.
This shows that the membrane modified with polyethylenimine can be used as an ion exchange membrane. Subsequently, this has also confirmed the chemical modification of membrane. To further confirm this, the polyethylenimine-coupled membrane was subjected to ATR/FT-IR examination. Figure 1a shows the ATR/FT-IR spectra of unmodified membrane, which reveals its chemical structure. It shows several characteristic peaks, the peak at 3,475 cm−1 corresponds to the –OH groups of closed ring structure of the polymer unit. The peak at 2,869 cm−1 corresponds to C–H bond whereas the peak at 1,374 cm−1 corresponds to C–N bond. The sharp peak at 1,074 cm−1 corresponds to –C–O (stretch). However, in comparison to the spectra of unmodified membrane, the spectra of modified membrane shows new peak at 1,596.06 cm−1 which corresponds to primary amine (Fig. 1b). This has confirmed the coupling of polyethylenimine to the membrane. All these results have strongly suggested the suitability of polyethylenimine-coupled membrane in bioseparation.
ATR/FT-IR spectra of unmodified and modified membrane. [All the spectra are given as it is obtained from the instrument except enlargement; X-axis, frequency; Y-axis, transmittance; A unmodified membrane with x- and y-axis values; B polyethylenimine-treated membrane; C glutaraldehyde-treated membrane]
Effect of Glutaraldehyde on Adsorption
The above results have clearly indicated that the polyethylenimine-coupled membrane can be used as an ion exchanger. This suggests that the adsorption of PA obtained on the polyethylenimine-coupled membrane was mainly due to ionic interactions. Therefore, the possibility of leakage of adsorbed PA from polyethylenimine-coupled membrane was higher during its repeated use. To overcome this problem, polyethylenimine-coupled membrane was treated with glutaraldehyde prior to PA adsorption. We have used glutaraldehyde because it has been widely used as a common crosslinker or grafter for biomaterials due to its reaction with amino group of organic compound at ambient temperature [25]. It was reported that the reaction of glutaraldehyde with amino group of matrix leads to the introduction of aldehyde group onto the matrix that can bind enzymes [29]. This reaction has been termed as the activation of the matrix and the binding of an enzyme is generally considered to occur via a Schiff base formation [29]. This suggests that the glutaraldehyde treatment of polyethylenimine-coupled membrane prior to PA adsorption can significantly reduce the chances of its leakage during repeated use. The similar reaction scheme has been used earlier for an enzyme immobilization [26]. To confirm the binding of glutaraldehyde to the polyethylenimine-coupled membrane, it was subjected to the ATR/FT-IR examination. This will help in understanding the changes that occur after the glutaraldehyde treatment in chemical structure of polyethylenimine-coupled membrane. Figure 1c shows new peak at 1,716.49 cm−1, which closely corresponds to aldehyde. This suggests the binding of glutaraldehyde to the polyethylenimine-coupled membrane. This was also substantiated with the increase in PA adsorption to the glutaraldehyde-treated polyethylenimine-coupled membrane. In comparison with the polyethylenimine-coupled membrane, the glutaraldehyde-treated polyethylenimine-coupled membrane showed 33 % higher (172.5 ± 4.2 μg cm−2) PA adsorption.
Effect of PA Binding on Flux
As high volumetric throughput is one of the most important advantages of affinity membrane chromatography [14]. Therefore, the evaluation of flux pattern of PA-coupled membrane was studied. Although decrease in flux due to blockage of pores after the coupling of macromolecules such as polyethylenimine and PA to the membrane is desirable. However, the operational flux has to be sufficient to achieve the basic purpose of the use of membrane in affinity bioseparation. In general, this aspect has been neglected largely and almost negligible information is available in the literature. The decreased in flux after the PA immobilization to the chitosan/cellulose composite membrane was reported, but it was not quantified [14]. However, different flux has been reported for the unmodified membranes, which were later developed as affinity membrane. The flux of chitosan/cellulose composite membrane was reported to be 8.9 ml/min/cm2 at a pressure drop of 5 psi [14]. Whereas, 2.12-mL min−1 cm−2 flux at a pressure drop of 10 psi was reported for chitosan composite membrane [30]. Similarly, the developed cellulose affinity membrane has showed 5-mL min−1 flux for 4.1-cm2 surface area [2]. On the contrary, 950 ± 21.5 LMH flux was obtained at 0.5-bar out pressure for the PA-coupled membrane developed by us. This appears to be higher flux reported so far for the PA-coupled membrane used in bioseparation. However, the flux obtained for the PA-coupled membrane (950 ± 21.5 LMH) was 45.5 ± 1.2 % less than the flux of unmodified membrane (1,746 ± 25.3 LMH). The decrease in flux after the membrane modification can be attributed to the blockage of membrane pores. However, flux obtained after the binding of PA to the membrane (950 ± 21.5 LMH) appears to be sufficient to achieve the high volumetric throughput. This also suggests that the modified membrane has sufficient open pores to obtain high volumetric throughput. This was further confirmed by the SEM examination of the modified membrane, which was used for the IgG purification. Figure 2, clearly shows the open pores, this strongly suggests the suitability of the developed membrane to obtain high volumetric throughput in bioseparation.
Utility of PA-Coupled Membrane
The utility of PA-coupled membrane for the purification of IgG from blood was checked using human serum and commercial IgG preparation as a feed. The commercial IgG preparation was loaded on the PA-coupled membrane using adsorption buffer pH 7.0 and eluted with different acidic range buffer of pH levels 4.5, 4.0, 3.0, and 2.3. In general, acidic pH has been used for the elution of IgG adsorbed on the PA-coupled different matrix [2, 16, 31]. In the present study, PA-coupled membrane showed significant adsorption of commercial human IgG (182.4 ± 3.2 μg cm−2). However, split elution was obtained all over the acidic pH range. The elution obtained at pH levels 4.5 and 4.0 was 35.0 ± 0.7 and 31.0 ± 0.5 %, respectively. Whereas, the elution obtained at pH levels 3.0 and 2.3 was 25.0 ± 0.4 and 9.0 ± 0.2 %, respectively. The similar elution pattern was observed for human serum. However, 42 % less (105.8 ± 2.2 μg cm−2) adsorption was obtained for the human serum than the commercial human IgG. This can be attributed to the presence of other several proteins in serum, which might have blocked the adsorption sites available on PA-coupled membrane. Secondly, although the elution pattern of human serum was similar to commercial human IgG, it was however quantitatively different. At pH levels 4.5 and 4.0, the elution was 48.0 ± 1.3 and 36.0 ± 0.8 %, respectively, whereas at pH levels 3.0 and 2.3, it was 12.0 ± 0.3 and 4.0 ± 0.1 % elution respectively.
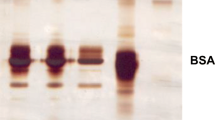
The split elution obtained for commercial human IgG and human serum has indicated the need of further study to enhance the recovery. Thus, the next experiment was carried out using pH levels 4.0 and 2.3 as an eluent. The elution of adsorbed commercial human IgG, which was carried out with pH levels 4.0 and 2.3 has showed 85.4 ± 2.6 and 13.3 ± 0.6 % elution, respectively. Whereas, the elution of adsorbed human serum, which was carried out with pH levels 4.0 and 2.3 has showed 84.5 ± 2.2 and 12.8 ± 0.26 % elution, respectively. As the maximum elution was obtained with pH 4.0, the next experiment was carried out using only pH 4.0 as an eluent. Figure 3, shows the purification pattern of IgG that was obtained at an elution pH 4.0 for commercial human IgG and human serum. This can be observed from the figure that the albumin and other proteins present in the human serum came down in the washing and the adsorbed IgG got eluted. Secondly, the figure also clearly shows the similar purification pattern for purified IgG from human serum and commercial preparation of IgG. This indicates that the adsorption of the IgG to PA-coupled membrane was highly selective. All these results have clearly indicated that the PA-coupled membrane developed in the present study is highly suitable for the purification of IgG from blood. Secondly, it has also strongly suggested the suitability of PA-coupled membrane for acidic pH operations, which is the basic need of any PA-coupled matrix that likely to be used in IgG purification.
SDS-PAGE of commercial human IgG and human serum obtained on protein A-coupled membrane. [1—low range molecular weight markers (in dalton): albumin, bovine serum, 66,000; ovalbumin chicken egg, 45,000; glyceraldehyde-3-phosphate dehydrogenase, rabbit muscle, 36,000; carbonic anhydrase, bovine erythrocytes, 29,000; trypsinogen, bovine pancreas, 24,000; trypsin inhibitor soybean, 20,000; α-Lactalbumin, bovine milk, 14,200. 2—feed, commercial human IgG. 3—wash I. 4—wash III. 5—eluent–pH 4.0. 6—empty. 7—feed, human serum. 8—wash I. 9—wash III. 10—Eluent, pH 4.0.]
Characterization of Adsorption
The time required for obtaining maximum adsorption is the critical parameter in an evaluation of affinity membranes. Therefore, IgG adsorption was studied using time as variable and the time required to reach the equilibrium adsorption concentration was determined. It was observed that the system required 3 h to reach the plateau (Fig. 4), which has showed 318.5 ± 5.9-μg cm−2 adsorption. However, the adsorption obtained at 6 h was 3.5 % higher (329.6 ± 6.4 μg cm−2) than the 3 h. As there was no significant difference in 3- and 6-h adsorption, 3 h was considered the time that required for obtaining equilibrium adsorption concentration. It appears that the binding sites available on the PA-coupled membrane have become almost saturated after 3 h. The curve pattern observed in the present study has been usually found in macromolecular adsorption. In general, the time required to reach the plateau is dependent on the nature of matrix and the factors such as hydrogen bonding, hydrophobic interaction, water structuring at the interface, and the configurational entropy of proteins at adsorbed sites [32].
To understand the phenomena of adsorption, adsorption isotherm was carried out with human IgG and the PA-coupled membrane. The experimental data was fitted in a Langmuir type isotherm and q m and K a was determined from the plot 1/q versus 1/c (Fig. 5), which corresponds to 154.8 ± 4.1 and 141.7 ± 3.4 μg cm−2, respectively. The Langmuir isotherm equation has been widely used for the interpretation of experimental data related to the binding behavior of biomolecule on ion exchange and affinity adsorbents [14]. The plot 1/q versus 1/c (Fig. 5) shows the linear isotherm over the entire concentration range studied with high R 2 values (0.972). This indicates that the adsorption character observed in the present study was consistent with the Langmuir adsorption model. However, the q m and K a value obtained in the present study may not be directly comparable with others due to different basis of calculation. Castilho et al. [1] has evaluated ten different affinity membranes through coupling of IgG-specific affinity ligand mainly PA.
They have reported the q m values, which were within the range of 2.48 ± 0.32 to 13.28 ± 0.73 mg mL−1 depending on the nature of membrane. In the present study, calculations were carried out on the basis of membrane area (in square centimeter), which was actually available for adsorption. However, the discrepancies in equilibrium adsorption concentration can be easily overcome using high membrane area for adsorption. This is the main advantage of the use of membrane in chromatographic applications in comparison with the conventional media [14]. The preparation process of the membrane used in the present study was simple and did not involve highly hazardous chemicals [17] in comparison to the regenerated cellulose-based membrane available in the market. Thus, this may compensate the extra cost that incurred due to increase in membrane area, which will be required to achieve desired q m .
Non-Specific Adsorption
In general, the affinity ligand such as PA can selectively bind the IgG that results in very pure product. However, the matrix and coupling agents that required for coupling the affinity ligands often influence the binding specificity. This result in non-specific binding that leads to poor purification efficiency. The blood plasma or serum, which is a primary source of IgG contains different proteins, mainly such as albumin, globulin and fibrinogen. Thus, in case of higher non-specific binding, it will be difficult to achieve the desired purity of the product. Therefore, it was highly desirable to understand the non-specific binding behavior of PA-coupled membrane. The HSA was used to study the non-specific binding, which shows 67.08 ± 1.3-μg cm−2 adsorption. This appears to be significantly comparable with the values reported so far for the non-specific adsorption. The non-specific binding for PA-coupled chitosan/coarse filter paper composite membrane [14], and polyethersulphone-supported membrane [30] was reported to be 0.65 and 2.62 mg cm−3, respectively.
Conclusion
The results obtained in the present study have clearly indicated the suitability of chemically modified ethyl cellulose-based membrane for the purification of IgG from blood. The observation such as the correlation of ATR/FT-IR analysis with the adsorption/desorption profile of human serum. Another observation such as the significant operational flux (950 ± 21.5 LMH) that was obtained after the binding of PEI, glutaraldehyde, and PA to the membrane and the open pores showed by SEM of membrane, which was used for IgG purification. In addition to these observations, low non-specific binding (67.08 ± 1.3 μg cm−2), higher equilibrium adsorption concentration (318.5 ± 5.9 μg cm−2), linear adsorption isotherm with high R 2 values (0.972) and selective IgG adsorption/desorption pattern showed by SDS-PAGE, clearly indicated a great potential of the present membrane in large-scale bioseparation.
References
Castilho, L. R., Anspach, F. B., & Deckwer, W.-D. (2002). Journal of Membrane Science, 207, 253–264.
Barroso, T., Temtem, M., Hussain, A., Aguiar-Ricardo, A., & Roque, A. C. A. (2010). Journal of Membrane Science, 348, 224–230.
Bresolin, I. T. L., Fioritti, R. R., & Bueno, S. M. A. (2011). Process Biochemistry, 46, 2277–2285.
Wongchuphan, R., Tey, B. T., Tan, W. S., Subramanian, S. K., Taip, F. S., & Ling, T. C. (2011). Process Biochemistry, 46, 101–107.
Suck, K., Walter, J., Menzel, F., Tappe, A., Kasper, C., Naumannb, C., Zeidler, R., & Scheper, T. (2006). Journal of Biotechnology, 121, 361–367.
Liu, S., Zeng, J., Tao, D., & Zhang, L. (2010). Cellulose, 17, 1159–1169.
Gemeiner, P., Polakovič, M., Mislovičová, D., & Štefuca, V. J. (1998). Chromatography B, 715, 245–271.
Boeden, H.-F., Pommerening, K., Becker, M., Rupprich, C., Holtzhauer, M., Loth, F., & Müller, R. (1991). Journal of Chromatography. A, 552, 389–414.
Hari, P. R., Paul, W., & Sharma, C. P. (2000). Journal of Biomedical Materials Research, 50, 110–113.
Chandel, A. K., Rao, L. V., Narasu, M. L., & Singh, O. V. (2008). Enzyme and Microbial Technology, 42, 199–207.
Malakian, A., Golebiowska, M., & Bellefeuille, J. (1993). American Laboratory, 25, 40P–40V.
Hou, K. C., Zaniewski, R., & Roy, S. (1991). Biotechnology and Applied Biochemistry, 13, 257–268.
Jia, L., Yang, L., Zou, H., Zhang, Y., Zhao, J., Fan, C., & Sha, L. (1999). Biomedical Chromatography, 13, 472–477.
Yang, L., & Chen, P. (2002). Journal of Membrane Science, 205, 141–153.
Charcosset, C. (1998). Journal of Chemical Technology and Biotechnology, 71, 95–110.
Castilho, L. R., Deckwer, W.-D., & Anspach, F. B. (2000). Journal of Membrane Science, 172, 269–277.
Adikane, H. V., & Thakar, D. M. (2010). Applied Biochemistry and Biotechnology, 160, 1130–1145.
Wei, X., Yunjun, L., Xuejing, S., Jingru, L., Yin, Y., Yong, L., & Yongbin, J. (2008). Journal of Membrane Science, 325, 592–598.
Shi, P., Zuo, Y., Zou, Q., Shen, J., Zhang, L., Li, Y., & Morsi, Y. S. (2009). International Journal of Pharmaceutics, 375, 67–74.
Larsson, M., Hjärtstam, J., Berndtsson, J., Stading, M., & Larsson, A. (2010). European Journal of Pharmaceutics and Biopharmaceutics, 76, 428–432.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Ho, Y.-S., Chiu, W.-T., & Wang, C.-C. (2005). Bioresource Technology, 96, 1285–1291.
Porath, J., & Axén, R. (1976). In K. Mosbach (Ed.), Methods in enzymology, vol, 44: immobilized enzymes (pp. 19–45). New York: Academic.
Fang, F., Yu, L., Binyuan, Z., & Ke'ao, H. (2005). J Wuhan Univ Technol Mater Sci, 20, 20–23.
Park, D., Haam, S., Jang, K., Ahn, I., & Kim, W. (2005). Process Biochemistry, 40, 53–61.
Beeskow, T. C., Kroner, K. H., & Anspach, F. B. (1997). Journal of Colloid and Interface Science, 196, 278–291.
Boussif, O., Lezoualc'h, F., Zanta, M. A., Mergny, M. D., Scherman, D., Demeneix, B., & Behr, J.-P. (1995). Proceedings of the National Academy of Sciences of the United States of America, 92, 7297–7301.
Ukeda, H., Ishii, T., Sawamura, M., & Kusunose, H. (1995). Analytica Chimica Acta, 308, 261–268.
Zeng, X., & Ruckenstein, E. (1996). Journal of Membrane Science, 117, 271–278.
Sugiura, T., Imagawa, H., & Kondo, T. (2000). Journal of Chromatography B, 742, 327–334.
Randy, G. L., & Sung, W. K. (1974). Journal of Biomedical Materials Research, 8, 251–259.
Acknowledgment
We thank DBT, New Delhi, India, for giving the financial support to carry out the work (BT/PR2937/PID/06/147/2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adikane, H.V., Iyer, G.J. Chemical Modification of Ethyl Cellulose-Based Highly Porous Membrane for the Purification of Immunoglobulin G. Appl Biochem Biotechnol 169, 1026–1038 (2013). https://doi.org/10.1007/s12010-012-0085-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-0085-y