Abstract
Chemical pretreatment of lignocellulosic biomass has been extensively investigated for sugar generation and subsequent fuel production. Alkaline pretreatment has emerged as one of the popular chemical pretreatment methods, but most attempts thus far have utilized NaOH for the pretreatment process. This study aimed at investigating the potential of potassium hydroxide (KOH) as a viable alternative alkaline reagent for lignocellulosic pretreatment based on its different reactivity patterns compared to NaOH. Performer switchgrass was pretreated at KOH concentrations of 0.5–2 % for varying treatment times of 6–48 h, 6–24 h, and 0.25–1 h at 21, 50, and 121 °C, respectively. The pretreatments resulted in the highest percent sugar retention of 99.26 % at 0.5 %, 21 °C, 12 h while delignification up to 55.4 % was observed with 2 % KOH, 121 °C, 1 h. Six pretreatment conditions were selected for subsequent enzymatic hydrolysis with Cellic CTec2® for sugar generation. The pretreatment condition of 0.5 % KOH, 24 h, 21 °C was determined to be the most effective as it utilized the least amount of KOH while generating 582.4 mg sugar/g raw biomass for a corresponding percent carbohydrate conversion of 91.8 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulose-to-ethanol production technology has been investigated intensively around the world over the last two decades. Lignocellulosic biomass is a complex substrate that typically contains 50–80 % [dry basis (db)] carbohydrates that are polymers of 5C and 6C sugar units. The two polysaccharides in lignocelluloses, cellulose (∼45 % db) and hemicellulose (∼25 % db), are bound together by lignin (∼25 % db), which is a complex three-dimensional polyaromatic matrix. Lignin is partly covalently associated with hemicellulose, thus preventing hydrolytic enzymes and acids from accessing some regions of the holocellulose to release the sugar units [1].
Of the various lignocellulosic feedstocks available, switchgrass (Panicum virgatum L.), a perennial warm-season grass native to North America [2], has received considerable attention for ethanol production because of its excellent growth in various soil and climatic conditions and its low requirements of agricultural inputs [3]. According to the study by Schmer et al. [4], switchgrass is capable of producing 5.4 times more renewable energy in the form of ethanol and other value added products than nonrenewable energy consumed, and greenhouse gas emissions from switchgrass-based ethanol are 94 % less than those from gasoline.
The process of ethanol production from lignocellulosic biomass constitutes three stages: (a) pretreatment of biomass to reduce lignin content and cellulose crystallinity, (b) hydrolysis of pretreated biomass for sugar generation, and (c) fermentation of sugars into ethanol. Pretreatment of biomass has been found to change its macromolecular structure and increase surface area and pore size, making it conducive for hydrolytic enzymes to attach themselves to the carbohydrate matrix for generating sugars, which are subsequently converted to ethanol through bacterial or yeast fermentation [5].
Pretreatment can be divided into three main categories: (a) physical, (b) chemical, and (c) biological. Physical pretreatment processes have proven to be energetically unviable and biological pretreatment methods can be expensive and time consuming [6–9]. Chemical pretreatment techniques on the other hands have been the most widely studied and alkaline pretreatment in particular has seen considerable success. Silverstein et al. [10] investigated chemical pretreatment of cotton stalks and reported that, among four pretreatment methods (NaOH, H2SO4, H2O2, and ozone pretreatments), NaOH pretreatment resulted in the highest level of delignification (65.63 % at 2 % NaOH, 90 min, 121 °C) with cellulose conversion of 60.8 %. Xu et al. [11] investigated sodium hydroxide pretreatment of switchgrass for ethanol production and reported that at the most suited pretreatment condition (50 °C, 12 h, and 1.0 % NaOH), the yield of total reducing sugars was 453.4 mg/g raw biomass, which was 3.78 times that from untreated biomass. The maximum lignin reductions were 85.8 % at 121 °C, 77.8 % at 50 °C, and 62.9 % at 21 °C, all obtained at the combination of longest residence time and highest NaOH concentration. Sodium hydroxide pretreatment of lignocellulosic materials results not only in significant lignin reduction but also excellent retention of the total reducing sugar content per gram of biomass treated [11]. Although NaOH is the most commonly investigated alkali reagent, other alkalis like calcium hydroxide [Ca(OH)2] have been investigated and shown to achieve maximum sugar yields of 433–462 mg/g raw biomass [9, 12]. Potassium hydroxide (KOH) pretreatment of rice straw [13] and poplar wood [7] has also been investigated. In their study comparing NaOH and KOH pretreatment of rice straw, Ong et al. [13] showed that, at equal hydrolytic enzyme loading, KOH treatment resulted in significantly higher sugars than NaOH treatment at similar conditions.
Potassium hydroxide is a relatively less explored pretreatment agent but could potentially be used for lignocellulose pretreatment due to its reported reactivity with carbon nanofibers and carbon nanostructures [14] and its ability to deacetylate biomass [7, 15]. Raymundo-Pinero et al. [14] studied the effect of KOH and NaOH, as carbon activating agents, on the structural pattern of carbon nanotubes and reported that NaOH could degrade the tubular structure of disoriented structures, whereas KOH degraded highly ordered tubular structures. Although a key aspect for attaining good sugar yield during enzymatic hydrolysis of pretreated biomass is low cellulose crystallinity and lignin content, significant levels of deacetylation can increase digestibility even at moderate lignin content and high crystallinity index [13]. However, if the lignin content is sufficiently low, crystallinity index and acetyl content do not have a significant impact on enzyme digestibility [7]. Hence, with this background, an attempt was made to study the effect of KOH during pretreatment on subsequent hydrolysis of switchgrass. A comparison between pretreatment effectiveness based on delignification and carbohydrate availability in samples treated at high and low treatment temperatures was made to better understand the mechanism of KOH in modifying lignocellulose structure. The effect of KOH concentration (0.5–2 %) at various combinations of residence times including 0.25, 1, 6–24, and 6–48 h at temperatures of 21, 50, and 121 °C, respectively, was investigated. Select samples with the greatest delignification or carbohydrate availability after pretreatment were hydrolyzed to estimate reducing sugar yield.
Materials and Methods
Biomass
“Performer” switchgrass obtained from the Central Crops Research station at Clayton, NC, USA was used as feedstock [16]. This switchgrass variety has been found to possess high nutritional value and digestibility, providing a dry matter yield of approximately 13,450 kg/ha. Switchgrass plants harvested up to 6 in. stubble in July 2007 were dried at 70 °C in a forced air oven in cloth bags, ground to pass a 2-mm sieve in a Wiley mill and stored at room temperature in zip-locked plastic bags at the Biological and Agricultural Engineering department at NC State University, Raleigh, NC, USA for use in various studies.
Pretreatment
Switchgrass samples were pretreated at 121, 50, and 21 °C. The 121 °C pretreatments were performed in an autoclave at 15 psi, corresponding with treatment times of 15, 30, and 60 min, while the 50 °C treatments were performed in a water bath for 6, 12, and 24 h. Pretreatments at 21 °C were performed at room temperature (maintained through a thermostat) for 6, 12, 24, and 48 h. All the temperature–time pretreatment combinations were performed at KOH concentrations of 0.5, 1, and 2 % (w/v) in a full factorial experiment design. Longer residence times were applied at lower temperatures to offset the impact of reduced chemical reaction rates and provide a comparison between pretreatment effectiveness at low and high temperatures. The pretreatment conditions selected for the study are summarized in Table 1.
Five grams of switchgrass in 50 ml of KOH solution was mixed in 125-ml serum bottles using glass rods, and the bottles were crimp sealed before pretreatment. Pretreated solids were carefully transferred to 250-ml polypropylene centrifuge bottles for separation of the prehydrolysate. The samples were washed using two strategies: dilute washed and washed, to remove any residual alkali and dissolved by-products that might inhibit enzymes during subsequent hydrolysis. For “dilute-washed” samples, after transferring bulk of the biomass-KOH slurry to the centrifuge bottle, the serum bottle was rinsed with 50 ml DI water to recover residual solids. The wash water was transferred to the centrifuge bottle and the total volume made up to 200 ml. The bottles were centrifuged at 4,000 rpm for 10 min, decanted, and the supernatant filtered through a Buchner funnel and flask assembly by vacuum filtration to recover all solids. The “washed” samples were prepared by transferring the pretreated solid-KOH slurry to the centrifuge bottle, centrifuging at 4,000 rpm for 10 min, and decanting the supernatant into the vacuum filtration assembly. The remaining solids were washed by adding the wash water from the serum bottle (50 ml) and an additional 50 ml DI water and centrifuged again. The supernatant was filtered as described previously. All solids accumulated on the filter papers in the filtration set up were quantified by oven-drying and considered in solid recovery calculations. Approximately 5 g of wet biomass was drawn from each pretreated sample and dried at 105 °C for estimation of solid recovery. A similar amount was placed for vacuum drying at 40 °C to obtain samples for composition analysis.
Hydrolysis
Select pretreated samples equivalent to 1.6 g (db) resuspended in 20 ml volume made up by 0.05 M citrate buffer (pH 5.0), 40 μg/ml tetracycline and Cellic® CTec2 cellulase enzyme complex (Novozymes North America, Franklinton) at a loading of 30 % (g enzyme protein/g biomass) were hydrolyzed in conical tubes for generation of reducing sugars. The enzyme complex is reported to have an activity of 108.3–168.8 floating-point unit/ml [17, 18] and protein content 117–185.2 mg protein/ml [17, 19]. To generate enough biomass for hydrolysis at the various conditions, pretreatments were performed in six replicates and two replicates each were combined randomly and mixed thoroughly to generate three larger replicates. This was done to avoid the impact of any scale changes during pretreatment of larger amounts. Untreated samples with equivalent enzyme loading were also hydrolyzed as control. Pretreated and untreated samples with no enzyme were prepared to determine the effect of soaking. Hydrolysis was performed for 72 h at 50 °C in a shaking water bath at 150 rpm. Upon termination of hydrolysis, the samples tubes were centrifuged at 4,000 rpm for 10 min, and the filtrate was collected for sugar analysis. The retentate was dried in the oven at 105 °C for estimation of residual solids after hydrolysis.
Analytical Methods
The chemical composition of switchgrass samples before and after pretreatment was analyzed using National Renewable Energy Laboratory’s Laboratory Analytical Procedures [20–22] for the measurement of ash, total solids, acid soluble lignin (ASL), and acid insoluble lignin (AIL). Briefly, AIL was measured by a two-step sulfuric acid hydrolysis, and filtrate from the AIL acid hydrolysis was utilized for the estimation of ASL and total sugars in untreated biomass and solids recovered after pretreatment. ASL was estimated through absorbance measurements at 205 nm in an ultraviolet–visible spectrophotometer (Shimadzu Pharmaspec UV-1700). Total reducing sugars in the AIL filtrate and enzyme hydrolysate were estimated by the 3,5-dinitrosalycylic acid (DNS) method [23, 24].
Statistical Analysis
All treatments in this study were conducted in triplicate. SAS 9.2 Software (Cary, NC, USA) was used for data analysis at 95 % confidence level. The experimental design was balanced and completely randomized, but with a rather complex factorial structure. There were a total of 31 different experimental conditions. These conditions were comprised of 30 combinations of three factors plus an untreated control. These 30 combinations can be broken down into three “design sectors.” The “short treatment times” sector comprised of nine design points: treatment times of 0.25, 0.5, and 1 h crossed with the three concentrations at the higher temperature of 121 °C in a complete 3 × 3 layout. In the “intermediate times” sector, 18 design points came from a complete, crossed three-factor layout (3 × 2 × 3), with the three factors concentrations (0.5, 1, and 2 %), temperatures (22 and 50 °C), and treatment times (6, 12, and 24 h). In a “long treatment time” sector, a treatment time of 48 h with temperature fixed at 21 °C was observed across the three concentrations. Lastly, an untreated control was used, for a total of 31 conditions. In the subsequent analysis of variance, an orthogonal decomposition of the treatment sum of squares on 30 ° of freedom was obtained to investigate variability due to time, concentration, and temperature, separately within these sectors, while pooling information about variability across the entire experiment. There were n = 3 replicates per treatment combination for a total of 31 × (n − 1) = 62 ° of freedom. The decomposition of the treatment sum of squares for sugars into orthogonal components is given in Table 6.
Results and Discussion
Composition of Switchgrass
The initial composition of “Performer” switchgrass used in this study is presented in Table 2. The carbohydrate portion (represented by total reducing sugars) of switchgrass was estimated to be 67.3 %. Total lignin (including AIL and ASL), which is the major noncarbohydrate component, was determined to be 24.77 %, which was comparable to typical lignin contents of herbaceous species and agricultural residues [25]. Undefined components are believed to be mainly nonstructural compounds including protein, waxes, fats, resins, and chlorophyll [26, 27].
Effect of Pretreatment Conditions
Pretreatment conditions had varying effects on solid recovery, lignin reduction, and sugar availability in the biomass. Intensity of treatment increased with increasing KOH concentration and treatment temperature.
Solid Recovery
On average, solid recoveries after pretreatment ranged between 48.8–76.1 % at 121 °C, 65.9–79.2 % at 50 °C, and 66.6–84.6 % at 21 °C (Table 3). It was observed that lesser solids were recovered as intensity of the pretreatment increased. Statistically, the main effect of both treatment time and KOH concentration had significant (p < 0.05) impact on solid recovery. The interaction effect between temperature and concentration also had a significant (p < 0.05) impact on loss of solids. NaOH pretreatment of switchgrass followed a similar trend of increased solid loss with increasing intensity treatments in terms of temperature and high concentration [11]. However, fewer solids were recovered in that study compared to KOH pretreatment, with the highest solid recovery being 80 %.
Lignin reduction
Lignin is a three-dimensional complex aromatic that acts as a strong barrier for the release of sugars from lignocellulosic biomass. This makes it imperative to degrade lignin without major disruption of the reducing sugars needed for bioconversion into fuels and chemicals [28]. Statistical analysis indicated that at 121 and 50 °C, residence time had a significant impact (p < 0.05) on lignin reduction at all three KOH concentrations (Fig. 1). The corresponding maximum lignin reductions were 55.6 and 41.7 % obtained at 1 h, 2.0 % KOH, 121 °C, and 24 h, 2.0 % KOH, 50 °C, respectively. At 21 °C, residence time had significant impact (p < 0.05) on lignin reduction at higher concentrations, and the highest lignin reduction of 28.5 % was obtained at 48 h, 2.0 % KOH. Maximum lignin reductions at different temperatures were all obtained at the combinations of highest KOH concentration and longest treatment times, which indicated a close relationship between pretreatment severity and lignin reduction. It was observed that delignification (85 %) in the NaOH pretreated switchgrass [11] is more pronounced than the KOH-pretreated samples. Since increasing pretreatment intensity does not necessarily lead to higher sugar recovery due to greater biomass solubilization, lignin reduction, though important, alone may not be an appropriate indicator for overall pretreatment effectiveness.
Reducing Sugar Content
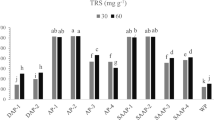
Carbohydrate (cellulose and hemicellulose), which is the key component in pretreated biomass for generation of fermentable sugars during hydrolysis, was estimated in this study through total reducing sugar measurement. The carbohydrate availability in pretreated biomass decreased with increase in the intensity of pretreatment conditions (concentration, temperature, and residence times; Fig. 2). The maximum carbohydrate retention in the 21 °C set was observed to be 66.2 % at 0.5 % KOH after 12 h of treatment. The highest carbohydrate availability after treatment at 50 and 121 °C was 64.1 and 60.6 %, respectively, observed with 0.5 % KOH, 24 h and 0.5 % KOH, 1 h pretreatments. The main effect of concentration and the interaction effect of temperature and concentration was significant (p < 0.05) on carbohydrate availability, whereas time did not have a significant main effect (p > 0.05) or a significant (p > 0.05) interaction effect with concentration on availability. It was observed that 2.2–35.5 % of the original untreated reducing sugar content was lost during various combinations of pretreatments depending on severity.
Selection of Optimal Pretreatment Conditions
Pairwise comparisons at a confidence interval of 95 % were made among all treatments at a specific temperature by the LS means procedure (SAS 9.2). Tukey method was used to provide a conservative estimate of significant differences among mean pairs. The comparisons were made between a manually chosen extreme response value and all other treatment means in the temperature set, to pick optimal values for subsequent enzymatic hydrolysis. Selections were based on maximum delignification and carbohydrate (reducing sugar) recovery. The combinations chosen relative to highest sugar values were 0.5 % KOH, 12 h, 21 °C, 0.5 % KOH, 24 h, 50 °C, and 1 % KOH, 1 h, 121 °C, and the combinations chosen for highest delignification were 2 % KOH, 48 h, 21 °C, 2 % KOH, 24 h, 50 °C, and 2 % KOH, 1 h, 121 °C.
Hydrolysis
Tables 4 and 5 represent the reducing sugar yields per gram of untreated biomass and percent conversion efficiency for samples obtained from the two washing strategies at the selected pretreatment conditions. A significant difference (p < 0.05) was observed between the percent conversions and sugar yields (mg/g) for samples from the two washing strategies hydrolyzed at 30 % enzyme loading. This may be attributed to decrease in enzymatic activity due to presence of inhibitory compounds generated by KOH (derivatives), resulting from lack of sufficient washing in the dilute washed samples. The pH of pretreated biomass–citrate buffer mixture prior to initiation of hydrolysis was however not significantly different (p > 0.05) from 5.0 (pH of citrate buffer) for both the dilute–washed and washed samples. A pH of 5.0 has been reported to be optimal for Cellic® Ctec2 by the manufacturer. It was observed that on average 35 % of the original biomass was left after hydrolysis across all pretreated samples.
Based on analysis of reducing sugars generated after hydrolysis of the select pretreated samples (Table 6), the optimal pretreatment providing significantly higher (p < 0.05) percent conversion of 102 ± 1.1 % was observed to be 2 % KOH, 48 h, 21 °C, washed set with 30 % (g enzyme protein/g dry biomass) enzyme loading. Conversion values of over 100 % have been reported in previous studies [29] and may be partially attributed to inexact measurements during DNS analysis besides suggesting that there may have been over estimation since no correction was made for sugars released due to soaking (0 % enzyme loading). The highest yield of 582.4 mg reducing sugar/g untreated biomass was observed with 0.5 % KOH, 12 h, 21 °C, washed. It was observed that sugar yields with the 30 % enzyme loading washed samples were higher compared to those from lime (433 mg/g) and NaOH (453 mg/g) pretreatment of switchgrass [11]. A yield of 462 mg sugar/g raw biomass from lime pretreated was also found to be lower than the value observed in this study [12]. These differences could be attributed to the reactivity patterns of KOH and NaOH on the biomass structure. Some variations may have also arisen from differences in the enzymes and enzyme loadings used.
There was no significant difference (p > 0.05) in the percent conversion values of the dilute washed samples hydrolyzed with and without enzyme. It was noted that the high enzyme loading (30 % g enzyme protein/g biomass) generated a high amount of sugars from the untreated switchgrass samples compared to previous studies [9, 11], which have utilized lesser loadings. This seems to suggest higher efficacy of the enzyme and its ability to generate considerable sugars from the untreated biomass. However, this aspect needs further exploration.
Conclusions
Pretreatment of ground switchgrass with KOH resulted in promising reducing sugar conversions of over 85 % after hydrolysis. There is a clear indication of the requirement for a post-pretreatment washing step for generation of maximum sugars from the pretreated biomass. A high sugar yield with the 0.5 % KOH, 12 h, 21 °C pretreatment indicates that even very low concentrations of KOH can be effective in generating high sugars during hydrolysis. Based on the sugar yield of 582.4 mg/g biomass at the above condition, using a theoretical ethanol yield of 0.51 lb ethanol/lb sugars and a factor of 1 gal ethanol/6.55 lb ethanol [30], ethanol yield can be estimated as 90.5 gal/t of raw switchgrass. Overall, the high theoretical ethanol yield from mild KOH pretreated samples suggests that this alkaline pretreatment reagent has considerable potential but needs to be extensively investigated for comprehensive cost analysis.
References
Carlo, N. H., Geertje, V. H., & Andre, P. C. (2005). Ethanol from lignocellulosic biomass: Techno-economic performance in short-middle and long term. Biomass and Bioenergy, 28, 384–410.
Cortese, L. M., Honig, J., Miller, C., & Bonos, S. A. (2010). Genetic diversity of twelve switchgrass populations using molecular and morphological markers. Bioenergy Research, 3, 262–271. doi:10.1007/s12155-010-9078-2.
Keshwani, D. R., & Cheng, J. J. (2009). Switchgrass for bioethanol and other value-added applications: A review. Bioresource Technology, 100, 1515–1523.
Schmer, M. R., Vogel, K. P., Mitchel, R. B., & Perrin, R. K. (2008). Net energy of cellulosic ethanol from switchgrass. Proceedings of the National Academy of Science, 105, 464–469.
Awolu, O. O., & Ibileke, I. O. (2011). Bioethanol production from brewer’s spent grain, bread wastes and corn fiber. African Journal of Food Science, 5(3), 148–155.
Belkacemi, K., Turcotte, G., de Halleux, D., & Savoie, P. (1998). Ethanol production from AFEX-treatedforages and agricultural residues. Applied Biochemistry and Biotechnology, 70–72, 441–462.
Chang, V., & Holtzapple, M. T. (2000). Fundamental factors affecting biomass enzymatic reactivity. Applied Biochemistry and Biotechnology, 84–86, 5–37.
Chen, Y., Sharma-Shivappa, R. R., Keshwani, D., & Chen, C. (2007). Potential of agricultural residues and hey for bioethanol production. Applied Biochemistry and Biotechnology, 142, 276–290.
Xu, J., Cheng, J. J., Sharma-Shivappa, R. R., & Burns, J. C. (2010). Lime pretreatment of switchgrass at mild temperatures for ethanol production. Bioresource Technology, 101, 2900–2903.
Silverstein, R. A., Chen, Y., Sharma-Shivappa, R. R., Boyette, M. D., & Osborne, J. (2007). A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresource Technology, 98, 3000–3011.
Xu, J., Cheng, J. J., Sharma-Shivappa, R. R., & Burns, J. C. (2010). Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy & Fuels, 24, 2113–2119.
Kaar, W. E., & Holtzapple, M. T. (2000). Using lime pretreatment to facilitate the enzymatic hydrolysis of corn stover. Biomass and Bioenergy, 18, 189–199.
Ong, L. G. A., Chuah, C., & Chew, A. L. (2010). Comparison of sodium hydroxide and potassium hydroxide followed by heat treatment on rice straw for cellulase production under solid state fermentation. Journal of Applied Sciences, 10, 2608–2612.
Raymundo-Piñero, E., Azaïs, P., Cacciaguerra, T., Cazorla-Amorós, D., Linares-Solano, A., & Béguin, F. (2005). KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organization. Carbon, 43, 786–795.
Kumar, R., & Wyman, C. E. (2009). Cellulase adsorption and relationship to features of corn stover solids produced by leading pretreatments. Biotechnology and Bioengineering, 103, 252–267.
Burns, J. C., Godshalk, E. B., & Timothy, D. H. (2008). Registration of “Performer” switchgrass. Journal of Plant Registrations, 2008(2), 29–30.
Eckard, A. D., Muthukumarappan, K., & Gibbons, W. (2012). Pretreatment of extruded corn stover with polyethylene glycol to enhance enzymatic hydrolysis: optimization, kinetics, and mechanism of action. Bioenergy Research, 5, 424–438. doi:10.1007/s12155-011-9162-2.
Kodaganti, B.P. (2011). Simultaneous saccharification and fermentation of Arundo donax—Comparison of feeding strategies. www.chemeng.lth.se/E655.pdf. Accessed 24 May 2012.
Eylen, D. V., Femke, V. D., Kabel, M., & Bont, J. (2011). Corn fiber, cobs and stover: Enzyme-aided saccharification and co-fermentation after dilute acid pretreatment. Bioresource Technology, 102, 5995–6004.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2005a). Determination of ash in biomass. Laboratory Analytical Procedure (LAP). Golden: National Renewable Energy Laboratory.
Sluiter, A., Hames, B., Hyman, D., Payne, C., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., and Wolfe, J. (2005b). Determination of total solids in biomass and total dissolved solids in liquid process samples. Laboratory Analytical Procedure (LAP). Golden: National Renewable Energy Laboratory.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2008). Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP). Golden: National Renewable Energy Laboratory.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analitical Chemistry, 31, 426–428.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
McMillan, J. D. (1994). Pretreatment of lignocellulosic biomass. In M. E. Himmel, J. O. Baker, & R. P. Overend (Eds.), Enzymatic conversion of biomass for fuel production (pp. 292–324). Washington: American Chemical Society.
Kuhad, R. C., & Singh, A. (1993). Lignocellulose biotechnology: Current and future prospectus. Critical Reviews in Biotechnology, 13, 151–172.
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., & Templeton, D. (2005). Determination of extractives in biomass. Laboratory Analytical Procedure (LAP). Golden: National Renewable Energy Laboratory.
Fan, L. T., Gharpuray, M. M., & Lee, Y. H. (1987). Cellulose hydrolysis. In: S. Aiba, L. T. Fan, A. Fiechter, J. Klein, & K. de Schügerl (Eds.), Biotechnology monographs (p. 8). Berlin: Springer.
Ohgren, K., Bura, R., Saddler, J., & Zacchi, G. (2007). Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresource Technology, 98, 2503–2510.
Theoretical ethanol yield calculator. http://www1.eere.energy.gov/biomass/ethanol_yield_calculator.html.
Acknowledgments
The authors appreciate the input by Dr. Sanjeev Tyagi, Principal Scientist, Central Institute of Post Harvest Engineering and Technology (CIPHET), Ludhiana, Punjab, India, during the initiation of this study. They would like to acknowledge the partial financial support for this research provided by National Agricultural Innovation Project, Indian Council of Agricultural Research (ICAR).
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Sharma and V. Palled made equal contributions to this study.
Rights and permissions
About this article
Cite this article
Sharma, R., Palled, V., Sharma-Shivappa, R.R. et al. Potential of Potassium Hydroxide Pretreatment of Switchgrass for Fermentable Sugar Production. Appl Biochem Biotechnol 169, 761–772 (2013). https://doi.org/10.1007/s12010-012-0009-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-0009-x