Abstract
This study reports on the identification, characterization and purification of a new bacteriocin, named Bacthuricin F103, from a Bacillus thuringiensis strain BUPM103. Bacthuricin F103 production began in the early exponential phase and reached a maximum in the middle of the same phase. Two chromatographic methods based on high performance liquid chromatography and fast protein liquid chromatography systems were used to purify Bacthuricin F103. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis revealed that this bacteriocin had a molecular weight of approximately 11 kDa. It also showed a wide range of thermostability of up to 80 °C for 60 min and a broad spectrum of antimicrobial activity over a pH range of 3.0–10.0. This bacteriocin was noted, and for the first time, to exhibit potent antimicrobial activity against Agrobacterium subsp. strains, the major causal agents of crown gall disease in tomato and vineyard crops, and against several challenging organisms in food, such as Listeria monocytogenes and Bacillus cereus. Complete killing with immediate impact on cells was observed within a short period of time. The sequence obtained for Bacthuricin F103 by direct N-terminal sequencing shared considerable homology with hemolysin. Bacthuricin F103 was noted to act through the depletion of intracellular ions, which suggest that the cell membrane was a possible target to Bacthuricin F103.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteriocins are ribosomally synthesised bacterial peptides and proteins that inhibit strains that are, in most cases, closely related to the producing strain [1]. Bacteriocins are the most abundant of the microbial defence systems. They are produced by all major groups of bacteria, often at very high frequencies within a population, and exhibit extraordinary protein diversity levels [2]. They have often been reported to exhibit a number of attractive features that have caught the attention of several researchers seeking to develop new antibiotics, namely those pertaining to their natural origins, wide range of activities, ease of production and proteinaceous nature, which implies a putative degradation in the gastro-intestinal tract of man and animals [3, 4].
To date, nisin, which is produced by different Lactococcus lactis subsp., represents the most extensively studied bacteriocin [5]. In fact, during the last few decades, a large number of bacteriocins produced by lactic acid bacteria have been identified and classified into four major classes based on observed common characteristics [6]. By comparison, however, there is relatively little data on bacteriocins from other bacterial genera, namely the genus Bacillus, although Bacillus subsp. represents an important group of bacteriocinogenic micro-organisms in alkaline-fermented foods and beverages [7]. Bacillus thuringiensis is a member of the genus Bacillus that has been widely used as a biopesticide for the control of many insect pathogens in agriculture. Bacteriocins from B. thuringiensis have recently attracted special attention particularly due to their possible application as alternative natural food biopreservatives to the available chemical ones, and/or for pest control and grain preservation in agriculture. In fact, although the data on their toxicity are very scarce, research and their long-time intentional use strongly suggest that they can be safely used [8]. Accordingly, the production of bacteriocins has been reported from a number of B. thuringiensis strains [9–15].
Several studies have reported on the mode of action of bacteriocins. In fact, most bacteriocins seem to share a common mechanism of action whereby they act on the cells of target strains and form pores in the cell membrane. In fact, the addition of bacteriocin is reported to induce the loss of intracellular materials in target strains [16–19]. However, the target(s) of bacteriocins in sensitive bacteria from B. thuringiensis have not yet been clearly elucidated.
Considering the promising advances that bacteriocins may bring to the global research and industrial communities, the present study aimed to report on the identification, characterization and purification of a novel bacteriocin, named Bacthuricin F103, from a B. thuringiensis strain BUPM103, with a unique N-terminal sequence and, interestingly, a potent bactericidal activity against Listeria monocytogenes, Bacillus cereus and Agrobacterium tumefaciens.
Materials and Methods
Bacterial Strains and Media
The B. thuringiensis strain BUPM103 used in the present study was originally isolated from Tunisian soil samples [20]. The isolate was selected from among hundreds of other isolates available at a local laboratory B. thuringiensis strain collection due to its ability to produce bacteriocin. B. cereus ATCC 14579 was used as an indicator bacterium for the determination of antimicrobial activity. Other indicator strains, listed in Table 1, were used for their sensitivity to bacteriocin. The indicator strains were cultivated in Luria Bertani (LB) media [21] or in their appropriate media and temperatures. All Bacillus strains were grown at 30 °C in glucose-based medium [22] modified and optimized for bacteriocin production by Kamoun et al. [23].
Bacteriocin Activity Determination and Quantification
The B. thuringiensis strain BUPM103 was cultivated in a modified glucose-based medium [23] at 30 °C for 30 h. Bacteriocin activity was checked through the well diffusion method [24] using 107 cells of the indicator strain and 50 μl of the test samples. Arbitrary units per millilitre (AU/ml) of culture were determined according to the method described by Kamoun et al. [15]. The assays were performed using B. cereus ATCC 14579 as the indicator bacterium.
Production and Ammonium Sulphate Precipitation of Bacteriocin
The B. thuringiensis strain BUPM103 was grown for 8 h in a 2,000-ml flask containing 400 ml of a modified glucose-based medium (pH 7) at 30 °C under stirring at 120 rpm. Cells were removed by centrifugation (3,000×g for 10 min at 4 °C). Ammonium sulphate was added to the supernatant while stirring to reach a saturation of 60%. The precipitate was collected by centrifugation at 10,000×g for 30 min at 4 °C, dissolved in 10 mM phosphate buffer (pH 7) and extensively dialysed against the same buffer for 15 h in dialysis tubing (MW cut-off, 3,500). The obtained solution that contained the antimicrobial activity, designated as the ammonium sulphate precipitate (ASP), was sterilized by filtration through a 0.45-μm pore-size filter (Millipore). The sterilized sample was stored at −20 °C until further use.
Purification and Estimation of Molecular Mass
Two consecutive chromatography steps were applied to a sample from the ASP fraction. In fact, the use of gel filtration and anion-exchange chromatography is well suited for the purification of BUPM103 bacteriocin. Accordingly, after precipitation by ammonium sulphate, the sample was applied to a gel filtration column (Shodex, PROTEIN KW-802.5; 8 mm I.D. × 300 mml), equilibrated and washed using 50 mM of sodium phosphate buffer (pH 7.0) at a flow rate of 1 ml/min. The fractions collected were assayed for antimicrobial activity. The final purification step involved the application of the fractions harbouring antimicrobial activity to a HiTrap Q Sepharose HP column (Amersham Pharmacia Biotech AB SE-751 84, Uppsala, Sweden; diameter, 1 cm, length 5 cm) equilibrated with 20 mM Tris/HCl buffer pH 8.2. Proteins were eluted in the same buffer with a linear NaCl gradient (0–75 mM) at a flow rate of 3 ml/min. Peptides were monitored spectrophotometrically at 280 nm. Fractions harbouring bacteriocin activity were collected, dialysed, lyophilized and then used for SDS-PAGE analysis. The latter was performed using the Tris–glycine buffer, following Laemmli [25], with 20% acrylamide (acrylamide/bisacrylamide ratio 30/0.8) gel. Bacteriocin activity was determined in the gel as described by Kamoun et al. [15].
Biochemical and Physical Treatments of Antimicrobial Activity
In order to evaluate the sensitivity of the bacteriocin under investigation to proteolytic enzymes (proteinase K, trypsin, catalase, alcalase and neutrase; Table 2), the samples were assayed for 30 min at final concentrations of 1 mg/ml and appropriate temperatures. After incubation, the samples were assayed for antimicrobial activity. Untreated samples were taken as controls in all cases. Protein concentration was determined according to Bradford [26] using bovine serum albumin as a standard.
The activity of the bacteriocin was also assessed in terms of sensitivity to various temperatures (30 °C, 40 °C, 50 °C, 60 °C, 70 °C, 80 °C, 90 °C, 100 °C and 121 °C). Accordingly, 100 AU of BUPM103 bacteriocin was incubated at different temperatures for 60 min (Table 2), except for the temperature of 121 °C where it was incubated for 20 min. Moreover, and in order to determine its stability at different storage conditions, the purified bacteriocin sample was stored for 2 days at room temperature (25 °C) and for 1 year at low storage temperatures (−20 °C and −80 °C); its antimicrobial activity was compared to the fresh purified preparation.
Further separate experiments were performed to evaluate the sensitivity of the bacteriocin to pH ranges. Accordingly, samples of bacteriocin (100 AU) were adjusted to pH values ranging from 3.0 to 10.0 in 50 mM of different buffer solutions, namely citrate buffer (pH 3.0–6.0), phosphate buffer (pH 7.0) and Tris/HCl buffer (pH 8.0–10.0; Table 2). The residual activity was determined after 4 h of storage at 4 °C.
Resistance to lyophilization was also evaluated. Samples were dried overnight in a Bioblock lyophilisator (Christ, Alpha 1-2). The lyophilized samples were stored at 4 °C until further use. The powder was dissolved in 10 mM phosphate buffer (pH 7.0) before use.
Effect of Bacthuricin F103 on B. cereus and Antimicrobial Spectrum
The mode of action of Bacthuricin F103 was examined by harvesting 3 ml of an exponentially growing culture of the indicator strain B. cereus ATCC 14579. This was then washed twice and suspended in a sterile 10 mM phosphate buffer (pH 7). Bacthuricin F103 was subsequently added at concentrations of 50 (2.27 μg), 100 and 150 AU. The suspended cells (105 cells) were maintained at 37 °C during the assay, and aliquots were retrieved every 20 min for the enumeration of viable counts on LB agar. Cells of B. cereus ATCC 14579 in buffer alone were used as controls.
The antibacterial activity of BUPM103 was tested against several Gram-positive and Gram-negative bacteria by agar spot assay [9]. All strains used as indicators were subcultured in their appropriate media and temperatures (Table 1).
Measurement of Extracellular Ions
Ion leakage was used as an indicator of the loss of cell membrane integrity. Extracellular ion contents were determined as described by Gong et al. [27]. B. cereus ATCC 14579 cell suspension (105 cells) in 50 ml of phosphate buffer (10 mM, pH 7.0) was mixed with Bacthuricin F103 (150 AU/ml) and incubated at 37 °C. Samples were removed after 1 h and filtered through 0.22-mm pore size sterile membranes (Millipore). The ion efflux of the filtrates was determined by high performance liquid chromatography (HPLC) column (Metrosep C 2 100/4.0, Metrohm) at a flow rate of 1 ml/min using nitric acid (2 mM) for the elution.
Effect of Bacthuricin F103 on L. monocytogenes ATCC 7644 Growth in Raw Beef Meat
The in situ efficacy of the Bacthuricin F103 was evaluated against L. monocytogenes in a sample of minced beef meat using a slightly modified version of the procedure described by Dortu et al. [28]. Fresh beef muscle cuts were bought from a local supermarket (Sfax, Tunisia). The pieces of meat were sliced aseptically into portions of approximately 150 ± 0.1 g to make four different batches (B1, B2, B3 and B4). The batches were placed individually in bags. Batches B1 and B2 were surface contaminated with 2 × 102 CFU of L. monocytogenes per gramme of meat. Before the contamination of batch B2, Bacthuricin F103 was filtered through 0.45-μm pore size filters (Millipore) and then added at final concentrations of 150 (6.81 μg), 300 and 500 AU per piece of meat. Batch B1 served as a positive control and was, hence, contaminated with L. monocytogenes without addition of bacteriocin. Batch B3 was not contaminated and served as a negative control. Batch B4 was mixed with bacteriocin and was not contaminated with L. monocytogenes. All bags containing the samples of meat were stored at 5 °C during the course of the experiments. Samples of 10 g were aseptically taken from each batch every 2 days for L. monocytogenes enumeration (for batches B1, B2 and B3) and residual bacteriocin activity (for batches B2 and B4). The colony-forming units (CFU) of L. monocytogenes was determined on PALCAM agar (Fluka, Schnelldorf, Germany) after incubation at 37 °C for 48 h. Suspensions obtained from the samples treated with Bacthuricin F103 (B2 and B4) were centrifuged, and the supernatant was filter-sterilized to determine the residual activity of bacteriocin. Each assay was performed in triplicates.
Amino Acid Sequence Analysis
The amino acid analysis of the bacteriocin of BUPM103 under investigation was performed using a 420 A-130 A Derivatizer-Analyzer System (Applied Biosystems) after the hydrolysis of the pure bacteriocin in HCl 6 M at 110 °C for 24 h. The N-terminal of the purified bacteriocin was sequenced by automated Edman degradation using an Applied Biosystems 492 Procise protein sequencer equipped with a PTH 140C analyzer.
Statistical Analysis
All the results related to the determination of bacteriocin activities, O.D.(600nm) (optical density) and CFU were the average of triplicate experiments. CFU were statistically analysed by SPSS software (version 100), using Duncan test performed after analysis of variance (ANOVA). Bacteriocin activities were analysed by Microsoft Excel 2003 using the Student’s t test. Results were considered significant at the α ≤ 0.05 level.
Results and Discussion
Screening of Strains Producing Bacteriocins
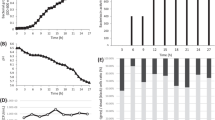
A collection of B. thuringiensis isolates was screened for their capacity to produce bacteriocin using the agar spot test [9]. The inhibitory activity in the strains culture supernatants was detected using the well diffusion method [24]. Strain BUPM103 was selected from among the other isolates because of the particular shape of its well-delimited bacteriocin inhibition halo towards the indicator-sensitive strain B. cereus ATCC 14579. Furthermore, BUPM103 bacteriocin production was noted to be dependent on the growth phase (Fig. 1) and, contrary to other bacteriocins, to reach its maximum at the mid-log phase culture. The results it exhibited differed from those noted for thuricin 7 whose activity, which could not be detected during the exponential growth phase, emerged at the end of the phase and reached a maximum during the mid-stationary phase [8]. Several previously reported bacteriocins were, however, noted to achieve maximal activity during the late exponential phase and during the stationary growth phase, such as tochicin whose activity that was detected at the mid-log growth phase reached a maximum at the early stationary phase and decreased after the late stationary phase [9]. The highest bacteriocin activity achieved for BUPM103 (160 AU/ml) occurred after 8 h of culture. The synthesis was then observed to undergo a gradual decrease during the late exponential phase and the stationary one, suggesting that the antibacterial molecule could be a secondary metabolite. This decrease in bacteriocin production was, in fact, previously described for thuricin [29] and bacthuricin F4 [15]. It could be attributed to the production of proteases by B. thuringiensis [30] or the occurrence of sporulation in several bacteria.
BUPM 103 bacteriocin production in batch culture. Kinetics of BUPM103 growth (triangles) and BUPM103 bacteriocin activity (squares) in culture supernatant samples. BUPM103 growth was monitored by the increase in O.D.(600 nm), and the bacteriocin activity was measured by the well diffusion method as arbitrary units per millilitre in culture supernatant samples. Error bars, which are sometimes obscured by the data symbols, represent ±SD (standard deviation)
Purification and Estimation of the Molecular Mass of Bacthuricin F103
Fractionated precipitation revealed that the highest activity obtained for Bacthuricin F103 occurred when an 8-h culture supernatant was precipitated with 60% ammonium sulphate. Bacthuricin F103 was concentrated approximately 40-fold from the culture medium by ammonium sulphate. This resulted in a 27-fold increase in its specific activity, which was 128 AU/mg in the growth medium and 3,444 AU/mg in ASP bacteriocin, with a recovery of 96.87% of its activity (Table 3).
A combination of two chromatographic methods, namely gel filtration and anion exchange chromatography, was employed to achieve a highly purified Bacthuricin F103. Bacteriocin activity was determined at each step in the purification process. The active bacteriocin fraction from gel filtration showed a specific activity of about 10,000 AU/mg, and the recovery rate at this stage was 62.5% (Table 3). The gel filtration fractions were pooled and further purified to homogeneity by fast protein liquid chromatography (FPLC; Fig. 2a). Upon FPLC, the activity eluted as a peak, which was recovered in three fractions as an NaCl concentration of approximately 16 mM (Fig. 2a). This final purification step resulted in a pure bacteriocin with a final specific activity that was approximately 172-fold greater than that in the culture supernatant and a recovery rate of up to 34.37% (Table 3). Table 3 summarizes the purification process of Bacthuricin F103.
BUPM103 bacteriocin purification. a Elution profile of bacteriocin using FPLC, indicating NaCl linear gradient and optical density at 280 nm of the eluted fractions. Antibacterial activity was detected in peak 1 indicated by the double-headed arrow. b Coomassie blue stained SDS-PAGE. c Direct detection of bacteriocin activity. The inhibition zone was observed after an overnight incubation at 37 °C. M: Rainbow protein molecular-weight marker (ovalbumin, 45,000 Da; carbonic anhydrase, 30,000 Da; trypsin inhibitor, 20,100 Da; lysozyme, 14,300 Da; aprotinin, 6,500 Da; insulin b chain, 3,500 Da; insulin a chain, 2,500 Da); lane 1: purified protein of peak 1, lane 2: FPLC purified bacteriocin showing an inhibition zone. The arrow indicates the bacteriocin corresponding band
The apparent molecular mass of the purified Bacthuricin F103 was estimated by SDS-PAGE. The analysis of the protein present in FPLC peak 1 and limited by the double-headed arrow (Fig. 2a) revealed only one protein band of about 11 kDa (Fig. 2b, lane 1) that could be stained with Coomassie blue, indicating the high purity of the purified monomeric bacteriocin. While the apparent molecular mass of Bacthuricin F103 was relatively different from thuricin 7 [8] (11.6 kDa) and tochicin [9] (10.5 kDa), it was significantly different from all the other previously reported B. thuringiensis bacteriocins, such as thuricin 439 [10] (3 kDa), thuricin S [11] (3.1 kDa), entomocin 110 [12] (4.8 kDa), thuricin H [13] (3.1 kDa), thuricin CD [14] (2.763/2.861 kDa), bacthuricin F4 [15] (3.1 kDa) and thuricin 17 [31] (3.1 kDa). Bacteriocin activity was determined in the gel; the portion of the SDS-PAGE showed an inhibition halo presented in Fig. 2c (lane 2).
Biochemical Properties of Bacthuricin F103
The sensitivity of Bacthuricin F103 with regards to proteolytic enzymes was investigated in an attempt to determine the biological nature of this antimicrobial compound produced by strain BUPM103. The findings revealed that the treatment of Bacthuricin F103 with a variety of proteases brought about either the elimination or reduction of its activity (Table 2). Unlike thuricin 7 [8] and tochicin [9] whose antibacterial activities were lost only with proteinase K, the antibacterial activity of Bacthuricin F103 was completely lost with both proteinase K and alcalase, which strongly suggest that it is proteinaceous in nature. With respect to trypsin and neutrase, however, Bacthuricin F103 showed different levels of sensitivity since its antibacterial activity was observed to undergo a decrease of 20% and 35%, respectively (Table 2). Catalase, on the other hand, had no observable effect on its antimicrobial activity, which indicates that the inhibitory activity was not due to hydrogen peroxide production.
Further assays aimed to investigate the thermostability profile of Bacthuricin F103. The findings indicated that Bacthuricin F103 could preserve 75% of its activity when treated at 70 °C (Table 2). It was also noted to remain active at 100 °C and above, preserving 20% of its activity at 100 °C and 10% after treatment for 20 min at 121 °C (Table 2). Thuricin 7 [8] and tochicin [9], whose molecular mass was very close to Bacthuricin F103, were, in fact, less stable, being inactivated when treated at 121 °C for 20 min. Rea et al. [14] reported on a similar reduction in thuricin CD activity at 90 °C and a complete loss of activity at 100 °C after 15 min. Likewise, a complete loss of thuricin 439 activity was observed following treatment at 121 °C for 15 min [10].
As far as the effects of storage conditions on recovery were concerned, bacthuricin F103 showed a recovery rate 100% under all storage conditions tested (−80 °C and 25 °C). The findings suggest that this compound do not degrade during room (25 °C) or low (−20 °C and −80 °C) storage temperatures and that the compound can be used experimentally, for at least several days, without concern about decline in activity. These results also suggest that Bacthuricin F103 may be considered a potential strong candidate for future use in commercial applications that might reduce or remove the current storage problems.
The pH stability of Bacthuricin F103 was also investigated. Like several other B. thuringiensis bacteriocins, Bacthuricin F103 retained most of its activity within the pH range of 3–10, with maximum activity at pH 7 (Table 2). Bacthuricin F103 also showed good stability at alkaline pH values for it retained 70% of its activity at pH 9.0 (Table 2). This feature is, in fact, highly valued particularly in alkaline-fermented food industries. When pH was below 7.0, however, the activity of Bacthuricin F103 was noted to decrease (only 30% of activity was measured at the pH range 3.0–5.0; Table 2).
Bacthuricin F103 was also noted to be resistant to lyophilization and resuspension since these conditions did not alter its antagonistic activity.
Bactericidal Action and Spectrum of Activity of Bacthuricin F103
The food-borne pathogen B. cereus ATCC 14579 was selected as a target in order to determine the inhibitory mode of action of Bacthuricin F103 and the potential gain effects that the latter might bring with regards to the control of this bacterium. The addition of Bacthuricin F103, at a concentration of 50 AU (2.27 μg), to 105 cells from a growing culture of the indicator B. cereus ATCC 14579 strain (the cell concentration used was much higher than those expected to occur in contaminated foods) revealed that the effect exhibited by this bacteriocin was bactericidal and not bacteriostatic. This is in agreement with the results previously reported for the majority of bacteriocins in several studies in the literature. In fact, Bacthuricin F103 was noted to lead to a fast decrease during the first 5 min, with log CFU per millilitre decreasing from 5 to 3.8, and then to a continuous decrease in cell viability, with a decline of approximately 4 logs in 3 h (Fig. 3). Interestingly, Bacthuricin F103 was observed to bring about the total growth inhibition of B. cereus cells after 180 min of incubation, which provides sound evidence for its efficiency for the control of this bacterium. The cells of B. cereus in buffer alone were unaffected (Fig. 3). The investigation of the bactericidal kinetics of Bacthuricin F103 with regards to the indicator strain B. cereus revealed that faster effects could be achieved at higher concentrations (100 or 150 AU; Fig. 3). A similar result was previously reported for B. thuringiensis BUPM4 [15]. In fact, the number of viable cells was reduced following similar kinetics, i.e. the more the activity values were increased, the more rapid the cell lysis became. The rapid effects achieved with Bacthuricin F103 provide further support for its strong candidacy for future industrial application as a food preservative. Further experiments that entail the testing of Bacthuricin F103 in model food systems would help determine the influence of the various parameters involved (e.g. initial cell concentration, incubation temperature, pH of the sample and food composition).
Bactericidal effect of Bacthuricin F103 on the growth of B. cereus ATCC 14579 cells. The results were expressed as survivals of B. cereus cells, after incubation of 105 cells in the presence of 50 (diamonds), 100 (squares) and 150 (triangles) AU of Bacthuricin F103, at 37 °C during different incubation periods. Circles: number of viable cells in the untreated control. Error bars, which are sometimes obscured by the data symbols, represent ±SD (standard deviation)
The inhibitory spectrum of Bacthuricin F103 was also investigated with regards to several spoilage and pathogenic bacteria of interest to agriculture and food industry using the agar spot assay. The antagonism of Bacthuricin F103 against different indicator strains is shown in Table 1. In fact, this bacteriocin showed a specific spectrum of activity particularly against B. thuringiensis, B. cereus, Bacillus subtilis, Bacillus ureus and Bacillus licheniformis, L. monocytogenes and A. tumefaciens. Such action is, in fact, highly valued for depressing B. cereus growth without interfering with other bacteria normally present in food preparations since B. cereus is easily spread in food, causing diarrheal and emetic food-borne illnesses, two of the most serious food safety concerns in the industrialized world [32].
Bacteriocin activity was also investigated against L. monocytogenes ATCC 7644 and the food isolate strain 2132, two pathogenic bacteria whose presence in food is a high risk factor for contamination and whose control is often reported to be highly difficult. From an application point of view, the control of the target cells of L. monocytogenes is very important since this bacteria can be found in a variety of raw and processed food products, such as meat, milk and dairy products, as well as in water, soil, agricultural and industrial waste material.
Bacteriocin activity was also detected against two phytopathogenic isolates of A. tumefaciens C58 [33] and B6 [34] that cause crown gall disease in several plant species including tomato. These findings are of great value for the nurserymen who are interested particularly in the biocontrol of the crown gall disease. To the authors’ knowledge, this is the first study to report on the activity of B. thuringiensis bacteriocin against L. monocytogenes, A. tumefaciens as well as B. cereus.
The inhibitory effects of Bacthuricin F103 were also observed against B. thuringiensis strains BUPM2, BUPM4, BUPM19, BUPM27, HD1 and HD73 (Table 1). This clearly shows that Bacthuricin F103 is highly active principally against species phylogenetically related to the producer strain. However, since Bacthuricin F103 was active against B. thuringiensis strains, BUPM103 was able to synthesize a bacteriocin that was different from the other B. thuringiensis strains available at the laboratory collection.
Mode of Action of Bacthuricin F103
Bacthuricin F103 was assayed for its ability to lead to the efflux of ions from B. cereus ATCC 14579. Accordingly, the supernatants of glucose-energized cells (0.5% w/v) of the control cells and of those treated with Bacthuricin F103 for 1 h were submitted to a Metrosep column. In the case of the cells that were not treated with Bacthuricin F103, the cells of B. cereus ATCC 14579 were noted to maintain extracellular ions as represented in peak 1. The subsequent addition of Bacthuricin F103 (150 AU/ml) to glucose-energized cells caused a dramatic loss of cellular ions as represented in peak 2, corresponding to the cationic ions obtained in the HPLC profile (Fig. 4). The difference between peak 1 and peak 2 indicated that Bacthuricin F103 induced massive leakage of ions from the cells. Thus, the biochemical effect(s) of Bacthuricin F103 were somehow similar to thuricin S [35].
Antilisterial Activity of Bacthuricin F103 in Raw Beef Meat
The concentration of L. monocytogenes in batch B1 (positive control) underwent a slight increase during storage from 2 × 102 to 10 × 102 CFU/g after 10 days (Fig. 5). This increase may be attributed to interaction with meat constituents. In batch B2, the application of different concentrations of Bacthuricin F103 led to the successful growth inhibition of L. monocytogenes. In fact, the addition of Bacthuricin F103 at a concentration of 500 AU led to the L. monocytogenes concentration to decrease by 1.61 log units after 6 days and to fall below the detectable level after 10 days (Fig. 5). At a concentration of 300 AU, Bacthuricin F103 brought about a lower, yet significant, decrease in the number of L. monocytogenes. After 10 days, the count was 1.8 log units lower than that of the control, and the CFU did not fall below the detection limit. At a concentration of 150 AU, Bacthuricin F103 did not reduce the numbers of L. monocytogenes significantly (Fig. 5).
Effect of Bacthuricin F103 on the growth of Listeria monocytogenes during storage at 5 °C in beef meat. The results were expressed as survivals of L. monocytogenes cells, after incubation of 2 × 102 CFU of L. monocytogenes per gramme of meat in the presence of 150 (squares), 300 (triangles) and 500 (circles) AU of Bacthuricin F103, during different incubation periods. Diamonds: number of viable cells in the untreated control. Error bars, which are sometimes obscured by the data symbols, represent ±SD (standard deviation)
Moreover, no bacterial growth was observed in batch B3, which was used as control. Batches B2 and B4 were used for bacteriocin residual activity. While no loss of bacteriocin activity was detected in batch B4, a slight and steady decrease (15%) was observed in terms of bacteriocin activity in batch B1 during storage with all the bacteriocin concentrations used. In fact, the inactivation of bacteriocin in meat products has been attributed to indigenous proteases [36]. The present study demonstrated that the application of 500 AU (22.7 μg) of Bacthuricin F103 per piece of meat in raw beef meat may help avoid contamination by L. monocytogenes.
Analysis of Bacthuricin F103 N-terminal Sequence
The purified fraction of Bacthuricin F103 was submitted to an N-terminal amino acid sequence analysis in order to determine its amino acid sequence and to confirm its authenticity. The results yielded the following sequence (AVLPYDDVNITNTGILYETIEPMPA). The amino acid composition indicated that the N-terminal sequence of Bacthuricin F103 consisted of 25 amino acids, of which about 52% are polar and basic amino acids. This sequence was also found to have a global negative charge, as deduced from the content of the sequenced fragment, which was consistent with its purification procedure using an anion exchange column.
The N-terminal sequence of Bacthuricin F103 was compared with the protein sequences present in SWISS-PROT database using a BLAST program. No homology with known bacteriocins was found. A part of Bacthuricin F103 N-terminal sequence was, however, found to share a 72% sequence homology with hemolysin from Fusobacterium nucleatum subsp. nucleatum ATCC 25586 (accession number gb|AAL94497.1|). F. nucleatum is a prominent member of the oral microbiota and represents one of the common causes of human infection [37]. Hemolysin plays an important role in the pathogenesis of many bacteria such as Staphylococcus aureus [38]. The pore-forming feature of the hemolysin so far investigated has been identified as a major mechanism by which proteinaceous exotoxins can damage cells. This similarity that Bacthuricin F103 shares with hemolysin and its mode of action provide further evidence that the cell membrane of the sensitive strain is a possible target for Bacthuricin F103. In view of that, the present study postulated that Bacthuricin F103 may be considered a novel B. thuringiensis bacteriocin that shares such a similarity.
Conclusions
The present paper reported on the identification, purification and characterization of a new bacteriocin, named Bacthuricin F103, from a B. thuringiensis strain BUPM103 isolated from Tunisian soil samples. The production of Bacthuricin F103 was observed to begin in the early exponential phase and to reach a maximum in the middle of the same phase, with the maximum production yield being recorded 8 h after growth. The bacteriocin was purified to homogeneity using a two-step protocol. The findings indicated that it has a proteinaceous nature for it showed sensitivity to proteolytic enzymes. It was also noted to exhibit a remarkable stability to heat, pH and storage conditions, remaining active in a wide pH range and up to 60 °C. Besides, it showed a rapid effect and a bactericidal mode of action on the indicator strain B. cereus ATCC14579. When tested for antimicrobial activity, it exhibited a broad inhibitory spectrum against related bacteria as well as against several food-borne pathogens, such as L. monocytogenes, B. cereus and the phytopathogenic bacterium A. tumefaciens. The findings presented in the current study demonstrated that Bacthuricin F103 may be applied in raw beef meat to avoid L. monocytogenes contamination.
The N-terminal sequence of Bacthuricin F103 shares high similarity with hemolysin, suggesting that the cell membrane of the sensitive strain may be a possible target for Bacthuricin F103. The interaction of Bacthuricin F103 with the membrane of target cells leads to pore formations, which allow for the efflux of ions and, thereby, ensure the efficient killing of target bacteria.
Compared to other bacteriocins from B. thuringiensis, Bacthuricin F103 is easily distinguished by its production kinetics, bactericidal kinetics, relative thermostability, molecular mass, N-terminal sequence and inhibitory spectrum. The combination of all the above-mentioned properties revealed that Bacthuricin F103 may be considered a promising novel bacteriocin with strong potential for future industrial application. Accordingly, further studies are currently underway in our laboratories to gain further insights into its genetics, namely its structural genes and regulatory elements.
References
Tagg, J. R., Dajani, A. S., & Wannamaker, L. W. (1976). Bacteriological Reviews, 40, 722–756.
Riley, M. A. (1998). Annual Review of Genetics, 32, 255–278.
Cleveland, J., Montville, T. J., Nes, I. F., & Chikindas, M. L. (2001). International Journal of Food Microbiology, 71, 1–20.
Asaduzzaman, S. K., & Sonomoto, K. (2009). Journal of Bioscience and Bioengineering, 107, 475–487.
Delves-Broughton, J. (1990). Food Technology, 44, 100–117.
Klaenhammer, T. R. (1993). FEMS Microbiology Reviews, 12, 39–86.
Wang, J., & Fung, D. Y. C. (1996). Critical Reviews in Microbiology, 22, 101–138.
Cherif, A., Ouzari, H., Daffonchio, D., Cherif, H., Ben Slama, K., Hassen, A., et al. (2001). Letters in Applied Microbiology, 32, 1–5.
Paik, H. D., Bae, S. S., Park, S. H., & Pan, J. G. (1997). Journal of Industrial Microbiology & Biotechnology, 19, 294–298.
Ahern, M., Verschueren, S., & Sinderen, D. V. (2003). FEMS Microbiology Letters, 220, 127–131.
Chehimi, S., Delalande, F., Sablé, S., Hajlaoui, M. R., Van Dorsselaer, A., Limam, F., et al. (2007). Canadian Journal of Microbiology, 53, 284–290.
Cherif, A., Chehimi, S., Limem, F., Hansen, B. M., Hendriksen, N. B., Daffonchio, D., et al. (2003). Journal of Applied Microbiology, 95, 990–1000.
Lee, H., Churey, J. J., & Worobo, R. W. (2009). FEMS Microbiology Letters, 299, 205–213.
Rea, M. C., Sit, C. S., Clayton, E., O’Connor, P. M., Whittal, R. M., Zheng, J., et al. (2010). Proceedings of the National Academy of Sciences of the United States of America, 107, 9352–9357.
Kamoun, F., Mejdoub, H., Aouissaoui, H., Reinbolt, J., Hammami, A., & Jaoua, S. (2005). Journal of Applied Microbiology, 98, 881–888.
Chikindas, M. L., García-Garcerá, M. J., Driessen, A. J., Ledeboer, A. M., Nissen-Meyer, J., Nes, I. F., et al. (1993). Applied and Environmental Microbiology, 59, 3577–3584.
Gonzalez, B., Glaasker, E., Kunji, E. R. S., Driessen, A. J. M., Suarez, J. E., & Konings, W. N. (1996). Applied and Environmental Microbiology, 62, 2701–2709.
Castellano, P., Raya, R., & Vignolo, G. (2003). International Journal of Food Microbiology, 85, 35–43.
Zhou, K., Zhou, W., Li, P., Liu, G., Zhang, J., & Dai, Y. (2008). Food Control, 19, 817–822.
Jaoua, S., Zouari, N., Tounsi, S., & Ellouz, R. (1996). FEMS Microbiology Letters, 145, 349–354.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: a laboratory manual (2nd ed.). New York: Cold Spring Harbor Laboratory Press.
Zouari, N., & Jaoua, S. (1997). Biotechnology Letters, 19, 825–829.
Kamoun, F., Zouari, N., Saadaoui, I., & Jaoua, S. (2009). Preparative Biochemistry & Biotechnology, 39, 1–13.
Jack, R. W., Tagg, J. R., & Ray, B. (1995). Microbiological Reviews, 59, 171–200.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Gong, H.-S., Meng, X.-C., & Wang, H. (2010). Journal of Basic Microbiology, 50, 1–9.
Dortu, C., Huch, M., Holzapfel, W. H., Franz, C. M. A. P., & Thonart, P. (2008). Letters in Applied Microbiology, 47, 581–586.
Favret, M. E., & Yousten, A. A. (1989). Journal of Invertebrate Pathology, 53, 206–216.
Zouari, N., & Jaoua, S. (1999). Enzyme and Microbial Technology, 25, 364–371.
Woo-Jin, J., Mabood, F., Souleimanov, A., Zhou, X., Jaoua, S., Kamoun, F., et al. (2008). Journal of Microbiology and Biotechnology, 18, 1836–1840.
Granum, P. E. (2009). Bacillus cereus. In M. P. Doyle, L. R. Beuchat, & T. J. Montville (Eds.), Food microbiology fundamentals and applications (2nd ed., pp. 373–381). Washington: ASM.
Penyalver, R., Oger, P. M., Su, S., Alvarez, B., Salcedo, C. I., López, M. M., et al. (2009). Molecular Plant-Microbe Interactions, 22, 713–724.
Rogler, C. E. (1981). Plant Physiology, 68, 5–10.
Chehimi, S., Pons, A. M., Sablé, S., Hajlaoui, M. R., & Limam, F. (2010). Canadian Journal of Microbiology, 56, 162–167.
Davidson, P. M., & Harrison, M. A. (2002). Food Technology, 56, 60–78.
Burnside, K., Lembo, A., de Los Reyes, M., Iliuk, A., Binhtran, N. T., Connelly, J. E., et al. (2010). PLoS ONE, 5, 1–16.
Karpathy, S. E., Qin, X., Gioia, J., Jiang, H., Liu, Y., Petrosino, J. F., et al. (2007). PLoS ONE, 8, 1–14.
Acknowledgements
This work was supported by grants from the Tunisian Ministry of Higher Education, Scientific Research and Technology. The ‘Embassy of France’ at Tunis, Tunisia also provided a fellowship to Fakher Kamoun for a 3-month training in the U.G.M.E. INRA, La Minière, Versailles, France. We would like to express our gratitude to Mr. Mohamed Zribi for the supply of PALCAM medium. Special thanks are also due to Mr. Anouar Smaoui from the English Unit at the Sfax Faculty of Science for carefully proofreading and editing the manuscript of the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamoun, F., Fguira, I.B., Hassen, N.B.B. et al. Purification and Characterization of a New Bacillus thuringiensis Bacteriocin Active Against Listeria monocytogenes, Bacillus cereus and Agrobacterium tumefaciens . Appl Biochem Biotechnol 165, 300–314 (2011). https://doi.org/10.1007/s12010-011-9252-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9252-9