Abstract
An exopolysaccharide-producing Antarctic yeast strain was selected and identified as Cryptococcus laurentii AL100. The physiological properties of the strain and its ability to utilize and biotransform different carbon sources (pentoses, hexoses, and oligosaccharides) into exopolysaccharide and biomass were investigated. Sucrose was chosen as a suitable and accessible carbon source. The biosynthetic capacity of the strain was studied in its dynamics at different sucrose concentrations (20, 30, 40, and 50 g/L) and temperatures (22 and 24 °C). The maximum biopolymer quantity of 6.4 g/L was obtained at 40 g/L of sucrose, 22 °C temperature and 96-h fermentation duration. The newly synthesized microbial carbohydrate was a heteropolysaccharide having the following monosaccharide composition: arabinose, 61.1%; mannose, 15.0%; glucose, 12.0%; galactose, 5.9%; and rhamnose, 2.8%. It was characterized by polydispersity of the polymer molecule, 60% of it having molecular mass of 4200 Da. The exopolysaccharide demonstrated good emulsifying and stabilizing properties with regard to oil/water emulsions and a pronounced synergistic effect with other hydrocolloids such as xanthan gum, guar gum, and alginate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The search for new microorganisms with no previous biotechnological application has determined the scientists' interest in the inhabitants of extreme niches for the past two to three decades. Antarctica offers a unique combination of cold weather, strong winds, humidity, long dark and light periods, high UV radiation, food deficit, and insufficient water amounts for living organisms [1]. Samples of soil, plants (moss, lichen, grass), water, ice, sediments, penguin molten feathers, etc. are collected from these natural regions and used as sources for the isolation and study of psychrophilic microorganisms [2–4]. A great number of bacteria, yeast, fungi, and single-celled algae existing in the cold environment are interesting both scientifically and biotechnologically, with regard to the production of biologically active substances [5–10].
A large number of microorganisms produce polysaccharides of different composition, structure, interesting physicochemical properties, and functional characteristics. Xanthan, gellan, dextran, hyaluronan, alginate, curdlan are synthesized by bacterial producers and produced commercially for use in the food industry, medicine, agriculture, and wastewater treatment [11–13]. Fungal producers have yielded the polysaccharides pullulan, scleroglucan, lentinan, whose properties are interesting for medical applications. The polysaccharides mannans, glucomannans, glucans, galactomannans, and phosphomannans have been produced by yeast belonging to the following genera: Candida [14, 15], Rhodotorula [16, 17], Sporobolomyces [8, 18, 19], and Cryptococcus [20, 21].
Cryptococcus is the most predominant yeast group in the Antarctic. In this genus, Cryptococcus laurentii and Cryptococcus albidus are considered ubiquitous and have been reported by almost all researchers of Antarctica [2, 4, 5]. In contrast to Cryptococcus neoformans, C. laurentii is a non-pathogenic yeast [22].
The maximum growth temperature of some psychrophilic yeast is 25 °C, therefore it enables the study of biological potential by periodic and continuous cultivation within a temperature range suitable for the synthesis of biologically active substances, and permits the development of laboratory technologies.
The pharmaceutical, cosmetic and food industries have increasingly turned their interest to the use of biopolymers from natural sources. Polysaccharides synthesized by microorganisms have been extensively applied as emulsifiers, stabilizers, binding and gelling agents, coagulants, thickeners, and suspending agents in different areas [23–26]. Little information on polysaccharides produced by psychrophilic yeast and their physicochemical properties is available in the literature [20, 24]. The capacity of yeast to synthesize exopolysaccharide (EPS) of various composition and properties makes it possible to select psychrophilic strains as well.
The aim of this paper was to study a selected psychrophilic exopolysaccharide producer, to identify it and to determine the chemical composition and emulsifying properties of the biopolymer.
Materials and Methods
Biological Material
Yeast isolate AL100 from soil samples taken from the region of the Bulgarian base on Livingston Island was selected as a suitable EPS producer [27].
Identification
Morphological, Cultural, Biochemical, and Physiological Characteristics
Yeast isolate AL100 was identified using the yeast classification criteria proposed by Kurtzman and Fell [28].
DNA Sequence Analysis
Isolation of the total yeast DNA of the selected producer was carried out according to Marmur's method [29]. Its 18S rRNA gene was amplified using universal eukaryotic primers PFf 5′ AGGGATGTATTTATTAGATAAAAAATCAA 3′ and PFr 5′ CGCAGTAGTTAGTCTTCAGTAAATC 3′ [30] sequenced with ABI 373A DNA sequencer (Macrogen, Korea). The sequences were automatically assembled and manually edited using Vector NTI version 10 software package (Invitrogen, USA). The obtained sequences were compared to the known sequences by using the BLAST network service [31] to determine their approximate phylogenetic affiliations and orientation.
Media and Growth Conditions
The basal medium for the testing of carbon sources contained (grams per liter): (NH4)2SO4: 2.5; KH2PO4: 1.0; MgS04·7 H20: 0.5; NaCl: 0.1; CaCl2·2 H20: 0.01, and yeast extract: 1.0. The pentoses (xylose, ribose, arabinose), hexoses (galactose, glucose, fructose, mannose, and rhamnose) and oligosaccharides (raffinose, trehalose, and sucrose) were added in a 10-g/L concentration. Sucrose was chosen as a suitable carbon source and studied in different concentrations (grams per liter): 20, 30, 40, and 50. The initial pH was adjusted to pH 5.3, and the media were sterilized for 30 min at 112 °C. The inoculum was obtained on a rotary shaker (220 rpm) in 500-ml Erlenmeyer flasks containing 50 ml of Sabouraud medium (Merck, Germany) on a shaker at 22 °C, 48 h. The medium was inoculated with 10% (w/v) of inoculum. The cultivation was carried out in 500-ml Erlenmeyer flasks containing 50 ml of the medium on a rotary shaker (220 rpm) at 22 and 24 °C for 120 h.
Isolation of Crude EPS
Whole cell cultures were centrifuged 6,000×g and the supernatant was used for polysaccharide determination. The EPS was precipitated with two volumes of 96% ethanol at 4 °C for 24 h, washed twice with ethanol, dried, and weighed.
EPS Substance Production by C. laurentii AL100 for Analytical Purposes
The strain was cultivated on a culture medium containing (grams per liter): sucrose: 40; (NH4)2SO4: 2.5; KH2PO4: 1.0; MgS04·7 H20: 0.5; NaCl: 0.1; CaCl2·2 H20: 0.01; and yeast extract: 1.0, at 22 °C and pH 5.3 for 96 h. The EPS was isolated as described above.
Analytical Methods
The total carbohydrate amount in the crude EPS was determined using the phenol-sulphuric acid method [32]. The protein amount in the solution of non-hydrolysed polysaccharides was determined according to the Lowry method. The ash content was estimated after calcination for 2 h and glowing the polymer at 550 °C for 3 h. The EPS and the dry biomass were determined using the weight method after drying to constant mass at 65 and 105 °C. An enzyme test (Megazyme, Ireland) was applied to determine reducing sugars.
Neutral sugars were measured as alditol acetates after hydrolysis of samples. The polysaccharides (20 mg) were pretreated with 2 M trifluoroacetic acid (TFA) for 3 h at 120 °C before conversion to alditol acetates according to the method developed by Blakeney et al. [33]. The monosaccharide composition of the polysaccharides was assessed on a 6890 GC System Plus gas-mass chromatograph system/5793 Mass Selective Detector (Hewlett Packard) with an SP 2380 capillary column (0.2-μm film, 0.25 mm × 30 m; Supelco) using the following temperature program: column temperature for 3 min, then 5 °C/min to 250 °C; injector temperature 250 °C, detector temperature 280 °C; helium as carrier gas at 1 ml/min. Peak identification was based on retention times, using myoinositol as internal standard.
The molar mass of the crude EPS was assayed through high-performance size-exclusion chromatography (HPSEC) on a Waters (Millipore) system. The assay was made on an Ultrahydrogel™ 500 column (7.8 × 300 mm; Waters) with bidistilled water as eluent at an elution rate of 0.8 ml/min. The column was calibrated using the P-82 Shodex standard (Showa Denko, Japan).
Preparation of Emulsion Model Systems
The emulsifying power of the model systems with exopolysaccharide by C. laurentii AL100 was established according to documented methods [34]. The sunflower oil used for the model emulsions was a commercially available product. The homogenization was carried out on a POLYTRON (Kinematica Gmbh Kriens-Luzern, Switzerland) at 3,000×g. The centrifugation was performed at 3,000×g for the duration of 20 min. Dispersity was determined according to the translucency index (T%) on a Shimadzu spectrophotometer, UV/VIS-12404 (Japan) at λ = 540 nm.
Synergistic Effect
The synergistic effect of the synthesized microbial polysaccharide in relation to emulsion stabilizing properties was studied in mixtures with: xanthan gum, guar gum, cellulose gum, sodium alginate (company products), and methyl ester of alginic acid (synthesized by means of acidic esterification with alcohol) [35].
The micrograph of the emulsions was made using a Nikon Microphot SA microscope (Japan) with an Olympus digital camera (Japan), magnification ×100.
Statistical Analysis
The data were analyzed using the Statistical Package for the Social Sciences for Windows software, Version 11.0. Statistically significant differences between groups were determined by analysis of variance. When the differences were significant, Duncan's multiple range test was performed. Means were considered significantly different at p < 0.05. The results in the figures have been presented as the means of three experiments ± standard deviation.
Results and Discussion
In a previous study on the metabolic potential of Antarctic yeast isolates for exopolysaccharide synthesis, isolate AL100 showed initial EPS quantity of over 5.0 g/L and was selected as an active biopolymer producer [27].
The isolate AL100 was identified according to its morphological, cultural, physiological, and biochemical properties. The morphological characteristics of the isolate were as follows: the colony was glossy with smooth surface and even margin, pale yellow in color; the cells were oval, spherical, single or double, 3.3–7.4 × 3.6–12.2 mm in size; the strain did not form ascospores, teliospores, arthrospores, or pseudomycelium; the true mycelium was filament-free. Its maximum growth temperature was 25 °C.
Physiological and biochemical studies were carried out using sugars, alcohols, acids, polysaccharides, and glucosides. The results of these characteristics indicated that isolate AL100 grew well on a medium containing d-glucose, d-galactose, sucrose, maltose, lactose, cellobiose, trehalose, melibiose, raffinose, melezitose, d-xylose, d-arabinose, d-arabinose, and d-ribose. It utilized mannitol, inositol, erythritol, ribitol, galactitol, xylitol, glycerol, sorbitol, succinate, lactic acid, salicin, citrate, starch, d-glucuronate; negative or weak reaction was observed for d-sorbose, d-glucosamine, methanol, Me-α-d-glucoside, cadaverine, and nitrite. Isolate AL100 showed a positive reaction in the DBB test, arbutin splitting, and urea hydrolysis. It did not ferment glucose or produce acids from glucose; it did not form starch-like compounds. It grew on a vitamin-free medium containing 50% glucose was positive and 10% NaCl and 5% glucose was negative.
On the basis of the taxonomic studies and the yeast identification criteria of Kurtzman and Fell (1998), isolate AL100 was identified as C. laurentii AL100.
According to the 18s rRNA gene sequence analysis, isolate AL100 firmly affiliated to the genus Cryptococcus, showing 98% similarity to the Cryptococcus magnus species. Regardless of the lower percentage of similarity to C. laurentii AL100, the data on the physico-biochemical profile of the strain and the ecological area of distribution of C. laurentii in Antarctica [36], we believe that it would be most appropriate to attribute the strain we investigated to the C. laurentii species. Furthermore, C. magnus (Lodder and Kreger-van-Rij) Baptist and Kurtzman (1976) is a synonym of C. laurentii (Kufferath) C.E. Skinner var. magnus Lodder and Kreger-van-Rij (1952) [28].
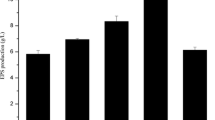
The results of the study on the physiological properties of C. laurentii AL100 for exopolysaccharide and biomass biosynthesis through utilization of different carbon sources—pentoses, hexoses, and oligosaccharides in 10-g/L concentration—have been shown in Fig. 1.
The EPS biosynthesized by the producer on xylose, ribose, and arabinose containing media was within the 2.5–3.5-g/L range, and the amount of accumulated biomass was 1.8–2.8 g/L. Ribose was a good carbon source transformed by the culture into 3.5 g/L biopolymer and a small amount of biomass (1.7 g/L) whereas the biomass obtained on xylose and arabinose containing media was 2.8 and 2.7 g/L, respectively, and the polymer synthesized was 2.5 and 2.8 g/L.
The study of the hexose effect on the EPS synthesis by the strain showed that galactose, glucose, fructose, and rhamnose were transformed into around 2.5 g/L of polysaccharide, while the exopolysaccharide yield reached 3.0 g/L with mannose. These monosaccharides, with the exception of rhamnose, were well utilized by the culture, and the biomass quantity ranged between 3.0 and 4.0 g/L. The oligosaccharides raffinose, fructose, and rhamnose were transformed by C. laurentii AL100 into a polymer, the quantity of which exceeded 3.0 g/L. Biomass was over 3.00 g/L on the trehalose and sucrose containing media, and 2.0 g/L on the raffinose containing medium.
The research continued with the use of sucrose as a good and reliable carbon source for the biosynthetic process carried out at 22 and 24 °C. The effect of sucrose in 20, 30, 40, and 50 g/L concentration in the medium on the exopolysaccharide biosynthesis, biomass accumulation, and pH change was investigated (Fig. 2).
The polymer biosynthesis increased significantly after 48 h corresponding to the end of the lag phase or the beginning of the exponential phase of the producer development. The polysaccharide was formed almost simultaneously with cell growth, except for the medium containing 20 g/L of sucrose. The EPS concentration and biomass data showed maximum values at 40 g/L sucrose 96 h after the start of the fermentation process, then the polymer content decreased slowly, which may be attributed to molecular depolymerization and inclusion of its products into the producer's metabolism.
The amount of EPS synthesized with 20 g/L sucrose in the culture medium reached 3.0 g/L at the 48th hour when the sucrose was under 1.0 g/L and the glucose had been used up. With 30 g/L sucrose in the medium, the EPS amount was 4.3 g/L at the 96th hour with fully hydrolyzed sucrose and utilized glucose. The maximum EPS synthesis of 6.4 g/L was observed on a culture medium with 40 g/L sucrose at the 96th hour, with a fully utilized carbon source. The biosynthetic process with C. laurentii AL100 and 50 g/L sucrose in the medium yielded a smaller amount of EPS and fermentation was prolonged to the 120th hour. There was a proportional relationship between the EPS and biomass synthesized and the high sucrose concentrations in the culture medium which could be explained by the fact that sucrose was the only carbon source building the biopolymer's carbohydrate skeleton.
A typical feature of the production of EPS by yeast is the significant pH change, which proves to be a regulating factor in EPS biosynthesis [18, 19, 36]. In the course of its metabolism, the C. laurentii AL100 strain changed the culture medium pH from the initial pH 5.3 to pH 2.2 after 24 h and these pH values were preserved until fermentation was complete.
The results of the study of the dynamics of sucrose hydrolysis and glucose and fructose utilization during C. laurentii AL100 cultivation at 22 °C have been shown in Fig. 3. The data on the glucose and fructose amounts resulting from the sucrose hydrolysis provided information on the carbon source utilization during the biosynthetic process. It was found that the sucrose was hydrolyzed as early as the beginning of the growth cycle, and insignificant non-hydrolyzed sucrose amounts were registered at the 72nd hour. Intense glucose utilization occurred simultaneously with sucrose hydrolysis until almost full depletion at the 48th hour, whereas fructose utilization ran more slowly. As seen from the utilization dynamics of the main nutrient, the period of intense EPS biosynthesis was observed when sucrose hydrolysis, glucose, and fructose utilization were complete. These results were consistent with our previous conclusions [8].
The data on C. laurentii AL100 cultivation at 24 °C indicated that the temperature was not suitable for biomass accumulation and EPS synthesis, and that was related to the disorders occurring in the metabolic synthesis (data have not been presented).
The productivity of the psychrophilic C.laurentii AL100 strain was 6.4 g/L EPS on a culture medium containing 40 g/L sucrose, at 22 °C for 96 h, whereas the Sp.salmonicolor AL1 Antarctic yeast strain yielded 5.6 g/L of polysaccharide on a medium supplied with 50 g/L sucrose, at 22 °C, for 120 h [8], and Adami and Cavazzoni found that the maximum fermentation yield of crude polysaccharides ranged from 6 to 12 g/L after 6–9 days at 25 °C with 60 g/L glucose [18].
The chemical composition of the crude EPS from C.laurentii AL100 is shown in column 2 in Table 1. As seen from the data, it contained 79.1% carbohydrate, 11.7% protein, and 9.2% ash. The newly synthesized microbial carbohydrate was a heteropolysaccharide of the following monosaccharide composition: arabinose: 61.6%, mannose: 15.0%, glucose: 12.6%, galactose: 5.9%, and rhamnose: 2.8%. The exopolysaccharide had a new monosaccharide composition different from that of the biopolymers of psychrophilic yeast previously produced by us [8, 20].
In their study on yeast of the Cryptococcus species, Golubev and Vdovina stated (without quoting any values), that glucose, galactose, mannose, hylose, and glucuronic acid built up their EPSs. The EPS produced by the C.laurentii strain cultivated on a glucose-containing culture medium included mannose, hylose, and glucuronic acid in its composition [37]. The exact chemical composition of other non-purified EPSs synthesized by different mesophilic and psychrophilic yeast reported by other authors has been presented in Table 1 (columns 3–9). The majority of the strains synthesized EPS with high carbohydrate content (74.3–92.5%), protein content (3.3–11.8%), and different amounts of ash. Mannose and glucose were the main components of the yeast polymers. The C.laurentii AL100 strain we investigated differed in its monosaccharide composition from all the others due to the presence of arabinose. This drew our attention to the study of its emulsifying properties.
HPSEC was used to establish the heterogeneity of the EPS which contained several fractions with molecular masses ranging from 2.08e+06 Da to 210 Da, 60% of the polysaccharide having molecular mass of 4,200 Da (Fig. 4). The microbial polymer had a low molecular mass compared to our other polysaccharides [8, 20].
The newly synthesized polysaccharide was characterized according to its emulsifying and stabilizing capacity for oil/water emulsions with a view to its practical application (Table 2).
The emulsifying capacity of the exopolysaccharide was investigated by means of model emulsions made with 0.5%, 1.0%, 1.5%, 2.0%, and 2.5% preparation concentration in the water medium and 50% oil phase of the oil/water type. The quality of the resultant emulsion was studied using the dispersity and stability indices. Table 2 presents the results on emulsion stability during centrifugation. The data show that the EPS from C. laurentii AL100 had good emulsifying and stabilizing properties and demonstrated a pronounced lipophilic effect of the oil/water interface. The oil phase was tightly bound; the separated oil was up to 5.0%.
The intact emulsion quantity was not directly dependent on the preparation concentration and the indices did not differ significantly in the different tests.
The investigated EPS synthesized by C. laurentii AL100 differed in the dispersity and stability indices of the emulsions obtained in comparison with the glucomannan synthesized by Sporidiobolus salmonicolor AL1, and this may be explained by the different monosaccharide composition, the polydisperse character of the polymer molecules and the lower molecular mass. Using 2.5% glucomannan, a stable oil/water emulsion was obtained and 100% preserved during centrifugation. The disperse system with mannan synthesized by Rhodotorula acheniorum MC had 72% stability under the same conditions. Laboratory products in cream form made with glucomannan have been tested for emulsion stability at different temperatures with a view to their application to the cosmetic industry [24]. Yun and Park used an extracellular polysaccharide synthesized by Bacillus sp. to obtain emulsions which had a stronger emulsifying effect than those made with xanthan [26].
Therefore, it was necessary to study the effect of some hydrocolloids on the emulsifying and stabilizing properties of the investigated biopolymer. The tests were conducted with 2.0% concentration of EPS synthesized by C. laurentii AL100, with the addition of xanthan, guar gum, cellulose, sodium alginate, and methyl ester of alginic acid in 0.7% concentrations in relation to the water medium. The results presented in Table 3 showed that in the presence of the minimum quantities of the polysaccharides added, the stability and dispersity of the resultant emulsions improved significantly, i.e., a pronounced synergistic effect was observed in relation both to the emulsifying properties and to the stability of dispersion systems. In all experiments, the separated oil percentage was zero, and the intact emulsion percentage ranged between 70 and 100. It is very important to note that the separated water percentage was much lower, even zero in some cases. The best results were achieved with xanthan and guar gum. As regards dispersity (index T%), the resultant emulsions did not differ significantly: T = 50–70%. It is worth mentioning that the hydrocolloids added, i.e., xanthan, guar gum, cellulose, sodium alginate, and methyl ester of alginic acid, are weak emulsifiers, except for the polymer synthesized by C. laurentii AL100.
The synergistic action of the microbial biopolymer with the hydrocolloids xanthan, guar gum, cellulose gum, methyl ester of alginic acid, and sodium alginate was studied (Table 3). The results showed that when different polysaccharides were added to the investigated EPS, a marked synergistic effect was observed in relation both to emulsifying properties and to stability. It is necessary to point out that the hydrocolloids studied, i.e., xanthan, guar gum, cellulose gum, and sodium alginate, are weak emulsifiers with the exception of the methyl ester of alginic acid.
Figure 5 shows a micrograph of the model emulsion obtained during the synergistic action of the 2.0% microbial biopolymer with 0.7% xanthan, which demonstrates that they are monodisperse systems.
Conclusion
The conditions for EPS biosynthesis by the C. laurentii AL100 psychrophilic strain were established and the monosaccharide composition and molecular mass of the EPSs were determined. The EPS demonstrated good emulsifying capacity for oil/water emulsion systems. It showed a marked synergistic effect in relation to its emulsifying and stabilizing properties with the hydrocolloids xanthan gum, guar gum, cellulose gum, and alginates. The established physicochemical properties of the biopolymer synthesized showed that it could be used for the manufacture of emulsion products for the cosmetic and food industry independently or in combination with other polysaccharides.
References
Raspor, P., & Zupan, J. (2006). Yeasts in extreme environments. In C. Rosa & G. Peter (Eds.), Biodiversity and ecophysiology of yeasts (pp. 371–417). Berlin: Springer.
Scorzetii, G., Petrescu, I., Yarrow, D., & Fell, J. (2000). Cryptococcus adeliensis sp.nov., a xylanase producing basidiomycetous yeast from Antarctica. Antonie van Leeuwenhoek, 77, 153–157.
Tosi, S., Casado, B., Gerdol, R., & Caretta, G. (2002). Fungi isolated from Antarctic mosses. Polar Biology, 25, 262–268.
Guffogg, S., Thomas-Hall, S., Holloway, P., & Watson, K. (2004). A novel psychrotolerant member of the hymenomycetous yeasts from Antarctica: Cryptococcus watticus sp.nov. International Journal of Systematic and Evolutionary Microbiology, 54, 275–277.
Vishniac, E. (2006). Yeast biodiversity in the Antarctic. In C. Rosa & G. Peter (Eds.), Biodiversity and ecophysiology of yeasts (pp. 419–439). Berlin: Springer.
Gerday, C., Aittaleb, M., Bentahir, M., Chessa, J., Claverie, P., Collins, T., et al. (2000). Cold-adapted enzymes: from fundamentals to biothechnology. Biochimica et Biophysica Acta, 18, 103–107.
Pavlova, K., Angelova, G., Savova, I., Grigorova, D., & Kupenov, L. (2002). Studies of Antarctic yeast for β-glucosidase. World Journal of Microbiology & Biotechnology, 18, 569–573.
Pavlova, K., Koleva, L., Kratchanova, M., & Panchev, I. (2004). Production and characterization of an exopolysaccharide by yeast. World Journal of Microbiology & Biotechnology, 20, 435–439.
Zlatanov, M., Pavlova, K., & Grigorova, D. (2001). Lipid composition of some yeast strains from Livigston Island, Antarctica. Folia Microbiologica, 46, 402–406.
Dimitrova, S., Pavlova, K., Lukanov, L., & Zagorchev, P. (2010). Synthesis of coenzyme Q10 and β-caroten by yeasts isolated from Antarctic soil and lichen in response to ultraviolet and visible radiations. Applied Biochemistry and Biotechnology, 162, 795–804.
Margaritis, A., & Pace, G. (1985). Microbial polysaccharides. In H. Blanch, S. Drew, & D. Wang (Eds.), Comprehensive biotechnology, 1005–1044. Pergamon Press: Oxford.
Sutherland, I. (1998). Novel and established applications of polysaccharides. Trends in Biotechnology, 16, 41–45.
Vanhooren De Baets, S., Bruggeman, G., & Vandamme, E. (1998). Plastics made by bacteria or their enzymes. Biologi Italiani, 1, 7–11.
Chiura, H., Iizuka, M., & Yamamoto, T. (1982). A glucomannan as an extracellular product of Candida utilis. I. Production and characterization of a glucomannan. Agricultural and Biological Chemistry, 46, 1723–1733.
Chiura, H., Iizuka, M., & Yamamoto, T. (1982). A glucomannan as an extracellular product of Candida utilis. II. Structure of a glucomannan characterization of oligosaccarides obtained by partial hydrolysis. Agricultural and Biological Chemistry, 46, 1733–1742.
Petersen, G., Nelson, G., Cathey, C., & Fuller, G. (1989). Rheologycally Interesting Polysaccharides from Yeasts. Applied Biochemistry and Biothechnology, 20(21), 845–867.
Pavlova, K., & Grigorova, D. (1999). Production and properties of exopolysaccharides by Rhodotorula acheniorum MC. Food Research International, 32, 473–477.
Adami, A., & Cavazzoni, V. (1990). Exopolysaccharides produced by some yeast strains. Annali di Microbiologia ed Enzimologia, 40, 245–253.
Elinov, N., Ananieva, E., & Vitovskaya, G. (1992). Features of the biosynthesis and characteristics of the exoglycanns in yeasts of the genus Sporobolomyces. Microbiologiya, 60, 466–470.
Pavlova, K., Panchev, I., Kratchanova, M., & Gocheva, M. (2009). Production of an exopolysaccharide by Antarctic yeast. Folia Microbiologica, 54, 343–348.
Vorotynskaya, S., Vitovskaya, G., & Ananyeva, E. (1992). Studies on the properties of polysaccharydes produced by the yeasts Cryptococcus luteolus (Saito) skinner. Microbiologiya Fitopatologiya, 26, 367–371.
Inge, N., Boyaert, V., Sofie, L., Do Maeseneire, H., & Vandamme, E. (2009). Extracellular polysaccharides produced by yeasts and yeast-like fungi. In T. Satyanarayana, G. Kunze (Eds), Yeast biotechnology: Diversity and applications (pp. 661–664). Berlin: Springer.
Tizard, I., Carpenter, R., McAnalley, B., & Kemp, M. (1989). The biological activities of mannans and related complex carbohydrates. Molecular Biotherapy, 1, 290–296.
Kuncheva, M., Pavlova, K., Panchev, I., & Dobreva, S. (2007). Emulsifying power of mannan and glucomannan produced by yeasts. International Journal of Cosmetic Science, 29, 377–384.
Willims, P., Hickey, M., & Mitchell, D. (2003). Fluid gels based on natural polymers for cosmetic applications. Cosmetics and Toiletries, 118, 51–59.
Yun, U., & Park, H. (2003). Phisical properties of an extracelular polysaccharide produced by Bacillus sp. CP 912. Letters in Applied Microbiology, 36, 282–287.
Rusinova-Videva, S., Pavlova, K., & Metcheva, R. (2009). Studies of Antarctic yeast isolates for exopolysaccharide synthesis. Biotechnology and Biotechnological Equipment, 23, 888–891.
Kurtzman, C., & Fell, J. (1998). The yeast: A taxonomic study (4th ed.). Amsterdam: Elsevier Scientific Publisher.
Marmur, J. (1961). A procedure for the isolation of deoxyribonucleic acid frommicroorganisms. Journal of Molecular Biology, 3, 208–218.
Jaeger, E., Carrol, N., Choudhury, S., Dunlop, A., Towler, H., Matheson, M., et al. (2000). Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. Journal of Clinical Microbiology, 38, 2902–2908.
Altschul, S., Gish, W., Miller, W., Myers, E., & Lipman, D. (1990). Basic localalignment search tool. Journal of Molecular Biology, 215, 403–410.
Dubois, M., Gilles, K., Hamilton, Y., Rebers, P., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Blakeney, A., Harris, Ph, Henry, R., & Stony, B. (1983). A simple and rapid preparation of aldetol acetates for monosaccharide analysis. Carbohydrate Research, 113, 291–299.
Kratchanov, Ch, Stamov, S., Popova, M., & Kuntcheva, M. (1978). Emulgierfahigkeit ven Atylesterndes Sonnenblumenpektins. Zeitschrift fur Lebensmittel-Untersuchung und -Forschung, 167, 338–341.
Denev, P., Kuncheva, M., & Popova-Ivanova, M. (2005). Synthesis and characteristics of alginate acides. Agricultural and Food Chemistry, 4, 1143–1149.
Heald, P., & Kristiansen, B. (1985). Synthesis of polysaccharide by yeast-like forms of Aureobasidium pullulans. Biotechnology and Bioengineering, 27, 1516–1519.
Golubev, V., & Vdovina, H. (1974). Monosaccharide composition of extracellular polysaccharides of some Cryptococcus species. Microbiology (Russia), 43, 154–156.
Grigorova, D., Pavlova, K., & Panchev, I. (1999). Preparation and preliminary characterization of exopolysaccharides by yeast Rhodotorula acheniorum MC. Applied Biochemistry and Biotechnology, 81, 181–191.
Petersen, G., Shubert, W., Richards, G., & Nelson, G. (1990). Yeast producing exopolysaccharides with drag reducing activity. Enzyme and Microbial Technology, 12, 255–259.
Acknowledgements
The study was supported by Grant DTK 02/46 from the National Fund Scientific Investigation and the European Social Fund, Operational Programme “Human Resources Development”, contract BG051PO001-3.3.04/32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlova, K., Rusinova-Videva, S., Kuncheva, M. et al. Synthesis and Characterization of an Exopolysaccharide by Antarctic Yeast Strain Cryptococcus laurentii AL100 . Appl Biochem Biotechnol 163, 1038–1052 (2011). https://doi.org/10.1007/s12010-010-9107-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-9107-9