Abstract
Shortcut nitrification is the first step of shortcut nitrogen removal from swine wastewater. Stably obtaining an effluent with a significant amount of nitrite is the premise for the subsequent shortcut denitrification. In this paper, the stability of nitrite accumulation was investigated using a 1.5-day hydraulic retention time in a 10-L (working volume) activated sludge sequencing batch reactor (SBR) with an 8-h cycle consisted of 4 h 38 min aerobic feeding, 1 h 22 min aerobic reaction, 30 min settling, 24 min withdrawal, and 1 h 6 min idle. The nitrite production stability was tested using four different ammonium loading rates, 0.075, 0.062, 0.053, and 0.039 g NH4-N/g (mixed liquid suspended solid, MLSS) day in a 2-month running period. The total inorganic nitrogen composition in the effluent was not affected when the ammonium load was between 0.053 and 0.075 g NH4-N/g MLSS · day (64% NO2-N, 16% NO3-N, and 20% NH4-N). Under 0.039 g NH4-N/g MLSS · day, more NO2-N was transformed to NO3-N with an effluent of 60% NO2-N, 20% NO3-N, and 20% NH4-N. The reducing load test was able to show the relationship between a declining free nitrous acid (FNA) concentration and the decreasing nitrite production, indicating that the inhibition of FNA on nitrite oxidizing bacteria depends on its levels and an ammonium loading rate around 0.035 g NH4-N/g MLSS · day is the lower threshold for producing a nitrite dominance effluent in the activated sludge SBR under the current settings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural fertilizers have been identified as a primary culprit behind the eutrophication phenomenon booming in lakes and gulfs [1]. Among the fertilizers used, the wastewater flushed from the confined swine production facilities and then applied to the cropland is one of the major responsible agricultural sources [2, 3]. When the scale of swine production keeps rising [4], manure that cannot be land applied according to the plant and soil testing has to be treated before discharge to avoid potential pollutions to water resources and the environment.
The energy input for aeration is one of the major costs associated with aerobic activated sludge treatment systems. The provision of aeration equipment generally represents less than 5% of the capital cost, but the energy consumption to run aeration represents approximately 25% of the annual operating costs [5], based on experiences from municipal wastewater treatment plants. Swine wastewater contains total nitrogen at a level normally one order of magnitude higher than that of municipal and/or domestic wastewater [6, 7]. The chemical oxygen demand (COD) equivalent of NH3 that can be calculated from Eq. 1 equals 3.76, while the COD of a typical organic carbon compound such as glucose (C6H12O6) is only 1.067 based on Eq. 2. This simple comparison attests that the oxygen demand of ammonium laden swine wastewater is much higher than an average municipal wastewater characterized mainly by its carbon content. From the above numbers, it is fair to say that, as opposed to municipal wastewater treatment, much more air needs to be supplied in aeration systems to treat swine wastewater.
In order to reduce the energy cost incurred by aeration, several advanced methods have been studied to improve nitrogen removal, such as the partial nitritation treatment for anaerobic digestion liquor of swine wastewater using swim-bed technology [8], the anaerobic ammonium oxidation (Anammox), and the partial denitritation in anaerobic nitrogen removal from piggery waste [9]. Regardless of what the next step is (it could be denitritation or Anammox), the first step is to produce an effluent with a significant amount of nitrite, which can be accomplished by a process called “shortcut nitrification” (also termed nitritation). Shortcut nitrification produces nitrite instead of nitrate from ammonium through controlling process parameters to allow the ammonia oxidizing bacteria (AOB) to grow faster than the nitrite oxidizing bacteria (NOB), thus omitting the step for nitrite to be oxidized to nitrate. Compared to the traditional nitrification and denitrification, the main advantages of shortcut nitrification and denitrification can be summarized as follows [10–13]: (1) 25% lower oxygen consumption in the aerobic stage, implying 60% energy savings; (2) lower electron donor requirement (up to 40%) in the anoxic stage; (3) 1.5 to two times higher denitrification rates with nitrite than with nitrate; (4) reduction of CO2 emission by 20%; and (5) 33–35% lower sludge production in the nitrification process and 55% in the denitrification process. With all these advantages, biological nitrogen removal via the nitrite pathway can be defined as an environmentally cleaner process, reducing aeration and chemical costs. The controlling parameter favoring nitrite production can be temperature because at a higher temperature (>25 °C), AOB have a greater specific growth rate than NOB [14]. It can also be the dissolved oxygen (DO) because at a lower DO, the AOB have a higher affinity for oxygen than NOB [15]. Free ammonia (FA; NH3) and free nitrous acid (FNA; HNO2) are found to have selective inhibition on NOB [16], which means a certain combination of reactor type and feeding strategy benefiting FA and FNA production can also be used as a controlling parameter for shortcut nitrification.
Our former research [17] has confirmed the possibility of accumulating nitrite from swine wastewater by using a continuous feeding strategy in an activated sludge sequencing batch reactor (SBR), in which ammonium loads were increased from 0.04 to 0.7 g NH4-N/L· day and an effluent with 13–23% of NH4-N, 15–21% of NO3-N, and 56–72% of NO2-N was generated. In order to test the nitrite production stability and locate a critical ammonium loading rate for producing a nitrite-dominant effluent under the current SBR system design, four different ammonium loading rates, 0.075, 0.062, 0.053, and 0.039 g NH4-N/g mixed liquid suspended solid (MLSS) day, were applied and the effluent inorganic nitrogen composition tested in a 2-month running period under the 1.5-day hydraulic retention time (HRT). In addition, a reducing load test in which ammonium loads was reduced from 0.081 to 0.011 g NH4-N/g MLSS · day was conducted to qualitatively visualize the relationship between the gradually reduced ammonium loads and the nitrite production efficiencies.
Materials and Methods
Manure Source
Raw swine wastewater was collected once a week from a reception sump of a finishing barn at the University of Minnesota Southern Research and Outreach Center, where fresh manure in a shallow pit inside the barn was flushed out biweekly. The test manure was the supernatant after settling and filtering (sieve opening, 2.5 mm) and was stored for normally less than 1 week before further treatment. The characteristics of swine wastewater were pH 8.21–8.33, COD 8,000–11,000 mg L–1, NH4-N 1,400–1,800 mg L–1; NO2-N and NO3-N were negligible. If not used immediately, the collected manure was stored at 4 °C or below.
Nitritation Sequencing Batch Reactor Setup
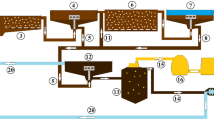
A 12-L lab-scale SBR with a working volume of 10 L, which was equipped with influent feeding/effluent discharging and air supply subsystems, was used in this study (Fig. 1). The SBR was operated at room temperature (20 ± 3 °C) without temperature control. The air was provided by a vacuum pressure pump (Barnant 60010-2392, USA) with the aeration intensity kept at 10 L min−1 to keep the biomass completely mixed without using a mechanical stirrer, and the running time of the air pump was controlled by a timer (BH-94460-45, Cole-Parmer Instruments Co., USA). Two peristaltic pumps (MasterFlex 7550-30, USA) were used for feeding and discharging, respectively. A mixer (Servodyne 50003-20, Cole-Parmer Instruments Co., USA) was installed in the influent tank for complete mixing at a constant rate of 80 rpm during the feeding period of SBR. The pumps and the mixer were automatically controlled by a computer program WIN LIN V1.2 (Cole-Parmer Instrument Co., USA).
Nitritation Sequencing Batch Reactor Operation
The startup of the production of a nitrite-dominant effluent was trial-tested by applying different ammonium concentrations to the system. When the MLSS in the SBR was initially around 1,800 mg/L with an influent ammonium concentration around 220 mg N/L, nitrite and nitrate were rarely produced, indicating that the nitrifying bacteria could not adapt to this ammonium load and all nitrifying activities were largely reduced, if not inhibited. The nitrite started to accumulate when the influent ammonium was firstly reduced to 65 mg N/L for 1 day (the corresponding nitrite concentration was 11.2 mg N/L) and then kept at around 100 mg/L for 2 days with a resulting nitrite concentration of 104 mg N/L on the fourth day. Then on the fifth day when the influent ammonium was 127 mg N/L, the effluent nitrite, nitrate, and ammonium concentrations were 104, 32.2, and 9.63 mg N/L, respectively, implying that nitrite became the dominant product in the effluent. The reason why the sum of the effluent nitrite, nitrate, and ammonium was greater than the influent ammonium is possibly due to remaining ammonium from previous cycles and newly converted ammonium from organic nitrogen in the swine manure. After startup, the reactor was fed with the collected swine wastewater at different dilutions to obtain different ammonium levels. The dilution was performed by blending swine wastewater with tap water, and the dilution rate was calculated by dividing the sum of volumes of the swine wastewater and added tap water by the volume of the swine wastewater. During this 2-month test period of the nitritation SBR, the 1.5-day HRT, 8-h cyclic operation mode was applied. This mode consisted of 4 h 38 min aerobic feeding, 1 h 22 min aerobic reaction, 30 min settling, 24 min withdrawal, and 1 h 6 min idle. The nitrite production stability was tested using four different ammonium loading rates, 0.075, 0.062, 0.053, and 0.039 g NH4-N/g MLSS · day by adjusting ammonium concentrations in the range of 498 to 1,018 mg N/L in the influent according to MLSS levels, which varied from 6,000 to 11,000 mg/L in this test period. Five effluent samples were collected every 2 days in the 2-month running period for each of the four ammonium loading rates. Sludge withdrawal was minimally performed in order to maintain the proliferated biomass in the system during this period, obtaining an SRT greater than 20 days for autotrophic nitrifiers to achieve complete nitrification [18].
After the data collection for stability analysis was done, a reducing load test was conducted. The influent ammonium concentration gradually decreased from 631 to 84 mg N/L (631, 491, 384, 298, 204, 117, and 84 mg N/L), and the corresponding ammonium load was decreased from 0.081 to 0.011 g NH4-N/g MLSS · day (0.081, 0.059, 0.046, 0.035, 0.024, 0.016, and 0.011 g NH4-N/g MLSS · day). Under each influent concentration, the reactor was run for 2 days (six cycles), in which three effluent samples were collected from the latter three cycles. Sludge withdrawal was conducted daily during this period in order to keep a relatively stable level of the MLSS at 5,400 ± 256 mg/L. The SRT, which was calculated by dividing the total solids in the reactor by total daily withdrawn solids, was around 30 days. In this experiment, the independent variable was the ammonium loading rate, and the dependent variable was the percent nitrite dominance in the system.
Analytic Methods
Liquid samples were obtained every 2 days from influent and effluent in the reducing load test and stability periods. After centrifuging at 5,000 rpm for 10 min, the supernatant was obtained for analyses of ammonium (NH +4 -N), nitrite (NO −2 -N), and nitrate (NO −3 -N). Measurements of NH +4 , NO −2 , and NO −3 were performed for both influent and effluent liquid samples following the Hach DR2800 Spectrophotometer Manual [19]. Nitrite (NO −2 -N) was measured by the diazotization method using TNT 839 vials (Hach, USA) and nitrate (NO −3 -N) by the cadmium reduction method using TNT 835 vials (Hach, USA). Ammonium (NH +4 -N) was measured by the Nessler method using Nessler’s reagent (Hach, USA). pH was measured by a portable pH meter (Orion 210A, Thermo Scientific, USA). MLSS were measured according to the standard methods [20]. The FA and FNA concentrations are calculated using Eqs. 3 and 4. T is in degree Celsius.
Statistical analysis was done by using SAS JMP V6.0. The comparison of different data sets was conducted by one-way analysis of variance (ANOVA) and Tukey–Kramer honestly significant difference (HSD) test embedded in JMP. The null hypotheses of no significant difference between data sets were rejected at 95% significance level when p < 0.05.
Results and Discussion
Stability Test
The nitrite production stability was tested using four different ammonium loading rates, 0.075, 0.062, 0.053, and 0.039 g NH4-N/g MLSS · day in a 2-month running period. The total inorganic nitrogen (TIN) composition in the effluent was calculated, and the results are shown in Table 1. The NO2-N in the effluent accounted for 65% of the TIN for daily ammonium loads of 0.075 and 0.053 and was slightly lower at 0.062 and 0.039 loading rates (63% and 60%), respectively. The NO3-N content was about 16% for all but one loading rate (20% for the 0.039 loading rate). NH4-N was about 20%, 21%, 19%, and 20% of the TIN for 0.075, 0.062, 0.053, and 0.039 daily loads, respectively. Statistical analysis was performed and the p values of the one-way ANOVA were 0.0024, 0.001, and 0.689 for NO2-N/TIN, NO3-N/TIN, and NH4-N/TIN, respectively, indicating significant difference existing for NO2-N/TIN and NO3-N/TIN but not for NH4-N/TIN under the four loading rates. Further comparisons for all pairs using Tukey–Kramer HSD suggest that for NO2-N/TIN and NO3-N/TIN, the significant different data set is from the load 0.039 at 95% significance level (Fig. 2). The ranges of NO2-N, NO3-N, and NH4-N under the four loading rates are shown in Table 2. Conclusively, the TIN composition in the effluent was not affected when the ammonium load was between 0.053 and 0.075 g NH4-N/g MLSS · day, but under 0.039 g NH4-N/g MLSS · day, the NO2-N percentage declined a little accompanied by a slight rise in NO3-N, suggesting that nitrite oxidizers were less inhibited when the ammonium load was kept under a certain threshold. Despite that, the process has accomplished the goal to make NO2-N become the dominant component of TIN in the effluent under all loads during the entire running period, namely successful nitrite accumulation from swine wastewater, which is the premise for conducting experiments in the second SBR using the high nitrite effluent as its influent. While the highest ammonium loading rate was 0.075 g NH4-N/g MLSS · day and the critical one was shown to be around 0.039 g NH4-N/g MLSS · day achieved in this study, broad range of ammonium loading rate under different reaction temperatures which was between 0.03 (20 °C) and 1.5 g NH4-N/g MLSS · day (36 °C) was found in research done by others. Peng et al. [21] suggested that the loading rate should be kept lower than 0.15 g NH4-N/g MLSS · day at 30 °C as for the wastewater containing seawater with a removal efficiency above 85%. The critical loading rate of NH4-N reduced to 0.08 and 0.03 g NH4-N/g MLSS · day for 25 °C and 20 °C, respectively. Ganiguéa et al. [22] studied startup and operation of a partial nitritation sequencing batch reactor treating urban landfill leachates. Stable partial nitritation was reached treating high ammonium loads (1–1.5 g NH4-N/g TSS · day based on 1,000 mg TSS/L) at 36 ± 1 °C and DO of 2 mg/L, achieving values for the conversion of ammonium to nitrite between 40% and 60%. The nitrification process stopped at the ammonium oxidation step without significant nitrate production being observed in spite of the high operating temperature (36 °C) and the high DO concentration in the reactor.
a One-way analysis of NO2-N/TIN by daily ammonium load; b one-way analysis of NO3-N/TIN by daily ammonium load; c one-way analysis of NH4-N/TIN by daily ammonium load. The centerlines of the means diamonds are the group means. The top and bottom of the diamonds form the 95% confidence intervals for the means [23]
Reducing Load Test
Based on the assumption that either FA or FNA, closely related to the ammonium loading rates, was responsible for inhibiting nitrite oxidizers, leading to the accumulation of nitrite, the 1.5-day HRT cycle was chosen for the reducing load test to qualitatively evaluate the relationship between the concentrations of free ammonia and free nitrous acid and the nitrite content in the effluent when the MLSS was kept at 5,400 ± 256 mg/L through the entire period. Figure 3a shows the profiles of influent NH +4 and effluent NH +4 , NO −2 , and NO −3 concentrations during the test. The influent NH +4 was gradually reduced from 630.1 to 84.1 mg/L. The corresponding changes in NO −2 production and the NO −2 /(NO −2 + NO −3 ) ratio in the effluent are shown in Table 3. The evolution of NO −2 /NO −3 ratio in the effluent is shown in Fig. 3b. After day 7, the NO −2 /NO −3 ratio experienced a sharp decline from about 3.0 to 2.4 and finally to 1.36. This change of the NO −2 /NO −3 ratio may be explained by examining Fig. 3c, d. The large standard deviations in Fig. 3d might be owing to the calculation stacking effect of deviations from both the NO −2 concentration and the pH. When the NH +4 load dropped from 0.035 to 0.024 g/g MLSS · day, the value of pH climbed from 6.2 to 6.4, accompanied by an abrupt decline of FNA from 1.2 to 0.6. From that point forward, the nitrite dominance environment in the system was no longer existing. According to the research done by Anthonisen et al. [16], NOB were inhibited by FA at levels above 0.1 mg/L, and all nitrifying bacteria were inhibited by FNA at levels above 0.2 mg/L. The FA concentration was always kept under 0.1 mg/L except in the final day, implying that FNA, instead of FA, was the acting inhibitor on NOB throughout the entire test period. FNA, when above 0.2 mg/L, did not show complete inhibition on the nitritation activities, so the conversion from ammonium to nitrite was still proceeding. The observations from this experiment demonstrate that the inhibition of FNA on NOB depends on its levels and would decrease under a certain level in a given system.
Conclusions
Shortcut nitrification and denitrification is a process based on the intermediate, i.e., nitrite, which exists in both nitrification and denitrification processes to treat wastewaters containing ammonium, especially meaningful for high ammonium wastewaters. Through the combination of an activated sludge SBR and a continuous feeding strategy, an environment favoring the nitrite accumulation from swine wastewater was established. The nitrite production stability was tested using four different ammonium loading rates, 0.075, 0.062, 0.053, and 0.039 g NH4-N/g MLSS · day in a 2-month running period. The TIN composition in the effluent was not affected when the ammonium load was between 0.053 and 0.075 g NH4-N/g MLSS · day (64% NO2-N, 16% NO3-N, and 20% NH4-N). Under 0.039 g NH4-N/g MLSS · day, 4% more NO2-N was transformed to NO3-N with an effluent of 60% NO2-N, 20% NO3-N, and 20% NH4-N. In the reducing load test, the NH +4 load was gradually decreased from 0.081 to 0.011 g NH4-N/g MLSS · day. When the NH +4 load dropped from 0.035 to 0.024 g NH4-N/g MLSS · day, the NO −2 /NO −3 ratio dropped from 0.75 to 0.70, accompanied by an abrupt decline of FNA from 1.2 to 0.6. From that point forward, the nitrite dominance environment in the system was no longer existing. Combining the results from both reducing load and stability tests, it is concluded that an ammonium loading rate around 0.035 g NH4-N/g MLSS · day is the lower threshold for producing a nitrite dominance effluent from the activated sludge SBR.
References
Howarth, R., Anderson, D., Cloern, J., Elfring, C., Hopkinson, C., Lapointe, B., et al. (2000). Nutrient pollution of coastal rivers, bays, and seas. Issues in Ecology, 7, 1–15.
Van Dyne, D. L., & Gilbertson, C. B. (1978). Estimating U.S. Livestock and poultry manure and nutrient production, U.S. Department of Agriculture, Economics, Statistics, and Cooperatives Service, ESCS-12. Springfield: National Technical Information Service.
Karlen, D. L., Cambardella, C. A., & Kanwar, R. S. (2004). Challenges of managing liquid swine wastewater. Applied Engineering in Agriculture, 20(5), 693–699.
Hunt, P. G., Vanotti, M. B. (2001). Coping with swine manure. Agricultural Research. July: 18–19.
Griffiths, P. C., Peters, M. P., & Farnell, B. A. (1989). Aeration system design for activated sludge processes [online]. In: Australian Water and Wastewater Association 13th Federal Convention: Investing in Water Futures, the Australian Water Industry in the 1990's; preprints of papers (pp. 233–237). Barton: Institution of Engineers, Australia. National conference publication (Institution of Engineers, Australia); no. 89/2. Availability: http://search.informit.com.au/documentSummary;dn=572050257811163;res=IELENG. ISBN: 0858254530.
Boursier, H., Beline, F., & Paul, E. (2005). Piggery wastewater characterisation for biological nitrogen removal process design. Bioresource Technology, 96, 351–358.
Zhang, Z. J., Zhu, J., King, J., & Li, W. H. (2006). A two-step fed SBR for treating swine wastewater. Process Biochemistry, 41, 892–900.
Yamamoto, T., Takaki, K., Koyama, T., & Furukawa, K. (2006). Novel partial nitritation treatment for anaerobic digestion liquor of swine wastewater using swim-bed technology. Journal of Bioscience and Bioengineering, 102(6), 497–503.
Ahn, Y. H., Hwang, I. S., & Min, K. S. (2004). ANAMMOX and partial denitritation in anaerobic nitrogen removal from piggery waste. Water Science and Technology, 49(5–6), 145–153.
Beccari, M., Marani, E., Ramadori, R., & Tandoi, V. (1983). Kinetic of dissimilatory nitrate and nitrite reduction in suspended growth culture. J WPCF, 55, 58–64.
Turk, O., & Mavinic, D. S. (1987). Benefits of using selective inhibition to remove nitrogen from highly nitrogenous wastes. Environmental Technology Letters, 8, 419–426.
van Kempen, R., Mulder, J. W., Uijterllnde, C. A., & van Loosdrecht, M. C. M. (2001). Overview: full scale experience of the SHARON process for treatment of rejection water of digested sludge dewatering. Water Science and Technology, 44, 145–152.
Ruiz, G., Jeison, D., & Chamy, R. (2003). Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration. Water Research, 37(6), 1371–1377.
Hellinga, C., Schellen, A. A. J. C., Mulder, J. W., van Loosdrecht, M. C. M., & Heijnen, J. J. (1998). The SHARON process: an innovative method for nitrogen removal from ammonium-rich wastewater. Water Science and Technology, 37, 135–142.
Wiesmann, U. (1994). Biological nitrogen removal from waste water. Advances in Biochemical Engineering, 51, 113–154.
Anthonisen, A. C., Loehr, R. C., Prakasam, P. B. S., & Srinath, E. G. (1976). Inhibition of nitrification by ammonia and nitrous acid. J. WPCF, 48, 835–852.
Wang, L., Zhu, J., & Miller, C. (2009). Nitrite accumulation from swine wastewater in sequencing batch reactor. Transactions of the ASABE, 52(4), 1363–1370.
Zimmerman, R. A., Bradshaw, A. T., Richard, D. (2004). Acclimation of nitrifiers for activated sludge treatment: A bench scale evaluation. WERF report: Treatment Processes (00-CTS-16ET), p. 72.
Inc, H. (2005). DR 2800 spectrophotometer procedures manual (1st ed.). Loveland: Hach.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). New York: APHA.
Peng, Y., Yu, D. S., Liang, D., & Zhu, G. (2004). Nitrogen removal via nitrite from seawater contained sewage. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 39(7), 1667–1680.
Ganiguéa, R., Lópeza, H., Balaguera, M. D., & Colprima, J. (2007). Partial ammonium oxidation to nitrite of high ammonium content urban landfill leachates. Water Research, 41(15), 3317–3326.
Sall, J., Creighton, L., & Lehman, A. (2005). JMP start statistics: A guide to statistics and data analysis using JMP and JMP IN Software. Cary: SAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Zhu, J. & Miller, C. The Stability of Accumulating Nitrite from Swine Wastewater in a Sequencing Batch Reactor. Appl Biochem Biotechnol 163, 362–372 (2011). https://doi.org/10.1007/s12010-010-9044-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-9044-7