Abstract
The commercial Saccharomyces cerevisiae Uvaferme 299 wine yeast was immobilized on cork pieces to produce a biocatalyst for use in dry red wine making. The immobilized biocatalyst was suitable for clarified and non-clarified grape must fermentation at ambient and low temperatures (0–25 °C). Fermentation times were low and very low amounts of residual sugar were detected, showing suitability for dry wine production. The presence of suspended solids in the non-clarified must did not affect the activity of the immobilized cells. Complete fermentation of sugars at 0 °C was possible with immobilized Uvaferme 299, although not a cryotolerant strain, indicating a cryoprotective effect of cork. The presence of cork did not affect the composition of major volatiles with methanol and acetaldehyde kept at low levels. Reduction of amyl alcohols on total volatiles was also observed in wines fermented at low temperatures. Differences in the headspace aroma profile in wines produced by immobilized and free cells were found by GC–MS analysis, but no cork taint compounds were detected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of immobilized cells in food fermentation processes has been extensively studied due to the well-known technical and economic advantages of immobilized cells compared to free cell systems [1]. Nevertheless, in the case of wine making, full industrial use is limited to the production of sparkling wines [2, 3]. To satisfy the demand for clean technologies and consumer acceptance, various food grade, natural materials like cellulose [4–6], gluten [7], and fruit pieces [8, 9] have been proposed as yeast immobilization supports for use in wine making and brewing processes. The immobilized cells were found suitable for rapid, ambient, or very low-temperature fermentation, producing wines and beers with fine and distinct characteristics. Taking into account that the raw material for wine making is grapes, the use of grape products as yeast immobilization supports, such as spent skins [10] or whole raisin berries [11, 12], was also evaluated. These materials are fully compatible with wine, safe, and their use led to products by faster fermentation, accelerated maturation, and good sensory characteristics. Furthermore, for such materials consumer acceptance is not an issue. For the same reasons, cork was also considered as support for yeast immobilization for use in wine making.
Cork is an inflammable natural product whose composition protects it from insect and microbial infection. In addition, due to its physical properties like flexibility, lightness, impermeability to polar liquids and gases, inertness, and resistance to heat, cork is the traditional and prevalent material used as wine bottle stopper [13]. Therefore, its contact with wine is acceptable since it has been traditionally used in wine making for centuries. The cork used for this purpose is made from the outer bark of the Querqus suber tree, which grows mainly in Mediterranean countries [13]. It consists of 14 sided cells with about 30 strong walls, which contain a waxy substance, suberin, which is highly impermeable to gases and water [14]. The porous structure of cork makes it ideal for cell entrapment. Therefore, the aim of this study was to investigate the suitability of cork as yeast immobilization support for use as biocatalyst in wine making at ambient and low temperatures.
Materials and Methods
Yeast Strain and Fermentation Media
Dried Saccharomyces cerevisiae Uvaferme 299, which is widely used in industrial wine making, was used in the present study. Saint Denis de l’ Hotel LSDH-BP 10 grape must from red grapes was used, which does not contain sucrose, pigments, or preservatives. It was adjusted to an initial 11.5°Be density (approximately 196 g of sugar per liter). Cylindrical commercial cork pieces were used as support for yeast immobilization.
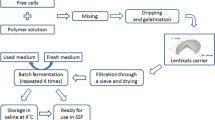
Yeast Immobilization and Fermentation Experiments
Total 25 pieces of cork (apparent volume 100 mL) were placed in a 500-mL glass cylinder and covered with 300 mL of clarified and pasteurized must in which 10 g of dried yeast were suspended. The total bioreactor volume was 400 mL. The system was allowed to ferment at 30 °C. The amount of cells immobilized on cork pieces at this stage was estimated by an indirect gravimetric method as described by Tsakiris et al. [11]. It was calculated that approximately 2.5 g of yeast (dry weight) were immobilized on the 25 pieces of cork.
Totally, 16 repeated fermentation batches of must were performed at variable temperatures (initially 25 °C, then reduced to 0 °C for immobilized cells or 6 °C for free cells, and then increased again to 15 and 25 °C). After the end of each fermentation batch, the produced wine was collected and the immobilized biocatalyst was washed with fresh grape must, and 300 mL of grape must were added for the next fermentation batch. In a similar way fermentation batches were carried out with free yeast cells, adding 300 mL of grape must and 2.5 g of dry yeast in a 500 mL glass cylinder. Each time, 7.5 g of wet weight yeast cells were maintained in the bioreactor and fresh must was added for the next fermentation batch. In the same manner, repeated fermentation batches were carried out with non-pasteurized and non-clarified must of the Greek variety Savvatiano to evaluate the effect of suspended solids on cell immobilization and fermentation kinetics.
Assays
Fermentation kinetics were performed by measuring the residual sugar (°Be density) at various time intervals. The final products were collected and analyzed for ethanol, residual sugar, acidity, and volatile by-products. Total acidity of the wines (expressed as g tartaric acid/L) was determined by titration with 0.1 N NaOH. Volatile acidity (expressed as g acetic acid/L) was determined by titration with 0.1 N NaOH of the distillates obtained after steam distillation of the wine samples. Residual sugar was determined by high-performance liquid chromatography (HPLC), using a HPLC Shimadzu chromatograph (Kyoto, Japan) with a SCR-101 N stainless steel column, a LC-9A pump, a CTO-10A oven at 60 °C and a RID-6A refractive index detector. Three times distilled water was used as mobile phase with a flow rate of 0.8 mL/min and 1-butanol (Sigma-Aldrich, Poole, UK) was used as an internal standard. Residual sugar concentrations were calculated using standard curves.
Determination of Volatiles
Ethanol and methanol were determined by gas chromatography on a Shimadzu GC-8A Gas Liquid Chromatograph (Kyoto, Japan) with a Porapac-S column and a FID detector. Nitrogen was used as carrier gas at 40 mL/min. The column, injector/detector temperatures were 130 and 210 °C, respectively. For the ethanol and methanol determination, a total volume of 2 μL for each sample was injected directly into the column. 1-Butanol (0.5% v/v) was used as internal standard. Ethanol and methanol concentrations were calculated using standard curves.
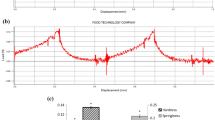
The volatile compounds acetaldehyde, ethyl acetate, 1-propanol, isobutanol, and amyl alcohols, were determined by gas chromatography on a Shimadzu GC-8A instrument with a stainless steel column packed with Escarto-5905 (5% squalene, 90% Cabowax-300, and 5% Bis(2-ethylhexyl) sebacate). Nitrogen was used as carrier gas at 20 mL/min. The injection port and FID detector temperatures were 210 °C, and the column temperature was 80 °C. The internal standard was 1-butanol (0.5% v/v). Samples of 4 μL were injected directly in the column, and the concentrations of the above compounds were calculated using standard curves (Fig. 1).
GC–MS Analysis
The volatile constituents of the wines produced at 15 °C by S. cerevisiae Uvaferme 299, free and immobilized on cork pieces, were determined by means of gas chromatography–mass spectroscopy (GC–MS) [15]. More specifically, the volatiles were isolated by the headspace solid-phase micro-extraction (SPME) technique. The fiber used for the absorption of volatiles was a 2 cm fiber coated with 50/30 mm divinylbenzene/carboxen on poly-dimethyl-siloxane bonded to a flexible fused silica core, (Supelco, Bellefonte, PA, USA). The conditions of headspace-SPME sampling were as follows: 10 mL liquid sample, 3 g NaCl and internal standard (4-methyl-2-pentanol) were transferred into a 20-mL headspace vial fitted with a teflon-lined septum sealed with an aluminum crimp seal. The contents were magnetically stirred for 5 min at 60 °C, and then the fiber was exposed to the headspace for 45 min. The length of the fiber in the headspace was kept constant. Desorption of volatiles took place in the injector of the gas chromatograph in the splitless mode, at 240 °C for 3 min. Before each analysis, the fiber was exposed to the injection port for 5 min to remove any volatile contaminants. GC/MS analysis was performed on a Shimadzu GC-17A gas chromatograph coupled to a Shimadzu MS QP5050 mass spectrometer. Helium was used as carrier gas (1.8 mL/min). Separation of compounds was performed on a capillary column (Supelco Wax-10, 60 m, 0.32 mm i.d., and 0.25 μm film thickness). Oven temperature was programmed at 35 °C for 6 min and then it was raised to 60 °C with a rate of 2 °C/min, held constant for 5 min, raised to 200 and 250 °C with a rate of 5 and 25 °C/min, respectively. Finally, it was held at 250 °C for 6 min. The injector and interface temperatures were set at 240 °C. The mass spectrometer was operated in scan range 45–400 m/z. Identification of the compounds was effected by comparing (1) the Kovtas retention indices based on the even n-alkanes (C10–C24) with those of standard compounds and by the literature Kovats retention indices and (2) MS data with those of standard compounds and by MS data obtained from NIST107, NIST21, and SZTERP libraries. The concentrations of volatiles were determined by comparisons with the area of the internal standard and not by separate pure standard solutions of each volatile compound (semi-quantitative analysis). Specifically, analysis was performed by dividing the peak area of a compound with the peak area of the internal standard and multiplying the result with the concentration of the internal standard (1.62 mg/L).
Results and Discussion
Yeast cells immobilized on cork pieces were found suitable for dry red wine making at ambient and low temperatures (25–0 °C). Repeated batch fermentations were carried out using grape must with an initial sugar concentration of 196 g/L, while the temperature was successively shifted from 25 to 0, to 15, and finally 25 °C. The fermentation temperature affected the fermentation times, with immobilized cells being able to ferment sugars completely at 15 and 25 °C within 7 and 5 days, respectively, as were the free yeast cells, yielding an alcohol concentration of about 11.5% v/v. In both cases the fermentation slowed down at 6 °C requiring fermentation times of at least 30 days (Tables 1 and 2 and Fig. 2), which was expected since the strain used was not cryotolerant. Fermentation below 6 °C was possible only with immobilized cells (Table 1, Fig. 2), which is an important observation indicating a cryoprotective effect of the carrier. This was not observed in previous studies using grape skins or grape raisins as immobilization supports [11, 12]. In all cases, very low concentrations of residual sugars were detected (<0.1 g/L) showing suitability of biocatalysts for low temperature wine making. The wines produced were of fine clarity and contained ethanol at concentrations similar to those of dry table wines. Acidities were also low (0.45 g/L expressed as acetic acid). In Fig. 2, it can be observed that there were no significant differences in the fermentation kinetics of non-clarified Savviatiano and Saint Denis grape musts using yeast immobilized on cork pieces. Therefore, the fermentation of a non-clarified must, as in industrial practice, using the proposed biocatalyst is feasible, yielding similar productivities with the clarified must, which is used in most research studies. This result indicates that the presence of suspended solids in the non-clarified must did not affect the efficiency of the biocatalyst by potential occlusion of the cork pores that would inhibit the action of yeast entrapped in their interior.
Volatiles
The major compounds defining the overall volatile effects on wine aroma are acetaldehyde, ethyl acetate, and higher alcohols such as 1-propanol, isobutyl alcohol, and amyl alcohols [16]. The effect of temperature and immobilization technique on the concentrations of these compounds in the produced wines is summarized in Tables 1 and 2. The fermentation temperature affected significantly the concentrations of amyl alcohols and acetaldehyde, while it did not affect ethyl acetate, 1-propanol, isobutyl alcohol, and methanol. Acetaldehyde, ethyl acetate, and higher alcohols were found at concentrations that contribute to pleasant flavors of wines [17, 18]. Acetaldehyde concentration was low although fermentations were carried out in the absence of SO2. The observed significant reduction of amyl alcohol concentrations with the drop of fermentation temperature (Fig. 3) agrees with several previous studies, and is important since it enhances the effects of minor aroma compounds [15, 19–21]. No significant differences were observed on the formation of volatiles between free and immobilized cells.
In total, 45 compounds were detected by SPME GC/MS analysis (Table 3), of which 33 were identified in the headspace of wines made by free cells and 40 in wines made by immobilized cells at 15 °C. Semi-quantitative analysis showed that the immobilized cells produced significantly higher concentrations of esters and other compounds that contribute to pleasant wine aromas.
The major ester detected by GC was ethyl acetate. Other esters present in all samples were those of ethyl esters of fatty acids, such as ethyl hexanoate, ethyl dodecanoate, and ethyl octanoate known as “fruity esters” [22], which were detected in higher concentrations in wines made with immobilized cells. Esters of aliphatic acids like decanoic, dodecanoic, tetradecanoic etc., were also detected, which are responsible for yeasty tones [23] and characterize wines of early aging [24]. Acetate esters other than ethyl acetate, like 3-methylbutyl acetate, hexyl, and octyl acetates, which impart pleasant fruity aromas were also detected [17]. Two important esters with pleasant aromas, 2-phenylethyl acetate (banana–apple aroma), and ethyl-9-decenoate [18, 25] were detected in all samples, but their concentrations were two times higher in wines made with immobilized cells.
Fatty acids, which mainly contribute to the complexity of wine aroma at concentrations not higher than their threshold values [25, 26], such as hexanoic, octanoic, and decanoic acid, were detected in all wines but again in higher concentrations in the case of immobilized cells.
Alcohols like 2-phenylethanol and terpene alcohols, as well as terpene compounds, are generally described with pleasant aromas [25], and they were detected in all wine samples. These compounds are mainly derived from the grape, are variety dependent, and are principal components that contribute to the characteristic aroma of a wine [27]. For example linalool (flowery) and β-citronellol (citrus, sweet, floral note), were detected, the second at levels much higher than its perception threshold (0.018 mg/L) [18, 27, 28]. Finally, important carbonyl compounds were detected, such as benzaldehyde (bitter almond) and β-damascenone (complex odor of flowers, tropical fruit, and stewed apple) [18, 24].
The SPME GC/MS analysis did not show presence of compounds that are responsible for the cork off-odor contamination of wine, known as cork taint [13]. The main compound responsible for this defect is 2,4,6-trichloroanisole [29]. However, other compounds such as 1-octen-3-ol, 1-octen-3-one, guaiacol, 2-methylisoborneol [30], 2-methoxy-3,5-dimethylpyrazine [31], and more recently 2,4,6-tribromoanisole [32] have also been reported as being responsible for cork taint. Their presence in wines is related to physical factors or to cork production treatments [33]. These compounds were not detected in the wines of the present study; therefore, the proposed technique does not aggravate wine with undesirable flavor compounds.
Conclusions
The above study demonstrates that cork pieces can be used for the development of a cost-effective wine-making process involving immobilization of yeast. They are a cheap, safe, and easily available raw material. Their use demands no pre-treatment and the immobilization technique is a natural process. The immobilized biocatalyst is operationally stable, which makes possible its use at commercial scale, even in very low-temperature processes without the need for cryotolerant strains. For industrial purposes it is also possible to use spent pieces from cork production and treatment, which are of negligible cost since only a very small proportion is recycled today by agglutination. Finally, for red wine making using the proposed technique (yeast cells immobilized on a solid support) a modification of the batch bioreactor can be used to separate the grape skins used for color extraction, from the biocatalyst and the fermenting grape must as shown in a previous study [34]. Color extraction is possible in such low fermentation conditions, but longer times of contact of the skins with the fermenting liquid are required, which is a minor drawback compared with the improved overall quality of wines produced at low temperatures.
References
Kourkoutas, Y., Bekatorou, A., Banat, I. M., Marchant, R., & Koutinas, A. A. (2004). Food Microbiology, 21, 377–397.
Fumi, M. D., Trioli, G., Colombi, M. G., & Colagrande, O. (1988). American Journal of Enology and Viticulture, 39, 267–272.
Colagrande, O., Silva, A., & Fumi, M. D. (1994). Biotechnology Progress, 10, 2–18.
Bekatorou, A., Soupioni, M. J., Koutinas, A. A., & Kanellaki, M. (2002). Applied Biochemistry and Biotechnology, 97, 105–121.
Ikonomopoulou, M., Kanellaki, M., Soupioni, M., & Koutinas, A. A. (2003). Applied Biochemistry and Biotechnology, 104, 23–36.
Iconomou, L., Kanellaki, M., Voliotis, S., Agelopoulos, K., & Koutinas, A. A. (1996). Applied Biochemistry and Biotechnology, 60, 303–313.
Bardi, E. P., Bakoyianis, V., Koutinas, A. A., & Kanellaki, M. (1996). Process Biochemistry, 31, 425–430.
Kourkoutas, Y., Komaitis, M., Koutinas, A. A., & Kanellaki, M. (2001). Journal of Agricultural and Food Chemistry, 49, 1417–1425.
Bekatorou, A., Sarellas, A., Ternan, N. G., Mallouchos, A., Komaitis, M., Koutinas, A. A., et al. (2002). Journal of Agricultural and Food Chemistry, 50, 7249–7257.
Mallouchos, A., Reppa, P., Aggelis, G., Koutinas, A. A., Kanellaki, M., & Komaitis, M. (2002). Biotechnology Letters, 24, 1331–1335.
Tsakiris, A., Bekatorou, A., Koutinas, A. A., Marchant, R., & Banat, I. M. (2004). Food Chemistry, 87, 11–15.
Tsakiris, A., Kourkoutas, Y., Dourtoglou, V. G., Koutinas, A. A., Psarianos, C., & Kanellaki, M. (2006). Journal of the Science of Food and Agriculture, 86, 539–543.
Ezquerro, O., & Tena, M. T. (2005). Journal of Chromatography A, 1068, 201–208.
Riboulet, J. M., & Alegoet, C. (1986). In aspects pratiques du bouchage des vins. Chaintre: Collection Avenir Œenology, Bourgogne Publications.
Kandylis, P., Goula, A., & Koutinas, A. A. (2008). Journal of Agricultural and Food Chemistry, 56, 12037–12045.
Regodón Mateos, J. A., Pérez-Nevado, F., & Ramírez Fernández, M. (2006). Enzyme and Microbial Technology, 40, 151–157.
Rapp, A., & Mandery, H. (1986). Wine aroma. Experentia, 42, 873–884.
Ribérau-Gayon, P., Glories, Y., Maujean, A., & Dubourdieu, D. (2000). Hanbook of Enology (Vol. 2). Baffins Lane: Wiley. pp. 41–54, 189, 193–196.
Loukatos, P., Kiaris, M., Ligas, I., Bourgos, G., Kanellaki, M., Komaitis, M., et al. (2000). Applied Biochemistry and Biotechnology, 89, 1–13.
Vidrih, R., & Hribar, J. (1999). Food Chemistry, 67, 287–294.
Kandylis, P., & Koutinas, A. A. (2008). Journal of Agricultural and Food Chemistry, 56, 3317–3327.
Miranda-Lopez, R., Libbey, L. M., Watson, B. T., & McDaniel, M. R. (1992). Journal of Food Science, 57, 985–993.
Molnar, I., Oura, E., & Suomalainen, H. (1981). Acta Alimentaria, 10, 27–36.
Pisarnitskii, A. F. (2001). Applied Biochemistry and Microbiology, 37, 552–560.
Etievant, X. P. (1991). In H. Maarse (Ed.), Volatile compounds in foods and beverages (pp. 483–533). New York: Marcel Dekker.
Jackson, R. S. (1994). In wine science: principles and applications. San Diego: Academic Press.
Vilanova, M., & Sieiro, C. (2006). Journal of Food Composition and Analysis, 19, 694–697.
Pena-Alvarez, A., Capella, S., Juárez, R., & Labastida, C. (2006). Journal of Chromatography A, 1134, 291–297.
Vasserot, Y., Pitois, C., & Jeandet, P. (2001). American Journal of Enology and Viticulture, 52, 280–281.
Darriet, P., Pons, M., Lamy, S., & Dubourdieu, D. (2000). Journal of Agricultural and Food Chemistry, 48, 4835–4838.
Simpson, R. F., Capone, D. L., & Sefton, M. A. (2004). Journal of Agricultural and Food Chemistry, 52, 5425–5430.
Chatonnet, P., Bonnet, S., Boutou, S., & Labadie, M. D. (2004). Journal of Agricultural and Food Chemistry, 52, 1255–1262.
Mazzoleni, V., Caldentey, P., Careri, M., Mangia, A., & Colagrande, O. (1994). American Journal of Enology and Viticulture, 45, 401–406.
Tsakiris, A., Sipsas, V., Bekatorou, A., Mallouchos, A., & Koutinas, A. A. (2004). Journal of Agricultural and Food Chemistry, 52, 1357–1363.
Mallouchos, A., Loukatos, P., Bekatorou, A., Koutinas, A. A., & Komaitis, M. (2007). Food Chemistry, 104, 918–927.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsakiris, A., Kandylis, P., Bekatorou, A. et al. Dry Red Wine Making Using Yeast Immobilized on Cork Pieces. Appl Biochem Biotechnol 162, 1316–1326 (2010). https://doi.org/10.1007/s12010-009-8905-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8905-4