Abstract
Xylanases have important applications in industry. Immobilization and stabilization of enzymes may allow their reuse in many cycles of the reaction, decreasing the process costs. This work proposes the use of a rational approach to obtain immobilized commercial xylanase biocatalysts with optimized features. Xylanase NS50014 from Novozymes was characterized and immobilized on glyoxyl-agarose, agarose-glutaraldehyde, and agarose-amino-epoxy support and on differently activated chitosan supports: glutaraldehyde-chitosan, glyoxyl-chitosan, and epoxy-chitosan. Two different chitosan matrices were tested. The best chitosan derivative was epoxy-chitosan-xylanase, which presented 100% of immobilization yield and 64% of recovered activity. No significant increase on the thermal stability was observed for all the chitosan-enzyme derivatives. Immobilization on glyoxyl-agarose showed low yield immobilization and stabilization degrees of the obtained derivative. The low concentration of lysine groups in the enzyme molecule could explain these poor results. The protein was then chemically modified with ethylenediamine and immobilized on glyoxyl-agarose. The new enzyme derivatives were 40-fold more stable than the soluble, aminated, and dialyzed enzyme (70 °C, pH 7), with 100% of immobilization yield. Therefore, the increase of the number of amine groups in the enzyme surface was confirmed to be a good strategy to improve the properties of immobilized xylanase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing interest for researching on xylanases in the latest years is due to the several industrial applications of this enzyme [1–3]. This enzyme can hydrolyze β-1,4 glycosidic linkages of the xylan, main compound of hemicelluloses, to produce short chain xylooligosaccharides. The main practical applications of xylanases are in the bread and baking industry, pulp bleaching, production of xylooligosaccharides, and in the conversion of biomass into fermentable sugars for the ethanol production [4–6]. The lack of pentose-fermenting microorganism represents, however, a limiting factor in converting lignocelluloses into bioethanol. Therefore, the most promising strategy is to integrate production of valuable compounds, especially xylose, from hemicelluloses to production of ethanol from cellulose [6–10].
However, the use of enzymes as catalyst for industrial application may require the modification of them, especially to ease the recovering of the enzyme and to obtain a long operational stability. These features could be achieved through its immobilization [2, 4, 11]. It has been shown, however, that in many cases, several immobilization protocols have to be tested in order to achieve the thermal stability of the enzyme derivative [12–14]. The immobilization from xylanases for the industrial use has been reported by many authors lately [1–5], but no significant thermal stabilization of these biocatalysts has been described. In fact, an adequate solid support, as well as appropriate immobilization conditions, must be carefully selected in order to promote thermal stability of the enzyme [12–14]. Covalent multipoint attachment of enzymes to solid supports has been shown to increase the resistance of enzymes to high temperatures and denaturants solvents [11, 15, 16]. Multipoint covalent immobilization requires the interaction of several residues of the same enzyme molecule with active groups of the support. The formed bonds will increase the rigidity of a small part of the molecule surface (e.g., 10–20%), which will make more rigid the overall three-dimensional structure. Then, enzyme stabilization is achieved. Aldehyde and/or oxirane groups in the support and amine groups in the enzyme are a good choice to promote the multipoint attachment of the enzyme molecule and, therefore, to obtain highly stable enzyme derivatives [17]. Oxirane groups may react with amine, acid, and thiol groups. The formation of several Schiff bases between amine groups of the same enzyme molecule and aldehyde groups of the support has been shown to be a successful strategy but will require the presence of several amine groups on the enzyme surface, that is, lysine residues [7, 11, 16]. If the enzyme has low number of lysine residues, the increase of the number of amine groups on the enzyme surface can be achieved by amination of the soluble enzyme, through reaction of the carboxylic groups of the protein surface with ethylenediamine [18].
Chitosan is a cheap and abundant linear polysaccharide composed of randomly distributed β-(1-4)-linked d-glucosamine (deacetylated) and N-acetyl-d-glucosamine (acetylated) units. This cationic biomaterial is produced by deacetylation of chitin, a polymer present in the cell wall of fungi and in the shells of shellfish (mainly crab, shrimp, lobster, and krill), wastes of the seafood industry. The water-insoluble chitin, after removal of more than 60% of the acetyl groups, is called chitosan and becomes soluble at acidic pH, due to the presence of the charged amine groups. At basic pH, the amine groups of chitosan become uncharged (pK a 6.5), and the polymer is insoluble in aqueous medium, forming a gel. The coagulation conditions as well as the desacetilation degree of the chitosan powder may also influence in the internal support structure [19]. Chitosan has reactive amino and hydroxyl groups which, after further chemical modifications, can make covalent bonds with reactive groups of the enzyme. Due to the amine groups, chitosan is a cationic polyelectrolyte (pK a 6.5), being insoluble in water but soluble at acidic solutions below pH about 6.5. The mechanical properties of this polymer can be improved by further cross-linking using bifunctional reactants, like glutaraldehyde [20]. In order to make bonds with the enzyme, the amine groups can directly react with glutaraldehyde to generate aldehyde groups, which will form the Schiff bases with the enzyme. The hydroxyl groups can be also activated by using epoxy reactants like glycidol and epichloridrin, for instance, followed by oxidation with sodium periodate, to generate reactive aldehyde glyoxil groups [17].
Agarose gels are easily handled and activated due to presence of hydroxyl groups. They have a high surface area, an adequate porous diameter for protein immobilization, and good mechanical properties [16]. Besides, the large surface of agarose gels allows the formation of several bonds between the aldehyde groups of the support and the deprotonated terminal and lysine amine groups of the same enzyme molecule. The higher the number of bonds between enzyme and support, the more rigid the enzyme structure, and therefore the higher the thermal stability of the immobilized enzyme. Glyoxyl-agarose has been successfully used for immobilization and stabilization of several enzymes [21–23]. Glyoxyl groups have low reactivity and require the formation of at least two bonds between enzyme and support, which implies to the need of immobilization not only using the terminal amine group but also the reactive (deprotonated) amine groups of the lysine residues of the enzyme. As the pK of lysine residues is 10.5, the immobilization has to be performed at alkaline pH values, and only enzymes that can tolerate that high pH values would take advantage of the immobilization in glyoxyl-agarose. As xylanases are used in the pulp bleaching, they may be stable at this alkaline immobilization condition.
The main purpose of this work was to test different supports (agarose and chitosan) and activating agents (glycidol, glutaraldehyde, epichloridrin, and ethylenediamine), which could bring not only the insolubilization of the enzyme but also the increase of its thermal stability. The influence of the amination of the soluble enzyme and of two different coagulating agents (NaOH and KOH) for chitosan on the immobilization results was also tested.
Materials and Methods
Materials
Crude commercial xylanase NS50014, with 500 U/mL of enzyme extract, was kindly donated from Novozymes A/S (Denmark). Birchwood xylan was purchased from Sigma-Aldrich S.A. (USA). Chitosan with desacetilation degree of 85.2% was purchased from Polymar Ind. Com. Imp. Exp. Ltda (Brazil). Agarose 6BCL beads was purchased from Amersham Pharmacia Biotech AB (Uppsala, Sweden), and 1-ethyl-3-(3-dimethyl aminepropyl) carbodiimide (EDAC), sodium borohydride, and ethylenediamine (EDA) were supplied by Sigma-Aldrich S.A. (St. Louis, MO, USA). Glutaraldehyde 25% (v/v) was purchased from Vetec- SP (Brazil); glycidol and epichlorohydrin (1-chloro-2,3-epoxy) were purchased from Sigma Chemical Company (St Louis, MO, USA). All other reagents were of analytical grade.
Methods
Determination of Xylanase Activity
Xylanase activity was determined according to the IUPAC definition [24] by measuring along the time of the release of reducing sugars from the hydrolysis of birchwood xylan (1% w/v) catalyzed by the enzyme. Reducing sugars were quantified using the dinitrosalicylic acid method [25]. Birchwood xylan (1% w/v) was incubated at 50 °C with diluted enzyme solutions in sodium acetate buffer 50 mM, pH 5.5, for 10 min. One unit of activity was defined as amount of enzyme required to release 1 µmol of xylose from birch wood xylan in 1 min under these conditions.
Protein concentrations were determined by Bradford method [26], with BSA as standard.
Dialysis and Amination of the Commercial Enzyme
In order to avoid interference of enzyme additives on the immobilization results, 10 mL of commercial xylanases was dialyzed using cellulose acetate membrane against 1 L of a 5 mM potassium phosphate buffer, pH 7, at 4 °C for 24 h.
Soluble xylanases were chemically modified according Hoare and Koshland [27]. Soluble enzyme (0.6 mL) was added to 5.4 mL of 1 M EDA at pH 4.75. Solid EDAC (10 mM) was then added to the solution. After 120 min of gentle stirring at 25 °C, the solution was dialyzed in cellulose acetate membrane against 1 L of a 5 mM potassium phosphate buffer, pH 7, at 4 °C for 24 h. The dialyzed enzyme was immediately immobilized. Amination in the presence of polyethylene glycol (PEG) was similarly performed. In this case, a concentration of 1:10 (mass of PEG per mass of protein) of polyethylene glycol was added to the enzyme solution.
During the amination processes, samples were withdrawn, and the enzyme activities as well as protein content were determined as described above.
Determination of Temperature and pH Values for Maximum Activity of Xylanases
Enzyme activities of dialyzed and non-dialyzed enzyme were determined as described above, at different pH (from pH 3 to pH 11, at 50°) and temperature values (from 20 °C to 90 °C, pH 5.5).
Preparation of 2.5% Chitosan Gel
Chitosan powder was mixed to a 5% (v/v) acetic acid solution and stirred during 30 min. After that, solutions of 0.1 M KOH or 0.1 M NaOH were added, and the total volume was kept under agitation for 4 h. The particles formed were washed with distilled water and vacuum-filtered.
Activation of the Supports (Agarose and Chitosan Gels)
Glyoxyl-agarose
The agarose gel was activated as previously described [16]. Agarose gel was suspended in water (30 mL/105 g of agarose) and kept in an ice bath. NaOH solution (1.7 N), containing 28.5 mg of borohydride per milliliter of solution, previously cold prepared, was added to the agarose suspension (50 mL of NaOH solution/105 g of agarose). Afterward, glycidol was added dropwise (36 mL/105 g of agarose). The suspension was gently stirred at 25 °C for 16 h. Following that, the glyceril support was washed with water, filtered under vacuum, and sucked dry. Glyceril-agarose was suspended in water (895 mL/105 g of glyceril-agarose), and sodium periodate was added (3.21 g/105 g of glyceril-agarose). The suspension was kept under gentle stirring for 2 h in order to produce the possible maximum amount of glyoxyl groups in the support.
Glutaraldehyde-chitosan
Chitosan particles were reacted with 5% (v/v) glutaraldehyde prepared in 0.1 M sodium phosphate buffer, pH 7.0, during 1 h of agitation and 25 °C (v gel/v total of 1:10). After that, the support was washed with distilled water.
Epoxy-chitosan
Chitosan particles (10 g) were suspended in 100 mL of dimethylformamide and kept under gentle stirring for 30 min at 60 °C. Afterward, 0.8 g of KOH was dissolved in 3 mL of isopropanol and 10 mL of epichlorohydrin and added to the chitosan suspension. This suspension was kept under gentle stirring for 12 h at 60 °C. After that, the supports were washed with distilled water.
Glutaraldehyde-agarose
This support was prepared as described by Fernández-Lafuente et al. [28]. Glyoxyl-agarose (35 g) was suspended in 200 mL of 2 M ethylenediamine, pH 10.05, and kept under gentle stirring for 2 h at 25 °C. Sodium borohydride (10 mg/mL) was added. After 2 h of reaction, the particles were filtered and successively washed with 0.1 M sodium acetate buffer, pH 4, 0.1 M sodium borate buffer, pH 9, and distilled water. This support (20 g; MANAE-agarose) were suspended in 22.4 mL of 0.2 M sodium phosphate buffer, pH 7, and 33.6 mL of 25% (v/v) glutaraldehyde and kept under gentle stirring for 14 h. The particles were then washed with distilled water.
Amino-epoxy-agarose
Agarose (7 g) was suspended in 30 mL of 0.8 M NaOH containing 340 g of sodium borohydride. Acetone (11.4 mL) and 5.7 mL of epichlorohydrin were added and kept under agitation for 8 h and 25 °C. Further additions of epichlorohydrin were performed after 2 and 4 h [29]. The support obtained (epoxy-agarose) was washed with distilled water. For 10 g of this support, 60 mL of 2% (v/v) ethylenediamine, pH 7, was added and kept under agitation for 2 h.
Immobilization of Xylanases
The immobilization enzyme course was monitored by measuring the enzyme activity in the supernatant and in the whole suspension at different time intervals. Additionally, controls with soluble enzyme were used to determine the possible inactivating effect of the pH, temperature, or dilution on the enzyme during the immobilization. In all cases, the suspensions were prepared using relation 1:10 (volume of support/volume of suspension) and gently stirred at 25 °C at different times. The load of the supports was from 1 to5 mg of protein per gram of support to avoid mass transfer limitations.
Immobilization on glutaraldehyde supports
It was carried out in 25 mM sodium phosphate buffer, pH 7.0, for 3 h. To end the immobilization reaction, the preparations were washed with excess 25 mM sodium phosphate buffer, pH 7, and stored at 4 °C until further use.
Immobilization on glyoxyl supports
For the immobilization on highly activated glyoxyl-agarose support, the enzyme was diluted in 0.1 M sodium bicarbonate buffer, pH 10.05, and incubated for 24 h with the support. To end the reaction, sodium borohydride was added to the reaction up to a final concentration of 1 mg/mL and incubated for further 30 min under stirring [11]. Finally, the immobilized preparation was washed with an excess of 25 mM sodium phosphate, pH 7, and stored at 4 °C until further use.
Immobilization on heterofunctional amino-epoxy supports
Immobilization on amino-epoxy supports were performed in two steps as previously described [29]. The enzyme solution in 5 mM sodium phosphate buffer, pH 7.0, was incubated with the support for 12 h. After that, the gel was filtered and incubated with 0.1 M sodium bicarbonate at pH 10 for 12 h. To end the reaction, the remaining epoxy groups were blocked by incubation with 3 M glycine or aspartic acid, pH 8.0, for 12 h. The preparation was then thoroughly washed with 25 mM sodium phosphate buffer, pH 7, and stored at 4 °C until further use.
The parameters of immobilization procedure were defined as immobilization yield (IY), which is the ratio between the amount of immobilized enzyme and the amount of enzyme offered to immobilization, and the recovered activity (RA), which the ratio between the measured derivative activity and the theoretical immobilized activity.
Irreversible Thermal Inactivation of Xylanases
Soluble enzyme and suspensions of the derivatives prepared in 0.05 M sodium phosphate buffer, pH 5.5, were kept at 70 °C. For the derivatives of the previously aminated xylanases, the assay was performed in 0.05 M sodium phosphate buffer, pH 7, at 70 °C. Aliquots were withdrawn periodically, and their enzymatic activity was measured as described previously until half-life time (τ 1/2) could be determined.
Acid Hydrolysis of Soluble Xylanases and Amino Acid Analysis
Soluble xylanases (1 mg protein) were hydrolyzed in HCl (6.7 N) at 105–115 °C for 24 h. The mixture was filtrated and dried. The amino acid extract was dissolved in 0.2 N sodium citrate buffer, pH 2.2, for further amino acid analysis. Amino acids were analyzed by high-performance ion-exchange chromatography as previously described [11].
Results and Discussion
Characterization of Crude and Dialyzed Xylanases
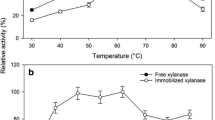
Crude and dialyzed enzyme contained, respectively, 13.4 and 7 mg of protein/mL. Enzyme activities of commercial and dialyzed xylanases were measured at different temperature (Fig. 1) and pH values (Fig. 2). Results shownin Fig. 1 indicate that commercial and dialyzed enzymes showed similar activity profiles at different temperatures. However, results in Fig. 2 indicates that the non-dialyzed xylanases showed to be quite stable at pH values in the range from 4 to 10, while the dialyzed xylanases presented a sharp profile with maximum activity around 7. That could be explained by the possible presence of additives in the commercial enzyme solution that protects it from activity lost.
Immobilization of Xylanases on Chitosan Gel
Influence of the Preparation Conditions of the Chitosan Particles and Immobilization Time
Chitosan 2.5% (w/v) gel particles were prepared using 0.1 M NaOH or 0.1 M KOH as coagulating agents. As mentioned before, the choice of the coagulating agent may influence on the characteristics of the chitosan gels [19]. Results presented in Table 1 indicated that the use of 0.1 M KOH as coagulating agent led to RAs higher than the ones obtained with the particles coagulated in NaOH solution. Probably, KOH led to a different internal structure of the gel particle with a better geometrical congruency between enzyme and support or larger porous. The IY was not influenced by the coagulant agent, but it was greatly influenced by the immobilization time (3 or 24 h). Although glutaraldehyde is more reactive at pH 10, and a higher enzyme load had been offered when the immobilization time was 3 h, only about 1.45 mg of protein was immobilized, while after 24 h, offering 2.5 mg of protein, around 98% of immobilization yield was obtained. The high reactivity of glutaraldehyde at pH 10 may have led to an intense polymerization of this reactant and increase of the support cross-linking between glutaraldehyde and amine groups of chitosan. Therefore, a smaller number of porous larger than the enzyme was produced at pH 10. Furthermore, a decrease of aldehyde groups available to react with the enzyme may have occurred, decreasing the immobilization rate. These results indicate that immobilization on chitosan activated with glutaraldehyde should be done at pH 7.
Influence of the Activating Agents and Methods
Chitosan matrices were activated using glutaraldehyde (glutaraldehyde-chitosan), glycidol (glyoxyl-chitosan), or epichloridrin (epoxy-chitosan). The results are shown in Table 2. The glyoxyl-chitosan supports presented the lowest immobilization yield and recovered activity. That could be explained by a poor concentration of lysine residues on the enzyme surface. Glyoxyl groups have low reactivity, which implies to the need of the formation of at least two bonds between the aldehyde groups in the support and the amine groups of the enzyme. At pH 7, only the terminal amine group of the enzyme is available to react. Immobilization through glyoxyl groups needs to be performed at pH above 10, in order to have lysine residues uncharged and, therefore, available to react. Therefore, a low number of lysine residues would imply to a low immobilization yield. The lowest recovered activity obtained when the support was glyoxyl-chitosan seems to be related to the presence of amine groups in the support. These groups may be reacting with the enzyme in a way that leads to an inactive form. Ionic interaction between positively charged amine groups of the support and acidic group of the enzyme could change the tridimensional structure of the enzyme. That could also explain the lowest stabilization factor obtained with glyoxyl activation. This hypothesis is reinforced by the results obtained with the activation with glutaraldehyde and epichlorohydrin. These agents are more reactive than glycidol. Therefore, during the activation procedure, they may be also reacting with the amine groups of chitosan, which would not be available to make the ionic interaction with the enzyme.
Immobilization of Xylanases on Agarose Gels
Agarose gels were activated with glycidol (glyoxyl-agarose), ethylenediamine, and glutaraldehyde (glutaraldehyde-agarose) or glycidol, ethylenediamine, and epichloridrin (amino-epoxy-agarose). The obtained results, shown in Table 3, also reinforced the previous hypotheses that there is an ionic interaction between amine groups in the support with the enzyme, which leads to an unstable enzyme form of the immobilized enzyme. It can be observed that when glyoxyl-agarose, which does not contain amine groups, was the support, the immobilized enzyme presents the same thermal stability of the soluble enzyme. On the other hand, when ethylenediamine was used, the support presents amine groups a less active form, which is obtained after immobilizing the enzyme. These results indicated that glyoxyl-agarose is the best support to immobilize xylanases. However, it did not obtain any increase in the thermal stability of the enzyme with immobilization. That may be explained by a low number of lysine residues in the enzyme. Therefore, it was decided to test a chemical modification of the xylanases in order to transform the carboxylic groups of the glutamic and aspartic residues in amine groups.
Amination of the Xylanases
Two different procedures where used for amination of the enzyme. In one case, the enzyme was chemically modified with no use of additives (crude aminated enzyme). In the other case, it was added PEG (1:10, mass of PEG per mass of protein) in order to protect the enzyme structure from excessive modification. The residual activities of the enzyme during the amination process are shown in Table 4.
As expected, the presence of PEG in the reaction medium protected the enzyme, decreasing the lost of activity due to amination, when compared with the reaction with no use of additives. It can be noticed, however, that the losses during the dialysis were in both cases around 40%.
Immobilization Rate of Xylanases
After amination assays, the enzyme immobilization was followed by measuring the enzyme activity in the supernatant. The immobilization yield was calculated, the activity of the immobilized enzyme was measured, and the recovered activity was calculated accordingly. Blank solution for the non-dialyzed xylanases showed that the enzyme did not lose activity in 24 h, while blank solution for the dialyzed enzyme showed that about 32% of the enzyme deactivated, due to pH conditions. The immobilization kinetics of both crude and dialyzed enzymes is shown in Fig. 3. Results of Fig. 3 indicated that crude and dialyzed enzyme, without amination, had a similar number of enzyme units that disappeared from the supernatant (theoretically immobilized). That suggests that the additives present in the crude xylanase solution do not interfere in the immobilization process. In both cases, the amount of enzyme immobilized was very small (10% and 13%). The immobilization yields of both chemically modified enzymes (in the presence and absence of PEG) were, on the other hand, after 5-h reaction, very high (96% and 100% ,respectively), which indicated that all the enzyme was quickly immobilized on the support, probably due to the presence of amino groups, which were introduced through amination reaction.
Thermal Inactivation of Xylanases
The half-life times of soluble and immobilized enzymes were investigated at 69 °C, pH 7. The results obtained for soluble, crude, and dialyzed xylanases are shown in Fig. 4. As can be observed, the dialysis step led to a lower thermal stability for the enzyme. The half-life times (τ 1/2) were 90 and 45 min for crude and dialyzed xylanases, respectively. Probably, many protein preservative substances that are usually present in the crude commercial enzyme solution are lost during the dialysis, and the enzyme will be more fragile to the action of external factors as extreme pH values and high temperatures [12, 15, 16].
The thermal inactivation of the dialyzed immobilized enzyme was then compared to the one obtained for the crude immobilized xylanases. As the presence of additives showed to be important for the thermal stability of the enzyme and these additives were not present anymore after enzyme immobilization, it was possible that the low stabilization factor observed for the crude immobilized enzyme was due to the absence of additives. In Fig. 5, the thermal stability of the immobilized enzymes, without the interference of additives, can now be compared. Similar residual activity profiles are observed for both forms, crude and dialyzed enzyme derivatives. Half-life times for both immobilized enzymes were the same, which was about 3.5 h. Therefore it can be concluded that the immobilization process did not really lead to an increase in the stability factor. The low number of lysine residues in the xylanases may be responsible for this result. A significant increase in the thermal stability of the enzyme is expected to occur when multipoint attachment is obtained. Increase in the half-life of the immobilized enzyme of 500 times to Alcalase, 8,000 times to Penicylin G Acylase, and 9,000 times to chymotrypsyn were already reported [12, 17]. However, this kind of immobilization requires a high number of amine groups on the enzyme surface. Amine groups were then introduced in the enzyme structure through amination. After the amination step, the enzyme was covalently immobilized on glyoxyl-agarose.
Figure 6 shows results of thermal inactivation for the immobilized xylanases, aminated in the presence of PEG and aminated in absence of PEG and soluble xylanases, crude, and dialyzed. A significant increase of the thermal stability of the aminated forms can be observed. It can also be noticed that the presence of PEG as protective agent for the enzyme was not favorable for the stabilization, although it was important to diminish the lost of activity during the amination step, as discussed in “Immobilization of Xylanases on Chitosan Gel”. The presence of PEG probably did not allow the complete chemical modification of the carboxylic groups, and a lower number of amine groups were added to the enzyme surface. The xylanases were then partially aminated. When no PEG was used, a complete chemical modification could be achieved, which allowed a more intense multipoint covalent attachment of the enzyme to the support. The half-life times at 70 °C and pH 7 were 6 h for the partially aminated immobilized xylanases and 30 h for the complete aminated enzyme. The multipoint covalent attachment represented a stabilization gain of 40 times, when compared with the dialyzed xylanase.
Conclusions
Immobilization of xylanases was performed in different prepared supports of chitosan and agarose gels. There was no significant gain on thermal stability for the derivatives immobilized on chitosan supports, and the best result was obtained when utilizing chitosan activated with epichlorohydrin. This derivative presented 100% of immobilization yield, 46% of recovered activity, and a stabilizing factor of 1.3. Glyoxyl-agarose showed to be a good support, but poor results were obtained with the immobilization of xylanase. The hypothesis of a low number of lysine residues on the enzyme surface was then investigated. Amine groups were introduced on the enzyme structure using ethylenediamine, in the presence and absence of PEG. The use of PEG aimed to protect the enzyme against a too high grade of chemical modification. The aminated enzyme, in the absence of PEG, after immobilization, led to xylanases 40-fold more stable than the soluble dialyzed enzyme at 70 °C and pH 7. Therefore, the increase of the number of amine groups in the enzyme surface was confirmed to be a good strategy to improve the properties of immobilized xylanase.
References
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Applied Microbiology and Biotechnology, 56, 326–338.
Ali, Z., Jiang, Z., Li, L., Deng, W., Kurakabe, I., & Li, H. S. (2005). Process Biochemistry, 40, 2707–2714.
Shah, S., & Gupta, M. N. (2008). Biochemical et Biophysical Acta, 1784, 363–367.
Gawade, P. W., & Kamat, M. Y. (1998). Journal of Biotechnology, 66, 165–175.
Cano, A., & Palet, C. (2007). Journal of Membrane Science, 291, 96–105.
Válquez, M. J., Alonso, J. L., Domíngues, H., & Parajó, J. C. (2000). Trends in Food Science & Technology, 11, 387–393.
Kristensen, J. B., Börjesson, J., Bruun, M. H., Tjerneld, F., & Jörgensen, H. (2007). Enzyme and Microbial Technology, 40, 888–895.
Tamanini, C., & Hauly, M. C. O. (2004). Ciências Agrárias, 25(4), 315–330.
Kastner, J. R., Eiteman, M. A., & Lee, S. A. (2001). Glucose repression of xylitol production in Candida tropicalis mixed-sugar fermentations. Biotechnology Letters, 23, 1663–1667.
Hahn-Hägerdal, B., Galbe, M., Gorwa-Grauslund, M. F., Liden, G., & Zacchi, G. (2006). Trends in Biotechnology, 24, 550–556.
Manrich, A., Galvão, M. A., Jesus, C. D. F., Giordano, R. C., & Giordano, R. L. C. (2008). International Journal for Biological Macromolecules, 43, 54–61.
Mateo, C., Palomo, J. M., Lorente, G. F., Guisán, J. M., & Fernandez-Lafuente, R. (2007). Enzyme and Microbial Technology, 40, 451–1463.
Lopez-Gallego, F., Betancor, L., Hidalgo, A., Mateo, C., Guisán, J. M., & Fernandez-Lafuente, R. (2004). Journal of Biotechnology, 11, 219–227.
Lopez-Gallego, F., Montes, T., Fuentes, N. A., Grazu, V., Betancor, L., Guisán, J. M., et al. (2005). Journal of Biotechnology, 116, 1–10.
Wong, S. S., & Wong, L. J. C. (1992). Enzyme and Microbial Technology, 14, 866–874.
Guisán, J. M. (1988). Enzyme and Microbial Technology, 10, 375–382.
Adriano, W. S., Mendonça, D. B., Rodrigues, D. S., Mammarella, E. J., & Giordano, R. L. C. (2008). Biomacromolecules, 9, 2170–2179.
Fernández-Lafuente, R., Rosell, C. M., Alvaro, G., & Guisán, J. M. (1992). Enzyme and Microbial Technology, 14, 489–495.
Berger, J., Reist, M., Mayer, M. J., Felt, O., & Gurny, R. (2004). European Journal of Pharmaceutics and Biopharmaceutics, 57, 35–52.
Krajewska, B. (2004). Enzyme and Microbial Technology, 35, 126–139.
Tardioli, P. W., Fernandez-Lafuente, R., Guisan, J. M., & Giordano, R. L. C. (2003). Biotechnology Progress, 19, 565–574.
Tardioli, P. W., Pedroche, J., Giordano, R. L. C., Fernandez-Lafuente, R., & Guisán, J. M. (2003). Biotechnology Progress, 19, 352–360.
Rodrigues, D. S., Mendes, A. A., Adriano, W. S., Gonçalves, L. R. B., & Giordano, R. L. C. (2008). Journal of Molecular Catalysis B Enzymatic, 51, 100–109.
Ghose, T. K. (1987). Pure and Applied Chemistry, 59, 257–268.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Hoare, D. G., & Koshland, D. E. (1967). Journal of Biological Chemistry, 242, 2447–2453.
Fernandez-Lafuente, R., Rodriguez, V., & Guisán, J. M. (1998). Enzyme and Microbial Technology, 23, 28–33.
Bolivar, J. M., Mateo, C., Rocha-Martin, J., Cava, F., Berenguer, J., Fernandez-Lafuente, R., et al. (2009). Enzyme and Microbial Technology, 44, 139–144.
Acknowledgment
The authors would like to thank the financial support of the Brazilian Research Agencies CNPq, CAPES, and FAPESP and Novozymes for the donation of crude xylanases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manrich, A., Komesu, A., Adriano, W.S. et al. Immobilization and Stabilization of Xylanase by Multipoint Covalent Attachment on Agarose and on Chitosan Supports. Appl Biochem Biotechnol 161, 455–467 (2010). https://doi.org/10.1007/s12010-009-8897-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8897-0