Abstract
Distilled glycerides are obtained through distillation of the system mono-diglycerides which is produced from the esterification reaction between a triglyceride with glycerol. In this work, monoglycerides (MG) and diglycerides (DG) are produced through lipase-catalyzed glycerolysis of soybean oil using Candida antarctica B in a solvent-free system. To separate the products of the reaction in order to obtain essentially MG and an oil of DG, it is necessary to use a suitable process in order to preserve the stability of the components and to keep the products free of inappropriate solvents. So, after 24 h of enzymatic reaction, the mixture of acylglycerols and fatty acids was distilled into a centrifugal molecular distiller, since it provides a free solvent and lower temperature environment to increase the desired product concentration. Starting from a material with 25.06% of triglycerides (TG), 46.63% of DG, 21.72% of MG, 5.38% of free fatty acids (FFA), and 1.21% of glycerol, the MG purity in the distillate stream was 80% at evaporator temperature (T E) equal to 250 °C and feed flow rate (Q) equal to 10.0 mL/min. At these conditions, the MG recovery was 35%. The material collected in the residue stream presented DG-enriched oil with TG unhydrolyzed, residual MG, and low acidity (29.83% of TG, 53.20% of DG, 15.64% of MG, and 1.33% of FFA), which is suitable to replace TG oil in the human diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
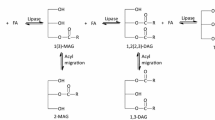
Monoglycerides (MG) and diglycerides (DG) are anionic oil-in-water emulsifiers commonly used in food, cosmetics, and pharmacy [1–3]. Often, mixtures of MG and DG are used in these applications, since they are cheap and give proper performance [4]. However, for uses like baked goods, confectionery products, ice cream, margarine, peanut butter, whipped desserts, whipped toppings (cosmetics, plastics), an emulsifier with high concentration of MG, called distilled monoglycerides [5] is necessary. Glycerides as DG can be used not only like emulsifiers together with the MG, but with a nutritional purpose, replacing the ordinary triglycerides (TG) oil in food. According to recent research, oil rich in DG presents similar cooking properties than TG oil, but nutritional studies in animals and in humans have shown that DG exhibit some beneficial effects mainly at the level of a reduction of body mass [6, 7]. These glycerides are produced by chemical or enzymatic routes, generally obtained from the glycerolysis of triglycerides, the hydrolysis of triglycerides, or the direct esterification of glycerol with fatty acids. However, even after the chosen reaction, it is a necessary process to separate as well as to concentrate these glycerides. The use of conventional distillation process is prohibitive because the low vapor pressures of the components and the thermal instability of the acylglycerols. Instead, molecular distillation is a gentle temperature separation process and so, adequate for obtaining products with high MG concentration and also an oil rich with DG with loss of nutritional properties.
Molecular distillation (MD) is generally accepted as a safety method to separate and to purify thermally unstable compounds and substances having low vapor pressure and higher molecular weight, without significant thermal decomposition [8]. MD, also known as short-path distillation, is characterized by a short exposure of the distilled liquid to the operating temperatures, a high vacuum in the distillation space, and a small distance between the evaporator and the condenser. This process has been employed for various applications. In lipid areas, MD has been used for recovering carotenoids from palm oil [9], recovering of tocopherols [10], fractioning polyunsaturated fatty acids after lipase-catalyzed hydrolysis [11], and separation of acylglycerols containing eicosapentaenoic acid and docosahexaenoic acid, and residues from saturated and monounsaturated ethyl esters after a lipase-catalyzed ethanolysis [12], among other applications.
In this work, a mixture of TG, DG, MG, free fatty acids (FFA), and glycerol (GL) obtained after the lipase-catalyzed glycerolysis reaction was fed in a centrifugal molecular distiller to obtain high concentration of MG and an oil rich in DG.
Materials and Methods
Materials
Commercial refined soybean oil was used. Glycerol (99%, 0.5% of water content) was supplied by Synth (São Paulo, Brazil). Lipase from Candida antarctica B (CA-IM, immobilized lipase), was generously supplied by Novozymes A/S (Bagsvaerd, Denmark).
Glycerolysis Reaction
The enzymatic glycerolysis reaction was carried out in a batch system. The reaction mixture consisted of glycerol and soybean oil, 2.0 wt.% of immobilized lipase (based on the soybean oil concentration), and an extra 3.5 wt.% of water (based on the glycerol amount). The molar ratio of glycerol to soybean oil (glycerol/oil) was 8:1. The temperature was controlled at 70 °C (optimal activation temperature for lipase from CA-IM). The reaction was stirred using a magnetic stirrer at 300 rpm. The reaction was stopped after 24 h by heating the reaction until 90 °C for 15 min, as described by Tuter et al. [13]. The final product separated into two liquid phases after the lipase-catalyzed glycerolysis: the lower phase containing glycerol, water, and lipases and the upper phase containing acylglycerols, FFA, and residues of GL.
Centrifugal Molecular Distillation
The model used in this work was a centrifugal distiller from Myers Vacuum Inc. (Kittanning, PA), with an evaporation surface area of 0.0046 m2. The feed temperature (T F), distillate and residue streams, and the condenser temperature (T C) were 60 °C. The typical pressure of the system was 24 Pa, and the evaporator rotation (rot) velocity was 1,350 rpm. A scheme of the equipment is illustrated in Fig. 1.
Analysis of Glycerides by HPSEC
The determination of the composition of the acylglycerols, FFA and GL, was performed using a gel-permeation chromatography, also called high-performance size-exclusion chromatography (HPSEC) [14]. The chromatographic system consists of an isocratic pump, model 515 HPLC Pump (Waters, Milford, MA), a differential refractometer detector model 2410 (Waters, Milford, MA), and an oven for columns maintained at 40 °C by a temperature control module (Waters, Milford, MA). Two HPSEC columns Styragel HR 0.5 and HR 1 (Waters, Milford, MA) were connected in series. These columns are packed with styrenedivinylbenzene co-polymer. The mobile phase used was HPLC-grade tetrahydrofuran from Tedia Inc. (Fairfield, OH), and the flow rate was 1 mL/min. The typical pressure at this flow rate was 450 PSI (3102 kPa). All the standards were obtained from Supelco, Inc. (Bellefonte, PA). The products of reaction, DG, MG, and FFA, as well as TG and GL are separated due to differences of molar weight.
Fatty Acid Composition Analysis
Gas chromatography is used to determine the composition of the GLA in the FFA and acylglycerol fractions. Following this, these fractions are converted into methyl esters by the method of Hartman et al. [15]. The analyses were carried through in a Varian chromatograph model STAR 3600CX. The identification of the fatty acids was carried through comparing the times of retention of the components present in each sample with the times of retention of the components present in the standard F.A.M.E. Mix C4-C24 (Supelco) and in oils of known composition.
Density of Lipids
The density (g.cm-3) of samples of oils and acylglycerols obtained from lipase-catalyzed reaction and after the molecular distillations stages were carried out in a Stabinger SVM 3000/Anton Paar viscometer, at different temperatures, according to ASTM D7042.
Results
Lipase-Catalyzed Glycerolysis Reaction
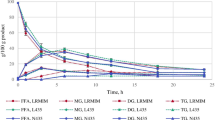
The effects of the amounts of lipase and water on the glycerolysis reactions, when using lipase from C. antarctica (CA-IM) in an enzymatic glycerolysis reaction, were investigated in previous work [4]. We verified that the lipase CA-IM was the best enzyme to produce MG and DG considering all the available studied lipases and the lower cost of production of MG and DG. So, in this work, the best conditions (lipase load, water content, glycerol/soybean oil molar ratio), already verified, of the glycerolysis reaction to produce a mixture of components to be then distilled were chosen. Figure 2 shows the kinetic behavior of the glycerolysis reaction employing the lipase CA-IM as biocatalyzer.
After 24 h, the reaction was stopped by heating up to 90 °C in order to inactivate the lipases and, when the agitation was stopped, two phases were formed. The upper phase is composed by acylglycerols TG, DG, MG, and AGL, and the lower phase is composed by GL, water, and lipases. The upper phase was separated for analysis and then fed into the molecular distiller.
The concentration (mass fraction, weight percent) of the acylglycerols after 24 h is 25% of TG, 46.6% of DG, 21.7% of MG, 5.3% of FFA, and 1.2% of GL.
Molecular Distillation
In the MD, the distillate stream is rich in lower molecular weight molecules, while the residue stream is rich in higher molecular weight molecules. The extension of this depends on the evaporator temperature (T E) and on the pressure inside the vacuum chamber.
A cascade system consists on re-feeding the molecular distiller with the residue material for each increase of T E. Thus, a maximum of MG recovery in the distillate stream might be reached, gradually decreasing the MG concentration in the material obtained in the residue stream. On other hand, at each step of distillation, the DG component remains more concentrated in residue stream. Experiments fixing the heating temperature and varying the feed flow rates were carried out.
The feed consists on a mixture of components resulted from glycerolysis reaction which presents different molecular weight molecules, as can be seen in the Table 1. The lower component of the acylglycerol mixture is GL, with molecular weight of 92 kg/mol, and it can be distillate with T E in the range 80–100 °C and 24 Pa. Components with higher molecular weight might be distilled at higher T E; however, components like DG and TG essentially are obtained in the residue stream because the high temperatures required to distill these components (above 280 °C) might cause degradation of these molecules.
The first step of the MD consists in a pre-distillation at 100 °C and 150 °C in order to decrease the GL and FFA concentrations in the residue stream. These compounds, GL and FFA, must be removed as much as possible because the presence of the GL affects directly the vacuum of the chamber of evaporation, and the similarity of the molecular weights of the different FFA and the different MG produced make the separation of both components hard. The mass fractions of the feed, distillate, and residue stream are presented in Table 1.
After the steps of the pre-distillation at 100 °C and 150 °C to eliminate and/or to decrease the GL and FFA of the residue stream, the T E was increased from 190 °C up to 250 °C together with variation of the flow rate (Q; Fig. 3). Compositions of the acylglycerols (TG, DG, MG, FFA, and GL) were monitored for the feed, distillate, and residue stream at each stage of T E, shown in Table 2. During the entire experiment, data of feed, distillate, and residue flow rate (mass and volumetric) were also measured. The Q, mass (grams per minute) and volumetric (milliliters per minute) flow, at different T E are shown in Table 3.
It can be verified in Fig. 3 that, when the process temperature and the feed flow rate increase, the concentration of MG in the distillate stream also increases. Increasing of temperature causes higher heat transfer rate, and higher molecules can be evaporated. At 190 °C, the increase from 6 to 10 mL/min in the feed flow rate provided an increase of 31.88% of MG. At 230 °C and 250 °C, the increase of the flow rate provided an increase of 16.7% and 4.1% of MG, respectively. When lower Q is used, preferential paths (channeling) can occur, and the change in heat transfer rate between the evaporator and the fluid is neglected. Increasing Q to 10 mL/min, the film formed at the surface of the evaporator is more uniform, providing an efficient transfer rate from evaporator to the fluid to be evaporated.
It was possible to concentrate distilled MG to 80%, obtained with a feed flow rate of 10 mL/min and 250 °C, when the last feed contained only 12.75% of MG. The residue stream also contains MG which did not distill in the experimental conditions.
The feed concentration is continuously changed at each increase of temperature, so that the recovery of MG at each step is different.
The recovery of determined component is based on the following Eq. 1:
where N is the recovered component.
The MG recoveries in different temperatures and the varying feed flow rates are presented in Table 4.
The MG recoveries are always higher when the feed flow rate is lower. This is due to the residence time of the molecules on the evaporator: increasing the period of contact of the molecules on the evaporator, the availability of the all molecules to evaporate (the higher and the lower molecules) is increased. In the same way, the concentration of DMG is lower when Q is lower due the availability of residence time that all molecules have to evaporate; it is what promotes the decrease of MG concentration on the mixture being distilled. When Q is higher, only the lower molecules, at least the MG molecule, can evaporate, increasing the concentration of MG on the distillate stream.
After the procedure of molecular distillation, in the residue stream, it was provided a final material rich in DG (>53%) presenting low acidity of 1.33%, 29.83% of TG, and 15.64% of MG. The separation of TG from DG is difficult due to the small vapor pressure difference between DG and TG [16]. Therefore, TG is not removed and remained in the final product. The DG-enriched oil with unhydrolyzed TG, residual MG, and low acidity has similar digestibility and energy value as TG oil. For this reason, the residue obtained was also studied as a great product and characterized together with TG oil. The structure of the DG-enriched oil is lower than TG oil (soybean oil), as can be seen in the Table 5, analyzing the molecular weight determined experimentally. On the other hand, the density (ρ) obtained is very similar to TG oil for all temperatures measured. MG produced had an opaque white color and was solid at room temperature and even at temperatures of 40 °C, was noted the presence of semi-solids, that make the procedure for measuring the density of this material impossible. The DG oil had a pale yellowish color, was semisolid at room temperature, and may find suitable applications as potential healthy functional cooking oil replacing TG oil in the human diet.
Conclusions
This work has shown the performance of the molecular distillation process for separating a mixture of acylglycerols and free fatty acids obtained from a previous lipase-catalyzed glycerolysis reaction of soybean oil to produce and concentrate MG, which has emulsifier properties and is DG-enriched to replace the TG oil in the human diet. Due to the enzymatic glycerolysis from vegetal source, the products (TG, DG, MG, and FFA) do not have hydrogenated fats and also, they are free of trans fats. This mixture was fed into the molecular distiller to separate and to concentrate MG through the difference in the molecule molecular weights. The first product could be redistilled and a cascade system was employed to recover almost all the MG produced. At 250 °C, it was possible to concentrate MG to 80% from a mixture that contained initially 21% of MG, without loss of its properties or changes in its structure. The material collected in the residue stream presented DG-enriched oil with unhydrolyzed TG, residual MG, and low acidity (29.83% of TG, 53.20% of DG, 15.64% of MG, and 1.33% of FFA). This DG oil presented a pale yellowish color, semisolid at room temperature and with suitable properties to replace TG oil in the human diet.
References
Kristensen, J. B., Xu, X., & Mu, H. (2005). Journal of the American Oil Chemists' Society, 82, 329–334.
Kaewthong, M., Sirisansaneeyakul, S., Prasertsan, P., & H-Kittikun, A. (2005). Process Biochemistry, 40, 1525–1530.
Chang, C., & Bodmeier, R. (1998). International Journal of Pharmaceutics, 173, 51–60.
Fregolente, P. B. L., Fregolente, L. V., Pinto, G. M., Batistella, B. C., Wolf-Maciel, M. R., & Maciel Filho, R. (2008). Applied Biochemistry and Biotechnology, 146, 165–172.
Cvengros, J. (2005). Chemical Engineering & Technology, 18, 49–58.
Meng, X., Zou, D., Shi, Z., Duan, Z., & Mão, Z. (2006). Lipids, 39, 37–41.
Taguchi, H., Nagao, T., Watanabe, H., Onizawa, K., Matsuo, N., & Tokimitsu, I. (2001). Lipids, 36, 379–382.
Fregolente, L. V., Fregolente, P. B. L., Chicuta, A. M., Batistella, C. B., Maciel Filho, R., & Wolf-Maciel, M. R. (2007). Chemical Engineering Research & Design, 85, 1524–1528.
Batistella, C. B., & Maciel, M. R. W. (1998). Computers & Chemical Engineering, 22, 53–60.
Watanabe, Y., Nagao, T., Hirota, Y., Kitano, M., & Shimada, Y. (2004). Journal of the American Oil Chemists' Society, 81, 339–345.
Yamane, T., Suzuki, T., Sahashi, Y., Vikersveen, L., & Hoshino, T. (1992). Journal of the American Oil Chemists' Society, 69, 1104–1107.
Torres, C. F., Nettekoven, T. J., & Jr Hill, C. G. (2003). Enzyme and Microbial Technology, 32, 49–58.
Tuter, M., Aksoy, H. A., Ustun, G., Riva, S., Secundo, F., & Ipekler, S. (2003). Journal of the American Oil Chemists' Society, 80, 237–241.
Schoenfelder, W. (2003). European Journal of Lipid Science and Technology, 105, 45–48.
Hartman, L., & Lago, R. C. A. (1973). Laboratory Practice, London, 22, 475–481.
Watanabe, Y., Shimizu, M., Sugiura, M., Sato, M., Korohi, J., & Yamada, N. (2003). Journal of the American Oil Chemists' Society, 80, 1201–1207.
Acknowledgements
The authors are grateful for the financial support of CNPq and FAPESP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fregolente, P.B.L., Pinto, G.M.F., Wolf-Maciel, M.R. et al. Monoglyceride and Diglyceride Production Through Lipase-Catalyzed Glycerolysis and Molecular Distillation. Appl Biochem Biotechnol 160, 1879–1887 (2010). https://doi.org/10.1007/s12010-009-8822-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8822-6