Abstract
The object of present study is to investigate the effects of 50 GHz microwave frequency electromagnetic fields on reproductive system of male rats. Male rats of Wistar strain were used in the study. Animals 60 days old were divided into two groups—group I sham exposed and group II experimental (microwave exposed). During exposure, rats were confined in Plexiglas cages with drilled ventilation holes for 2 h a day for 45 days continuously at a specified specific absorption rate of 8.0 × 10−4 W/kg. After the last exposure, the rats were sacrificed immediately and sperms were collected. Antioxidant enzyme (superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase), histone kinase, apoptosis, and cell cycle were analyzed in sperm cells. Result shows a significant decrease in the level of sperm GPx and SOD activity (p ≤ 0.05), whereas catalase shows significant increase in exposed group of sperm samples as compared with control (p < 0.02). We observed a statistically significant decrease in mean activity of histone kinase as compared to the control (p < 0.016). The percentage of cells dividing in a spermatogenesis was estimated by analyzing DNA per cell by flow cytometry. The percentage of apoptosis in electromagnetic field exposed group shows increased ratio as compared to sham exposed (p < 0.004). There were no significant differences in the G0/G1 phase; however, a significant decrease (p < 0.026) in S phase was obtained. Results also indicate a decrease in percentage of G2/M transition phase of cell cycle in exposed group as compared to sham exposed (p < 0.019). We conclude that these radiations may have a significant effect on reproductive system of male rats, which may be an indication of male infertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental exposure to electromagnetic fields and radiation (EMFs) has now steadily increased. Everyone is exposed to radiofrequency/microwave radiation emissions from wireless devices such as cellular phones and cordless phones, cellular antennas and towers, broadcast transmission towers, voice and data transmission for cell phones, pagers and personal digital assistants, and other sources of microwave radiations. The main source of exposure to 50 GHz radiations is emitted from microwave installation and equipment, used for a variety of purposes in addition to millimeter wave communication. Present study was designed to determine the effects of 50 GHz non-thermal microwave radiation on reproductive system. This frequency was so chosen because 50 GHz has a much smaller penetration depth which may cause an increase in the random molecular motion due to free-radical processes within the cell. The data presently available regarding the biological effects and medical applications of sub-millimeter wave therapy are not many and some need to be confirmed. So far, most of the investigations are confined to a frequency up to 10 GHz. Above range frequencies will have limited penetration into the body and to study their mode of interaction with system, including these at cellular and molecular levels, will be interesting to examine. Several studies have demonstrated that repair of tissue could be stimulated by millimeter wave, suggesting that such effects are mediated by activation of the organism’s own recovery mechanism. The mechanism to these effects is important to examine. It will be of further interest to establish their commonality with after-exposure parameters at comparatively lower frequencies. The study also aims to spread over the electromagnetic field (EMF) effect over a wider band of non-ionizing electromagnetic spectrum.
The increasing use of wireless communication devices has led to concerns that exposure to electromagnetic (EM) waves emitted by these devices may cause adverse health effects. Kesari and Behari [1] reported DNA double strand break and alteration in antioxidant enzyme activity by exposing the Wistar rat brain at 50 GHz frequency. Paulraj and Behari [2] indicated DNA single strand break and decrease in level of protein kinase at 2.45 GHz [3]. The present study on reproductive pattern is the continuation of our earlier study on brain system. The present study has been carried out with antioxidant enzyme (glutathione peroxidase, superoxide dismutase, and catalase), histone kinase, and cell cycle changes in sperm cells.
The present study has been performed in agreement with many studies, which were carried out in human as well as in animal model. The study of Fnu et al. [4] involving 361 men attending an infertility clinic suggested that use of cell phones adversely affects the quality of semen by decreasing sperm counts, motility, viability, and morphology, which might contribute to male infertility. In another study of Fejes et al. [5] on 371 men, it was suggested that prolonged use of cell phones might have negative effects on the sperm motility. In a similar pilot study of Agarwal et al. [6], they reported that the direct exposure of human semen samples to cellular phone radiation (in talking mode) decreased sperm motility and viability. In a small prospective study involving 13 normal men, GSM phone usage of 5 days for 6 h per day decreased the rapid progressive motility of sperm [7]. Investigators also found a decrease in sperm motility in semen samples of men exposed to a 900-MHz cell phone for 5 min [8]. Furthermore, adverse effect of microwave radiations on animal model already has been established, where a decrease in diameter of seminiferous tubule [9, 10], weight of testicular organs (i.e., caput, cauda, and corpus), sperm count [11], and destruction in Leydig cells were obtained.
The present study was incorporated in biological systems affected by electromagnetic radiation, where the mechanisms of tissue damage are thought to involve reactive oxygen species. Human spermatozoa represent a growing list of cell types that exhibit a capacity to generate reactive oxygen species (ROS). ROS are important mediators of sperm function. Mammalian spermatozoa membranes are rich in unsaturated fatty acid, which are sensitive to oxygen-induced damage mediated by lipid peroxidation (LPO) [12]. However, antioxidants are known as general compounds which dispose, scavenge, and suppress the formation of ROS or oppose their actions of the antioxidants where the enzymes glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) play significant roles [13]. ROS, including superoxide anion, hydrogen peroxide, and hydroxyl radical, act as subcellular messengers in such complex processes as mitogenic signal transduction, gene expression, and regulation of cell proliferation when they are generated excessively, or when enzymatic and non-enzymatic defense systems are impaired [14, 15]. The primary defense against oxidative stress in the cell with antioxidants includes SOD, GPx, and catalase. SOD specifically converts superoxide radicals to hydrogen peroxide, and CAT and GPx detoxify hydrogen peroxide to water [15, 16]. The above hypothesis shows that the ROS may be involved in the action of MW on biological system [17, 18]. It is important that antioxidant treatments in animals and humans could be beneficial in preventing or reducing some complications of low-frequency MW [18].
In the present study, we have also carried out histone kinase with cell cycle analysis. These parameters are correlated to each other. The most widely known protein kinases are those that catalyze the phosphorylation hydroxy amino acids, i.e., serine/threonine protein kinases and tyrosine kinases. Another type of protein kinase has been shown to phosphorylate proteins on the imidazole nitrogens of histidine. The best known group of these enzymes are the histidine kinases. Phosphorylation by protein kinases is a major signal transduction mechanism used by eukaryotic cells to regulate proliferation, gene expression, metabolism, motility, membrane transport, besides many others. The study of testicular cell suspensions in terms of rapidity and objectivity has been made by flow cytometry (FCM) [19]. The intensity of fluorescence per cell is measured by flow cytometry (FC) and, since it corresponds to its amount of DNA, the proportion of cells at each intensity represents the proportion of cells at different cell cycle phases—apoptosis, G0–G1, S, and G2–M [20].
In pursuance of our earlier work, we have decided to study the effects of 50 GHz electromagnetic field on the abovementioned parameters, which may be a positive indicator of tumor promotion.
Material
The Glutathione Peroxidase (GPx, catalog no. 703102), Catalase (catalog no. 707002), and Superoxide Dismutase (SOD, catalog no. 706002) antioxidant enzyme kit was purchased from the Cayman Chemical Company, Ann Arbor, MI, USA. dl-Dithiothreitol (DTT), aprotinin, leupeptin, pepstatin A, phenylmethanesulfonyl fluoride (PMSF), ethylene glycerol bis (2-aminoethyl)-N,N,N′,N′ tetra acetic acid (EGTA), adenosine-5′ triphosphate disodium salt hydrate (ATP), and β-glycerol phosphate disodium salt pentahydrate were purchased from Sigma-Aldrich, Germany. The other two are Histone H1 cat. no. 14-155 from Upstate, NY and propidium iodide (PI) purchased from HiMedia. P32 radioactive-labeled ATP was purchased from BRIT, Hyderabad, India. The rest of the chemicals were purchased from Thomas Baker Chemicals Limited, Marine Drive, Mumbai.
Methods
Animal Exposure
Male Wistar rats (60 days old and 190 ± 20 g body weight) were obtained from the animal facility of Jawaharlal Nehru University, New Delhi. These were divided into two groups: sham exposed (n = 6) and experimental group (n = 6). In the animal exposure and subsequent experimentation, a blind study was conducted and repeated. All animals were housed in an air-conditioned room, where the temperature was maintained at 25 °C with constant humidity (40–50%). Air circulation was constantly maintained to keep it in equilibrium with the room temperature. The animals were provided with standard food pallets (prepared by Brook Bond India Limited) and water ad libitum.
The protocol and study method was approved by the Institutional Animal Ethical Committee and the Committee for Purpose of Control and Supervision of Experiments on Animals.
Exposure Chamber
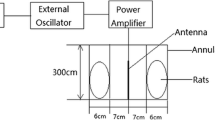
Two rats at a time were placed in a Plexiglas cage which was ventilated with holes of 1 cm. The inner dimensions of the animal holding cage were \( 31 \times 9.2 \times 8.5\;{\text{cm}}\;\left( {{\text{length}} \times {\text{breadth}} \times {\text{height}}} \right) \) and it was made in such a way that the animals can move freely. The chamber is lined with radar absorbing material (attenuation, 40 dB) to minimize the possibility of any reflections. At far field distance from horn antenna, it was found that the field is homogeneous in vertical plane of midline of the beam. Rats were exposed with 50 GHz continuous source through the antenna, 2 h a day for 45 days (Fig. 1). The power density at receiving end was measured (0.86 µW/cm2) and the nominal specific absorption rate (SAR) value was calculated (8.0 × 10−4 W/kg). The intensity of the 50 GHz field was measured at the plane of cage placement using a similar antenna as at the transmitting end. Since the animals were free to move in the exposure cage, it is suggested that both the animals received the same amount of dose during the period of investigation. The power received at the animal exposure cage was converted into SAR value by assuming a free field scenario, where the E field is parallel to orientation of the animal placement. Since the free space wavelength at this frequency is 0.6 cm, it is assumed that the exposure is limited to the subcutaneous surface. Every day, the cage was placed in the same position facing the horn antenna and the same number of rat positions was filled.
Specific Absorption Rate (SAR) Calculation
SAR was calculated by extrapolating the model values for small rats, following Durney et al. [21]. For a plane wave exposure having random polarization and power density of 0.86 µW/cm2, the SAR value turns out to be 8.0 × 10−4 W/kg.
Sample Preparation
In the present investigations, enzyme assay was used to determine the antioxidant enzyme activity (SOD, GPx, and catalase) in exposed as well as sham-exposed group of Wistar rats. Immediately after exposure period, rats were anesthetized by placing them in a glass jar containing cotton dipped in anesthetized ether. Animals were sacrificed and sperm was collected from epididymal region (caput and caudal). Caput and caudal were minced thoroughly with the help of scissors in cold buffer and pipette. The assay was performed with positive control for enzyme estimation.
Sperm Sample Preparation
Sperm cells were added to 5–10 ml of cold buffer (50 mM Tris HCl, pH 7.5, 5 mM EDTA, and 1 mM DTT) for GPx, 20 mM HEPES buffer (1 mM EGTA, 210 mM mannitol, and 70 mM sucrose) for SOD, and 5–10 ml of cold buffer (50 mM potassium phosphate, pH 7.0, containing 1 mM EDTA) for catalase. All the samples were centrifuged at 10,000×g for 15 min at 4 °C; supernatant was collected and enzyme assay was performed.
Estimation of Glutathione Peroxidase Activity
One hundred twenty microliters of assay buffer and 50 µl co-substrate mixture were added in non-enzymatic wells. One hundred microliters of assay buffer, 50 µl of co-substrate mixture, and 20 µl of diluted GPx were added in other wells as control sample whereas the same amount of assay buffer and co-substrate including 20 µl of sperm sample in place of GPx were added in all the wells. Immediately, reaction was initiated by adding 20 µl of cumene hydroperoxide to all the wells being used. Finally, well plate was placed in a micro-plate reader spectrophotometer and absorbance of the samples was taken at 340 nm.
Estimation of Superoxide Dismutase Activity
Twenty microliters of SOD standard was diluted with 1.98 ml of sample buffer. SOD standard wells were prepared by using 200 µl of the diluted radical detector and 10 µl of diluted standard. Sample wells were also prepared by adding 200 µl of the diluted radical detector and 10 µl of sample to the wells. The reaction was initiated by adding 20 µl of diluted xanthine oxidase to all the wells. The sample plate was kept in a micro-plate reader and absorbance was taken at 450 nm.
Estimation of Catalase Activity
One hundred microliters of assay buffer, 30 µl of methanol, and 20 µl of standard were added to wells, which contained 10 µl of formaldehyde and 9.99 ml of sample buffer, and formaldehyde wells were prepared. Control wells were prepared by adding 100 µl of diluted assay buffer, 30 µl of methanol, and 20 µl of diluted CAT. The sample wells were prepared by adding 100 µl of diluted assay buffer, 30 µl of methanol, and 20 µl of tissue samples. The reaction was initiated by adding 20 µl of diluted hydrogen peroxide to all the wells. Thirty microliters of potassium hydroxide was added to terminate the reaction. Thirty microliters of purpald (chromogen) was added to each well and thereafter incubated for 10 min at room temperature on a shaker. Ten microliters of potassium periodate was added to each well, incubated for 5 min at room temperature on shaker, and the absorbance of samples was taken at 540 nm.
All data are expressed as mean ± standard deviation (SD) and were analyzed by one-way analysis of variance. Probability value p < 0.05 was considered significant. The calculation/formulation was followed as per Cayman Chemical (catalog number mentioned in Material section), USA.
Preparation of Spermatozoa
Immediately after exposure period, rats were anesthetized by placing it in a glass jar containing cotton dipped in anesthetized ether, and epididymes were excised from testis. Seminiferous tubules were pulled out from testis and diced in phosphate-buffered saline (PBS) and kept at 37 °C in a water bath for 7 to 10 min. The spermatozoa released in PBS were collected carefully by pipetting and centrifuged at 500×g for 5 min to obtain the sperm. After this, histone kinase and cell cycle analysis were performed.
Histone Kinase (H1) Assay
Sperm was homogenized in 40 vol of ice-cold medium containing 10 mM Tris HCl, pH 7.4, 2 mM EDTA, 1 mM DTT, and protease inhibitors like aprotinin, leupeptin, pepstatin A (each 10 µl/ml), and 0.1 mM (PMSF). The homogenates were centrifuged at 1,000 ×g for 5 min at 4 °C. Thereafter, the supernatant was centrifuged at 12,000 ×g for 10 min at 4 °C. The pellet was taken and homogenized with ice-cold 20 mM HEPES, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and stored on ice at 4 °C. Protein concentration was measured by Lowry’s method [22]. An assay mixture was prepared by mixing 3 µl of γ[32P] ATP (spec. activity 3,000 Ci/mmol), 45 µl of 4 mM ATP, 222 µl of extraction buffer (80 mM b-glycerophosphate (pH 7.3), 20 mM EGTA, 15 mM MgCl2) and the aliquots stored at −20 °C. DTT (1 mM), protease inhibitors (1 mM PMSF, 10 µg/ml each of aprotinin, pepstatin, and leupeptin), and 30 µl of 20 mg/ml histone H1 were added to aliquots just prior to use. Twenty milligrams of each sample protein to be assayed was pipetted into labeled micro-well plates at 4 °C and mixed well by pipetting up and down several times. Each reaction was continued for 15 min by warming the micro-well plate to 37 °C in water bath. Reaction was stopped by transferring 10 µl of the reaction mixture to phosphocellulose P81 paper pre-cut into 1.5-cm squares. Paper filters were marked by carbon pencil (no ink) so they can be washed in the same beaker. The mixture was allowed to adsorb for a few seconds to slightly dry then dipped into a beaker containing 150 mM H3PO4 overnight. Filter papers were washed in 150 mM H3PO4 for 3 × 15 min at 20 °C. At final stage, filter papers were rinsed briefly in ethanol and air dried on paper towels. Strips were transferred into scintillation vials. Five milliliters of scintillation fluid was taken in vials and (c.p.m.) counted in Hewlett Packard Scintillation Counter.
Cell Cycle Estimation
Sperm cells (100 µl) of 1 × 106 cells/ml concentration were taken in falcon 12 × 75-mm polypropylene tube with snap cap and 1 ml ice-cold 70% ethanol was added per tube. The sample was incubated overnight at 4 °C. After the completion of incubation period, the sample was centrifuged at 1,000 ×g for 10 min at 4 °C and finally the supernatant was decanted. One hundred microliters of RNAase (100 U) was added to the pellet and pipetted well thereafter. Samples were incubated at room temperature for 20 min. Finally, samples were stained with 50 µl (25 µg/ml) propidium iodide (PI) in dark. Each sample was analyzed within half an hour after staining with PI.
Results
Antioxidant Enzyme Activity
Compared with the control group (5.03 ± 0.29), those exposed to the microwave showed a significant decrease (4.48 ± 0.10 nmol/min/ml; p < 0.003) in GPx activity (Fig. 2). A significant decrease in SOD activity (132.37 ± 5.7 U/ml; p < 0.006) as compared to control group (230.98 ± 25.67) was also observed (Fig. 3). However, the exposed group of animals showed significant increase in CAT activity (7.56 ± 0.71 nmol/min/ml; p < 0.02) as compared to the control group (6.74 ± 0.74 nmol/min/ml) (Fig. 4).
Histone Kinase Activity
The activity of histone kinase in sperm shows significant decline in microwave field exposed group (4,438.0 ± 111.21) as compared to sham exposed (4,605.05 ± 89.87) (Fig. 5). In the exposed groups, we found a statistically significant decrease in mean activity of histone kinase as compared to the control (p < 0.016). The decline in the level of histone kinase may also indicate that the level of G2/M phase has decreased.
Cell Cycle Analysis
Samples were analyzed with a FACS Calibur (Becton & Dickinson) FC with an argon-ion laser that produced 15 mW at 488 nm. The percentages of cells in each cell division phase (G0–G1, S, and G2–M) were estimated from data obtained from the FL2-A channel. In exposed group (32.27 ± 1.54), a significant increase (p = 0.004) was found in apoptosis as compared to sham exposed (23.72 ± 3.76). There was no significant change (p = 0.28) observed in G0/G1 phase in exposed (42.09 ± 13.35) as compared to the control ones (48.45 ± 3.64). S phase was significantly decreased (p = 0.026) in exposed group (5.37 ± 0.94) as compared to the control group (8.05 ± 2.33). However, in exposed groups, a statistically significant decrease (p = 0.019) was measured in G2/M phase (14.69 ± 1.28) as compared to the control group (18.41 ± 3.02). The different phases of sperm cell cycle were labeled with M1 (G0–G1), M2 (S), M3 (G2), and M4 (G2/M) as shown in Fig. 6.
Discussion
The results of the present study demonstrate for the first time that the EMF is able to affect activities of antioxidant enzymes, histone kinase, and cell cycle on reproductive pattern at 50 GHz exposure. Earlier, we have established the alteration of such parameters in brain using the same protocol. Experimental data demonstrate that exposure to EMF led to generation of ROS [23]. On the other hand, ROS are known to alter antioxidant enzyme activities. It has been proposed that moderate level of ROS can induce an increase/decrease in antioxidant, where as very high level of these reactants was shown to attenuate antioxidant enzyme activities [24]. ROS are able to damage many biomolecules including DNA, enzymes, lipids, and proteins. We can hypothesize that the changes in antioxidative enzyme activities, reduction in the level of histone kinase, and enhancement of apoptotic cells in cell cycle which have been observed in our study can be related to overproduction of ROS under EMF. In the case of antioxidant activity, we found a decrease in activity of SOD and GPx and increased activity of CAT after 2 h/day for 45 days at 50 GHz microwave exposure. This can be attributed to the enhancement in the generation of ROS and induction of the activity of CAT which metabolizes H2O2 to water. On the other hand, the increased amount of ROS could also be explanatory for a reduction in GPx and SOD activities after 45 days’ exposure. Moreover, ROS may cause a defect in sperm function through lipid peroxidation (LPO). This is because spermatozoa, unlike other cells, are unique in structure and function, and are susceptible to damage by LPO. ROS are produced by a variety of semen components including immotile or morphologically abnormal spermatozoa, leukocytes, and morphologically normal but functionally abnormal spermatozoa [25]. The SOD level in spermatozoa is positively correlated with sperm motility [26]. Also, catalase, which prevents ROS damage, has been found in both human spermatozoa and seminal plasma. Unfortunately, the precise mechanism involved in this effect is not clear, but the present paper could be useful in an establishment of EMF interaction mechanism with reproductive system.
In an addition to above, [18] hypothesized that the rat hepatocytes metabolized MW-induced ROS by two distinct pathways. One pathway involves the cytochrome P-450, which plays an important role in enzymatic activities, and leads to the formation of toxic peroxyl and alkoxy (reactive oxygen) radical. The second is a detoxification reaction that involves GPx producing oxidative glutathione [15]. These metabolic pathways could increase cellular free radicals, which may attack phospholipids, proteins, and nucleic acid. Thus, antioxidant activity and the inhibition of ROS generation are important in protecting the biological system from MW-induced damage [17, 27].
Free radicals induced cell damage may also be quantitatively determined by some measurement of histone kinase and cell cycle, which is an indicator of tumor promotion. In the present study, we have observed a large reduction in histone kinase activity in sperm during exposure. Such kinases, encoded by approximately 2% of eukaryotic genes, represent one of the major classes of cell-regulatory molecules [28]. Manning et al. [29] reported that the human genome carries 518 protein kinase genes (approximately 1.7% of all genes). It defines a large majority (approximately 90%) of these kinases belonging to the eukaryotic protein kinase (EPK) superfamily defined on the basis of a homologous kinase catalytic domain [30, 31]. The EPK domain interacts with both ATP and protein (peptide) substrates and functions to transfer the c-phosphate of ATP onto the hydroxyl group of a serine, threonine, or tyrosine amino acid residue within the peptide substrate. However, the maturation promoting factor (MPF) is a biologically recognized factor responsible for driving the cell cycle from the G to M phase as measured by cytoplasmic injection or cell fusion [32–36]. In general, initiation of mitosis (M phase) requires a histone kinase complex (MPF) consisting of a catalytic subunit (Cdc2 protein kinase) [37, 38] and a regulatory subunit (cyclin B) [39]. Assessment of the catalytic activity of a specific histone kinase plays an important role in elucidating signal transduction pathways that affect cell behavior. Hence, these results are in agreement with the findings that the activity of histone H1 kinase is closely related to the G2/M transition during the cell cycle [40]. Decrease of histone H1 kinase activity was realized just before the entry of differentiating cells into the M phase, thus suggesting a universal role of Cdc2/Cdk2 kinase to make the G2/M transition [41]. The study indicates the microwave effects on cAMP-independent protein kinases. The decrease in histone kinase activity may serve as a measure of the ability of an electromagnetic field to affect spermatogenesis, a cell cycle in sperm. Above results indicate that electromagnetic field induces apoptosis and may be a major cause of infertility. Recently, Kesari and Behari [42] reported that microwave (2.45GHz) exposure cause infertility by decreasing sperm count and an increase in apoptosis. Traditionally, exposure to electromagnetic field of the testis has been monitored by changes in the sperm count and spermatogenesis. In human, the molecular basis of male infertility is poorly understood. The recent evidence on the role of apoptosis during human spermatogenesis provides clues to the mechanisms at work.
Conclusion
The present study shows prolonged exposure to 50 GHz field radiation may decrease the level of histone kinase, GPx, and SOD. On the other hand, the level of CAT and apoptosis (cell cycle) were increased in the exposed group. Moreover, it was confirmed that a decrease in G2-M phase is directly related to histone kinase. We suggest that a reduction or an increase in antioxidant enzyme, histone kinase, and cell cycle observed in our study may be related to overproduction of ROS under microwave field exposure. Our findings conclude that these radiations may have significant effect on reproductive system of male rats. This is an indication of male infertility. The results of present study has been summarized in Fig. 7.
References
Kesari, K. K., & Behari, J. (2009a). Fifty-gigahertz microwave exposure effect of radiations on rat brain. Applied Biochemistry and Biotechnology, 158(2), 126–139.
Paulraj, R., & Behari, J. (2006). Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutation Research, 596, 76–80. doi:10.1016/j.mrfmmm.2005.12.006.
Paulraj, R., & Behari, J. (2004). Radiofrequency radiation effect on protein kinase C activity in rats brain. Mutation Research, 585, 127–131.
Deepinder, F., Kartikeya Makkar, K., & Agarwal, A. (2007). Cell phones and male infertility: dissecting the relationship. Reproductive Biomedicine Online, 15(3), 266–270.
Fejes, I., Zavaczki, Z., Szollosi, J., Koloszar, S., Daru, J., Kovacs, L., et al. (2005). Is there a relationship between cell phone use and semen quality? Archives of Andrology, 51, 385–393.
Agarwal, A., Deepinder, F., Sharma, R. K., Ranga, G., & Li, J. (2008). Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertility and Sterility, 89(1), 124–128.
Davoudi, M., Brossner, C., & Kuber, W. (2002). The influence of electromagnetic waves on sperm motility. Journal für Urologie und Urogynäkologie, 19, 18–32.
Erogul, O., Oztas, E., Yildirim, I., Kir, T., Aydur, E., Komesli, G., et al. (2006). Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study. Archives of Medical Research, 37, 840–843.
Dasdag, S., Ketani, M. A., Akdag, Z., et al. (1999). Whole-body microwave exposure emitted by cellular phones and testicular function of rats. Urology Research, 27, 219–223.
Dasdag, S., Akdag, M. Z., Aksen, F., et al. (2003). Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics, 24, 182–188.
Behari, J., & Kesari, K. K. (2006). Effects of microwave radiations on reproductive system of male rats. Embryo Talk, 1, 81–85.
Sikka, S. C. (1996). Oxidative stress and role of antioxidants in normal and abnormal sperm function. Frontiers in Bioscience, 1, e78–e86.
Miesel, R., Jedrzejez, K., Sanocka, D., & kKurpisz, M. K. (1997). Severe antioxidase deficiency in human semen samples with pathological spermiogram parameters. Andrologia, 29, 77–83.
Murphy, J. C., Kaden, D. A., Warren, J., & Sivak, A. (1993). International Commission for Protection against Environmental Mutagens and Carcinogens. Power frequency electric and magnetic fields: a review of genetic toxicology. Mutation Research, 296, 221–240.
Halliwell, B., & Gutteridge, J. M. C. (1999). Free radicals, other reactive species and disease. In B. Halliwell & J. M. C. Gutteridge (Eds.), Free radicals in Biology and Medicine (3rd ed., pp. 639–645). New York: Oxford University Press.
Nazıroglu, M., Karaoglu, A., & Aksoy, A. O. (2004). Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology, 195, 221–230.
Moustafa, Y. M., Moustafa, R. M., Beacy, A., Abou-El-Ela, S. H., & Ali, F. M. (2001). Effects of acute exposure to the radiofrequency fields of cellular phones on plasma lipid peroxide and antioxidase activities in human erythrocytes. Journal of Pharmaceutical and Biomedical Analysis, 26, 605–608.
Rush, G. F., Gorski, J. R., Ripple, M. G., Sowinski, J., Bugelski, P., & Hewitt, W. R. (1985). Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes. Toxicology and Applied Pharmacology, 78, 473–483.
Spano, M., & Evenson, D. P. (1993). Flow cytometric analysis for reproductive biology. Biology of the Cell, 78, 53–62.
Nunez, R. (2001). DNA measurement and cell cycle analysis by flow cytometry. Current Issues in Molecular Biology, 3(3), 67–70.
Durney, C. H., Iskander, M. F., Massoudi, H., Johnson, C. C. (1984). An empirical formula for broad band SAR calculations of prolate spheroidal models of humans and animal. In: J. M. Osepchuk (Ed.), Biological effects of electromagnetic radiation (pp. 85–90). New York: IEEE Press.
Lowry, O. H., Resebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folinphenol reagent. Journal of Biological Chemistry, 193, 265–275.
Chen, G., Upham, B. L., Sun, W., et al. (2000). Effect of electromagnetic field exposure on chemically induced differentiation of friend erythroleukemia cells. Environmental Health Perspectives, 108, 967–972.
Brydon, L., Petit, L., Delagrange, P., Strosberg, A. D., & Jockers, R. (2000). Functional expression of MT2 melatonin receptors in human PAZ6 adipocutes. Endocrinology, 142, 4264–4271.
Plante, M., de Lamirande, E., & Gagnon, C. (1994). Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertility and Sterility, 62(2), 387–393.
Alvarez, J. G., Touchstone, J. C., Blasco, L., & Storey, B. T. (1987). Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. Journal of Andrology, 8(5), 338–348.
Yen, G. C., Yeh, C. T., & Chen, Y. J. (2004). Protective effect of Mesona procumbens against tertbutyl hydroperoxide-induced acute hepatic damage in rats. Journal of Agricultural and Food Chemistry, 52, 4121–4127.
Hunter, T., & Plowman, G. D. (1997). The protein kinases of budding yeast: six score and more. Trends in Biochemical Sciences, 22, 18–22.
Manning, G., Whyte, D. B., Martinez, R., Hunter, T., & Sundersanam, S. (2002). The protein kinase complement of the human genome. Science, 298, 1912–1934.
Hanks, S. K., Quinn, A. M., & Hunter, T. (1988). The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science, 241, 42–52.
Hanks, S. K., & Hunter, T. (1995). The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB Journal, 9, 576–596.
Doree, M. (1990). Control of M-phase by maturation-promoting factor. Current Opinion in Cell Biology, 2, 269–273.
Maller, J. L. (1990). Xenopus oocytes and the biochemistry of cell division. Biochemistry, 29, 3157–3166.
Nurse, P. (1990). Universal control mechanism regulating onset of M-phase. Nature, 344, 503–508.
Meikrantz, W., & Schlegel, R. A. (1992). M-phase-promoting factor activation. Journal of Cell Science, 101, 475–481.
Jung, T., Moor, R. M., & Fulka, J. (1993). Kinetics of MPF and histone H1 kinase activity differ during the G2- to M-phase transition in mouse oocytes. International Journal of Developmental Biology, 37, 595–600.
Dunphy, W. G., Brizuela, L., Beach, D., & Newport, J. (1988). The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell, 54, 423–431.
Gautier, J., Norbury, C., Lohka, M., Nuese, P., & Mailer, J. (1988). Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2. Cell, 54, 433–439.
Labbe, J. C., Capony, J. P., Caput, D., Cavadore, J. C., Derancourt, J., Kaghad, M., et al. (1989). MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. Embo Journal, 8, 3053–3058.
Pawse, A. R., Margery, G. O., & Stocken, L. A. (1971). Histone kinase and cell division. Biochemical Journal, 122, 713–719.
Ozturk, M. A., Karcaaltincaba, M., & Criss, W. E. (1993). Cell cycle control part I cdc related kinases. Journal of Islamic Academy of Sciences, 6(4), 311–318.
Kesari, K. K., & Behari, J. (2009b). Effect of microwave at 2.45 GHz radiations on reproductive system of male rats. Toxicological and Environmental Chemistry. (in press).
Acknowledgements
Authors are thankful to the Council for Scientific and Industrial Research (CSIR) and Indian Council for Medical Research (ICMR), New Delhi, for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kesari, K.K., Behari, J. Microwave Exposure Affecting Reproductive System in Male Rats. Appl Biochem Biotechnol 162, 416–428 (2010). https://doi.org/10.1007/s12010-009-8722-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8722-9