Abstract
In this work, the agitation and aeration effects in the maximization of the β-galactosidase production from Kluyveromyces marxianus CCT 7082 were investigated simultaneously, in relation to the volumetric enzyme activity and the productivity, as well as the analysis of the lactose consumption and production of glucose, and galactose of this process. Agitation and aeration effects were studied in a 2 L batch stirred reactor. A central composite design (22 trials plus three central points) was carried out. Agitation speed varied from 200 to 500 rpm and aeration rate from 0.5 to 1.5 vvm. It has been shown in this study that the volumetric enzyme production was strongly influenced by mixing conditions, while aeration was shown to be less significant. Linear models for activity and productivity due to agitation and aeration were obtained. The favorable condition was 500 rpm and 1.5 vvm, which lead to the best production of 17 U mL−1 for enzymatic activity, 1.2 U mL−1 h−1 for productivity in 14 h of process, a cellular concentration of 11 mg mL−1, and a 167.2 h−1 volumetric oxygen transfer coefficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymatic hydrolysis of lactose, into glucose and galactose, is one of the most important biotechnological processes in the food industry due to the potentially beneficial effects on the assimilation of foods containing lactose, in order to avoid lactose intolerance in humans, as well as the advantages of industrial application because it can be applied to prevent crystallization [1].
Another advantage of this enzymatic process is that, during the lactose hydrolysis by β-galactosidase (EC 3.2.1.23), some galactooligosaccharides (GOS) are formed. These compounds are indigestible, acting as dietary fibers and promoting the growth of bifidobacteria in the intestine, with a subsequent favorable health effect in this organ and in the liver [2, 3].
Increasing industrial demand for lactase requires a development of methods to ensure the economic feasibility of lactose hydrolysis on commercial scales [4]. Throughout the years, with the intention of improving β-galactosidase production, this enzyme has been researched with the objective of obtaining optimized conditions for production. Therefore, the study of parameters, such as the agitation and the aeration, are important because they supply oxygen to the microorganisms during the process, influencing the substrate consumption, and enabling or inhibiting the synthesis of the products [5–7]. Besides, agitation controls nutrient transfer and the distribution of air and oxygen, while aeration determines the oxygenation of the culture and contributes to bulk mixing of the fermentation fluid, especially where mechanical agitation speed are low [8].
The study of the operational condition effects, such as temperature, agitation speed, and aeration rate, on enzyme production and other parameters in the process, such as microorganism growth, dissolved oxygen, pH, and sugars, can play an important role in understanding the enzyme production pattern and can provide information for scale-up [9, 10] and establish well-defined and optimized culture medium oxygenation conditions for yeast growth and β-galactosidase production [11].
Experimental design is a common practice in biotechnology and an important tool to determine the optimal process conditions. This technique is advantageous compared to the conventional method, which handles a single parameter per trial; as such, an approach fails to locate optimal conditions due to its failure to consider the effect of possible interactions between factors [12–16]. Various research workers have applied this technique, especially for the optimization of culture conditions [12–14] and for the determination of optimal values for processing parameters [15, 16]. However, data on the application of this tool simultaneously for the agitation and aeration effects, in the β-galactosidase production from Kluyveromyces marxianus, and the accompaniment of the resulting sugars of the reaction do not exist in literature.
The aim was to investigate, simultaneously, the agitation and aeration effects in the maximization of the β-galactosidase production from K. marxianus CCT 7082 in relation to the volumetric enzyme activity and the productivity; lactose consumption and the production of glucose and galactose of this process were accompanied during the fermentations, in the function of time, also. The goal in this study was to maximize the enzyme production in relation to the agitation speed and aeration rate conditions. In order to do so, the β-galactosidase activity and productivity were analyzed in the experimental design so as to compare the performance of this production.
Materials and Methods
Microorganism, Culture Medium and Inoculum
The microorganism used was K. marxianus CCT 7082, deposited in the Andre Tosello Foundation, Tropical Cultures Collection (Campinas, SP, Brazil). The strain was stored at 4ºC on malt and yeast extract agar (MY) slants, with the following composition (g L−1): yeast extract (3.0), malt extract (3.0), peptone (5.0), glucose (10.0), and agar (25.0). With the purpose of standardizing the inoculum for all trials, this stock culture was maintained at −18°C on MY broth and glycerol 20% (v/v). The inoculum culture was grown in a medium containing lactose as a carbon source, with the following composition (g L−1): (NH4)SO4 (1.2), KH2PO4 (5.0), MgSO4.⋅7H2O (0.4), yeast malt (1.0), and lactose (10.0). The cultivation conditions were 30ºC, 180 rpm for 14 h in an orbital shaker (Tecnal TE-420, Brazil) according to Pinheiro et al. [11].
β-Galactosidase Production in the Stirred Fermenter
The fermentations were carried out in a 2 L Biostat B fermenter (B. Braun Biotech International, Germany), with a working volume of 1.5 L, containing optimized culture medium by Manera et al. [14] ((NH4)SO4 (0.8), yeast malt (17.0), KH2PO4 (5.0), MgSO4⋅7H2O (0.4) and lactose (28.8)) to which 10% (v/v) inoculum was added. The pH was adjusted to 6.0 and not further controlled during the process. The temperature was kept at 30°C. The dissolved oxygen and the pH were determined using electrodes (Mettler-Toledo, Brazil) in the fermenter. In order to promote the agitation system, two Rushton type impellers was used. Samples were drawn at regular intervals of 2 h, throughout the time, and all fermentations were carried out for biomass, enzymatic activity, sugars, and ethanol.
β-Galactosidase activity and productivity were carried out in a central composite design (CCD; 22 trials plus three central points). The seven assays were studied with different combinations of agitation (200–500 rpm) and aeration (0.5–1.5 vvm), with triplicates in the central point (Table 1). The data was organized with the aid of Statistic 5.0 (Statsoft). The ranges used to define the agitation and aeration conditions to be studied in the stirred reactor were based in others researches [4, 16, 17].
Kinetic production of the β-galactosidase was evaluated and defined as the maximum volumetric enzyme activity (U mL−1) of each assay and the respective values of specific enzyme activity (U mg−1), productivity, Y P/X, and Y X/S in the same time of process in the maximum volumetric enzyme production, as presented in Table 1.
Process productivity and volumetric oxygen transfer coefficient (K L a) were determined in the present work. Productivity was defined as the variation between the maximum and the initial β-galactosidase activities and corresponding fermentation time. K L a was measured using a polarographic electrode by the gassing method for each combination of agitation and aeration from the CCD. This method consists of passing nitrogen gas until the oxygen concentration dropped to near zero [18].
Analytical Methods
Biomass Determination
The cell concentration was estimated by measuring absorbancy in a spectrophotometer (Quimis Q-108D, Brazil) at 620 nm and relating the readings to biomass dry weight with a calibration curve [17].
Enzymatic Activity Determination
Intracellular β-galactosidase was obtained by cellular disruption technique by abrasion using ultrasonic waves associated to glass pearls [19]. The β-galactosidase activity using o-nitrophenyl-β-d-galactopiranoside (Sigma-Aldrich) as a substrate was determined as described by Inchaurrondo et al. [20]. One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of o-nitrophenol (ONP) under the condition of the assay. Liberated ONP was measured spectrophotometrically (Quimis Q-108D, Brazil) at 420 nm.
Sugars and Ethanol Determination
Lactose, glucose, galactose, and ethanol concentrations were determined by high-performance liquid chromatography (Varian 9010 model, Bio Rad HPX 87H model column), at 30°C and at an elution solvent (H2SO4) flow rate of 0.7 mL min−1.
Results and Discussion
A CCD (22 trials plus three central points) was performed in order to investigate the effects of agitation speed and aeration rate in the volumetric β-galactosidase activity and productivity. The independent variables and their levels of the experimental design are presented in Table 1: assay 1 (200 rpm, 0.5 vvm), assay 2 (500 rpm, 0.5 vvm), assay 3 (200 rpm, 1.5 vvm), assay 4 (500 rpm, 1.5 vvm), and assays 5–7 (350 rpm, 1.0 vvm).
Figure 1 shows the fermentation runs in the stirred reactor: β-galactosidase activity, biomass, pH, dissolved oxygen, lactose, glucose, galactose, and ethanol as a function of time for each CCD assays. It has been verified in our work that all fermentations presented similar behavior in relation to substrate consumption, increase in biomass production, and enzymatic production, in agreement with those of by Belo and Mota [21] during the production of β-galactosidase from K. marxianus. The enzymatic activity peaks for the central point assays (assays 5–7) coincided with the beginning of the stationary phase at 16 h, and for the remaining assays (1–4), they took place at the end of the exponential growth phase, at 14 h.
Fermentation profile for β-galactosidase production from Kluyveromyces marxianus CCT 7082 in relation to (a) enzymatic activity and biomass, (b) pH and dissolved oxygen, (c) lactose and glucose, and (d) galactose and ethanol as a function for the time for each CCD assays (where assay 1: 200 rpm and 0.5 vvm; assay 2: 500 rpm and 0.5 vvm, assay 3: 200 rpm and 1.5 vvm, assay 4: 500 rpm and 1.5 vvm, and assays 5–7: 350 rpm and 1.0 vvm)
The biomass increment to high levels and the fall of the pH accompanied lactose consumption. In spite of the similarity in growth, the maximum cellular concentrations, enzymatic activity, and productivity obtained differed for each fermentation condition, reaching values between 1.9 and 10.5 mg mL−1, 3.2 and 14.7 U mL−1, 0.2, and 1.1 U mL−1 h−1, respectively.
The pH profile was similar for all experiments, and the results showed that a large variation of pH (6.0–5.0) did not occur, with fermentation beginning at pH 6.0 and finishing at values approximately 5.0. According to Schneider et al. [7], when the system is submitted to insufficient agitation conditions, the pH decreases due to the accumulation of CO2, from the microorganism metabolism.
The concentration of dissolved oxygen started to decrease at 2–4 h of process, corresponding to the beginning of the biomass increment. During the logarithmic growth phase, the oxygen was consumed faster. In agreement with Bandaiphet and Prasertsan [22], it was seen that the dissolved oxygen supplement must be at least equal to the oxygen demand. In this work, in the assays with less agitation, it could be observed that the oxygen supply in the fermentation did not correspond to the cellular demand, attaining low biomass and enzymatic activity.
Longhi et al. [23], in studying the follow-up of K. marxianus culture, observed that the dissolved oxygen concentration remained zero for approximately 10 h, while the cellular concentration remained constant; after this, the dissolved oxygen rose again. Similar behavior was verified in the present work; however, the dissolved oxygen elevation was not observed because the experiments were interrupted at 24 h, after maximum β-galactosidase activity.
For Feng et al. [10], a low agitation ensures insufficient oxygen supply for the development of the microorganism and, consequently, of the enzyme production. A high agitation increases the amount of dissolved oxygen, as well as the dispersion of the nutrients in the culture, because it provides an increase in the contact between substrate and microorganism and consequently provides an increase in biomass growth and enzyme production; as was verified in the present work when the assays illustrated in Fig. 1 were compared.
In this work, the ethanol concentration was low and only detected after 16 h of fermentation when the enzymatic activity began to decrease for all the assays and the cellular concentration remained constant until the end of the experiments. Mahoney et al. [24] and Gonzales-Siso [25] reported a decrease of β-galactosidase activity in the stationary phase. For these authors, an explanation about the decrease in β-galactosidase activity might be the enzyme stability. Ladero et al. [26] determined the pH effect on the stability of the β-galactosidase and verified that, in pH values near 5.0, the enzymes lose their activity. Another explanation was defended by Ornelas et al. [27], who studied β-galactosidase production. They verified that the enzyme activity increases during the exponential growth phase, and it declined at late exponential growth phase, suggesting that this decline could be attributed to the low lactose concentration in the culture medium.
It was interesting to observe that the galactose concentration was lower than the glucose concentration, suggesting that the first sugar could have been used in the making of GOS, which should be investigated in future works. Engel et al. [28], studying the GOS synthesis by β-galactosidase from Kluyveromyces lactis, and Chen et al. [29], studying three β-galactosidases from Aspergillus oryzae, K. lactis, and Bacillus sp. in the GOS production, verified that the galactose concentration was smaller than the glucose concentration, showing the galactose consumption by the microorganisms for GOS production.
Despite the volumetric production of β-galactosidase having varied quantities according to each studied condition, the specific enzyme activity (U mg−1) was seen to be similar in almost every fermentation, as can be verified in Table 1, keeping Y P/X almost constant. This occurred as the biomass increase enabled greater volumetric enzyme activity, reinforcing that the different studied agitation and aeration conditions influence biomass production and volumetric enzymatic activity (U mL−1) and not specific enzyme activity (U mg−1). The cell yield on substrate, Y X/S, and the product yield on substrate, Y P/X, were calculated at the maximum enzyme activity point, as shown in Table 1. With regard to Y X/S, in assay 4, we obtained a result 0.35, which is agreement with the one obtained by Lukondeh et al. [30] and Rech and Ayub [31] (0.4 and 0.38, respectively) when studying the β-galactosidase production in K. marxianus culture.
In our work, the value of the volumetric oxygen transfer coefficient (K L a; Table 1) varied from 48.8 to 167.2 h−1 according to the studied conditions of the CCD. It is interesting to observe that the minor values of enzyme activity and productivity (assays 1 and 3) corresponded to the lower values of K L a. In assay 4, as process conditions were more intense (500 rpm and 1.5 vvm), the value of K L a was the highest in the experimental design assays, which influenced the enzymatic activity positively as well as the productivity of the process.
Schneider et al. [7], studying the β-galactosidase production of K. marxianus, did not observe a proportionality between the K L a and the enzyme activity; therefore, when the K L a was increased to 30 for 63 h−1, the enzymatic activity increased to 4 for 22 U mL−1, and the increase in the K L a for 120 h−1 did not modify the enzymatic activity value. In the same study, these authors found an increased enzymatic activity of 22 for 33 U mL−1 and varied the combinations of agitation (rpm) and aeration (vvm) of 500/2.0 and 700/0.66, keeping the initial K L a constant around 60 h−1. An increase to 1,000/0.6 caused a reduction in the enzyme activity to 19 U mL−1. In this work, the absence of linearity behavior was also observed; therefore, the addition in the K L a value of 48.8 for 79.1 h−1, resulted in enzyme activity and productivity increase, and when the K L a increased to 91.6 h−1, the results were lower and increased again when the K L a was 167.2 h−1.
An increase in the variable levels from −1 to +1 for agitation and aeration exhibited an increasing effect of enzymatic activity and productivity. Main effects and interaction estimates for β-galactosidase activity and productivity were reported in Fig. 2. Both effects were significant on a 95% confidence level. In the experimental design, it has been shown that the enzyme production was strongly influenced by mixing conditions, while aeration was shown to be less significant. However, the aeration rate was not significant for enzyme productivity. It can be seen that agitation was the most relevant variable in the process. However, according to Schneider et al. [7], Silva-Santisteban and Maugeri [16], in studying K. marxianus culture, verified that an increase in the agitation speed can cause a brusque fall in the cellular concentration, despite the oxygen transfer increment, due to the shear effect, suggesting the importance of a future study on cellular viability during the fermentation process.
The dependent and independent variables were fitted to a linear model and examined in terms of goodness of fit. Therefore, an analysis of variance (ANOVA) was used to evaluate the adequacy of the fitted model (Table 2). Thus, the maximum values of β-galactosidase activity and productivity were used in this analysis. According to Haaland [32], the R 2 value provided a measure of how much the variability in the observed response values could be explained by the experimental factors and their interactions. A good model (values above 0.9 are considered very good) explains most of the variation in the response. The closer the R 2 value is to 1.00, the stronger the model and the better the response predictions. In this study, a high R 2 value (0.98 and 0.92) and a good performance of the F value (60.86 and 22.00) were obtained, being that these values are approximately seven and four times the F tabulate (9.28 and 6.94) for β-galactosidase activity and productivity, respectively.
According to Carvalho et al. [33], the central points provide additional degrees of freedom for error estimation, which increases power when testing the significance of effects. In our work, the pure error (Table 2) was very low, indicating a good reproducibility of the experimental design. Thus, the coded model was considered predictive, and it can be used to generate the response surface for the ANOVA effects on β-galactosidase activity and productivity, according to Figs. 3 and 4, respectively, and the linear model equations (Eqs. 1 and 2), respectively, to understand the interaction of the variables in the responses. Figures 3 and 4 show the response surface and contour diagrams for β-galactosidase activity and productivity, respectively, as a function of agitation and aeration, according to CCD.
These models describe the β-galactosidase activity (Eq. 1) and productivity (Eq. 2) as functions of agitation speed and aeration rate. In this study, the enzyme activity (Fig. 3) and productivity (Fig. 4) showed similarity in their behavior. The aeration above 1.25 vvm and high agitation speeds (500 rpm) were the best conditions for the process, being that 500 rpm and 1.5 vvm were considered the maximum conditions for the enzyme activity (14.7 U mL−1) and productivity (1.05 U mL−1 h−1) of this enzyme in 14 h. Therefore, to maximize β-galactosidase activity and productivity, both variables must be kept at the highest tested levels.
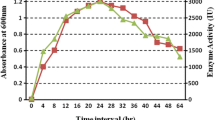
Figure 5 shows the average results referring to the kinetic fermentation (enzyme activity, biomass, pH, dissolved oxygen, lactose, glucose, galactose, and ethanol) of fermentation carried out in duplicate, using the optimized condition (500 rpm and 1.5 vvm) in the production of β-galactosidase from K. marxianus CCT 7082. In this maximized condition, approximately 17 U mL−1 of enzymatic activity, 1.2 U mL−1 h−1 of productivity and cellular concentration of 11 mg mL−1 were obtained.
Studies about β-galactosidase produced from K. marxianus CCT 7082 are important in the function of its application. According Sako et al. [34], β-galactosidase catalyze, under certain conditions, the formation of GOS, which are additives for the so-called healthy foods, and can be synthesized from lactose when this sugar acts as the acceptor and transgalactosylation is catalyzed by β-galactosidase. The presence of these compounds is considered desirable in foods; therefore, GOS formation during lactose hydrolysis favors the growth of intestinal bacterial microflora [2, 35].
Although β-galactosidase is the most employed enzyme for the GOS production, kinetic studies considering the hydrolysis of lactose and oligosaccharides synthesis by these enzymes are limited [36, 37], and most of the works in the literature consider only the hydrolysis reaction [38, 39]. Recent studies are being carried out by researchers of our group in order to apply the enzyme produced in the GOS production.
Conclusion
The methodology of experimental design was verified to be very useful to promote the β-galactosidase activity and productivity maximization. The enzyme production was strongly influenced by mixing conditions, while aeration was shown to be less significant. The conditions established for β-galactosidase maximized production, with 500 rpm and 1.5 vvm for agitation speed and aeration rate, respectively, obtaining approximately 17 U mL−1 enzymatic activity, 1.2 U mL−1 h−1 productivity in 14 h of process, a cellular concentration of 11 mg mL−1, and volumetric oxygen transfer coefficient of 167.2 h−1.
Abbreviations
- K L a :
-
Volumetric oxygen transfer coefficient (h−1)
- rpm:
-
Revolution per minute
- v/v:
-
Inoculum/broth volume
- vvm:
-
Air volume per broth volume per minute
- Y X/S :
-
Cell yield on substrate
- Y P/X :
-
Product yield on substrate
References
Jurado, E., Camacho, F., Luzón, G., & Vicaria, J. M. (2002). Enzyme and Microbial Technology, 31, 300–309. doi:10.1016/S0141-0229(02)00107-2.
Mahoney, R. R. (1998). Food Chemistry, 63, 147–154. doi:10.1016/S0308-8146(98)00020-X.
Boon, M. A., Janssen, A. E. M., & Riet, K. V. (2000). Enzyme and Microbial Technology, 26, 271–281. doi:10.1016/S0141-0229(99)00167-2.
Nor, Z. M., Tamer, M. I., Mehrvar, M., Scharer, J. M., Moo-Young, M., & Jervis, E. J. (2001). Biotechnology Letters, 23, 845–849. doi:10.1023/A:1010526831985.
Justen, P., Paul, G. C., Nienow, A. W., & Thomas, C. R. (1998). Biotechnology and Bioengineering, 59, 762–775.
Serrano-Carreón, L., Corona, R. M., Sanchez, A., & Galindo, E. (1998). Process Biochemistry, 33, 133–146. doi:10.1016/S0032-9592(97)00039-3.
Schneider, A. L. S., Merkle, R., Carvalho-Jonas, M. F., Jonas, R., & Furlan, S. (2001). Biotechnology Letters, 23, 547–550. doi:10.1023/A:1010338904870.
Giavasis, I., Harvey, L. M., & Mcneil, B. (2006). Enzyme and Microbial Technology, 38, 101–108. doi:10.1016/j.enzmictec.2005.05.003.
Milner, J. A., Martin, D. J., & Smith, A. (1996). Enzyme and Microbial Technology, 18, 507–512. doi:10.1016/0141-0229(95)00155-7.
Feng, Y., He, Z., Ong, S. L., Hu, J., Zhang, Z., & Ng, W. J. (2003). Enzyme and Microbial Technology, 322, 82–89.
Pinheiro, R., Belo, I., & Mota, M. (2003). Letters in Applied Microbiology, 37, 438–442. doi:10.1046/j.1472-765X.2003.01429.x.
Kalil, S. J., Maugeri-Filho, F., & Rodrigues, M. I. (2000). Process Biochemistry, 35, 539–550. doi:10.1016/S0032-9592(99)00101-6.
Burkert, J. F. M., Maugeri-Filho, F., & Rodrigues, M. I. (2004). Bioresource Technology, 91, 77–84. doi:10.1016/S0960-8524(03)00152-4.
Manera, A. P., Ores, J. C., Ribeiro, V. A., Burkert, C. A. V., & Kalil, S. J. (2008). Food Technology and Biotechnology, 46, 66–72.
Burkert, J. F. M., Maldonado, R. R., Maugeri-Filho, F., & Rodrigues, M. I. (2005). Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 80, 61–67. doi:10.1002/jctb.1157.
Silva-Santisteban, B. O. Y., & Maugeri-Filho, F. (2005). Enzyme and Microbial Technology, 36, 717–724. doi:10.1016/j.enzmictec.2004.12.008.
Rech, R., Cassini, C. F., Secchi, A., & Ayub, M. (1999). Journal of Industrial Microbiology & Biotechnology, 23, 91–96. doi:10.1038/sj.jim.2900692.
Galaction, A. I., Cascaval, D., Oniscu, C., & Turnea, M. (2004). Biochemical Engineering Journal, 20, 85–94. doi:10.1016/j.bej.2004.02.005.
Medeiros, F. O., Alves, F. G., Lisboa, C. R., Martins, D. S., Burket, C. A. V., & Kalil, S. J. (2008). Química Nova, 31, 336–339.
Inchaurrondo, V. A., Yantorno, O. M., & Voget, C. E. (1994). Biochemistry, 29, 47–54.
Belo, I., & Mota, M. (1998). Biotechnology and Bioengineering, 8, 451–455.
Bandaiphet, C., & Prasertsan, P. (2006). Carbohydrate Polymers, 66, 216–228. doi:10.1016/j.carbpol.2006.03.004.
Longhi, L. G. S., Luvizetto, D. J., Ferreira, L. S., Rech, R., Ayub, M. A. Z., & Secchi, A. R. (2004). Journal of Industrial Microbiology & Biotechnology, 31, 35–40. doi:10.1007/s10295-004-0110-4.
Mahoney, R. R., Nickerson, T. A., & Whitaker, J. R. (1974). Journal of Dairy Science, 58, 1620–1629.
Gonzales-Siso, M. I. (1994). Process Biochemistry, 29, 565–568. doi:10.1016/0032-9592(94)80019-7.
Ladero, M., Santos, A., García, J. L., Carrascosa, A. V., Pessela, B. C. C., & García-Ochoa, F. (2002). Enzyme and Microbial Technology, 30, 392–405. doi:10.1016/S0141-0229(01)00506-3.
Ornelas, A. P., Silveira, W. B., Sampaio, F. C., & Passos, F. M. L. (2008). Journal of Applied Microbiology, 104, 1008–1013. doi:10.1111/j.1365-2672.2007.03622.x.
Engel, L., Ebrahimi, M. P., & Czermak, P. (2008). Desalination, 224, 46–51. doi:10.1016/j.desal.2007.04.078.
Chen, C., Yu, M., Cheng, T., Sheu, D., Duan, K., & Tai, W. (2006). Biotechnology Letters, 28, 793–797. doi:10.1007/s10529-006-9002-1.
Lukondeh, T., Ashbolt, N. J., & Rogers, P. L. (2005). Journal of Industrial Microbiology & Biotechnology, 8, 284–288. doi:10.1007/s10295-005-0245-y.
Rech, R., & Ayub, M. A. Z. (2007). Process Biochemistry, 42, 873–877.
Haaland, P. D. (1989). Experimental design in biotechnology. New York: Marcel Dekker.
Carvalho, C. M. L., Serralheiro, M. L. M., Cabral, J. M. S., & Aires-Barros, M. R. (1997). Enzyme and Microbial Technology, 2, 117–123. doi:10.1016/S0141-0229(96)00245-1.
Sako, T., Matsumoto, K., & Ryuchiro, T. (1999). International Dairy Journal, 9, 69–80. doi:10.1016/S0958-6946(99)00046-1.
Shin, H. J., Park, J. M., & Yang, J. W. (1998). Process Biochemistry, 33, 787–792. doi:10.1016/S0032-9592(98)00045-4.
Bruins, M. E., Strubel, M., van Lieshout, J. F. T., Janssen, A. E. M., & Boom, R. M. (2003). Enzyme and Microbial Technology, 33, 3–11. doi:10.1016/S0141-0229(03)00096-6.
Neri, D. F. M., Balcão, V. M., Costa, R. S., Rocha, I. C. A. P., Ferreira, E. M. F. C., Torres, D. P. M., et al. (2009). Food Chemistry, 115, 92–99. doi:10.1016/j.foodchem.2008.11.068.
Albayrak, N., & Yang, S. T. (2002). Biotechnology and Bioengineering, 77, 8–19. doi:10.1002/bit.1195.
Nakkharat, P., & Haltrich, D. (2006). Applied Biochemistry and Biotechnology, 129, 215–225. doi:10.1385/ABAB:129:1:215.
Acknowledgements
The authors would like to thank CAPES/PROCAD for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, F.G., Filho, F.M., de Medeiros Burkert, J.F. et al. Maximization of β-Galactosidase Production: A Simultaneous Investigation of Agitation and Aeration Effects. Appl Biochem Biotechnol 160, 1528–1539 (2010). https://doi.org/10.1007/s12010-009-8683-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8683-z