Abstract
Forest biomass is a promising resource for future biofuels and bioproducts. Pre-pulping extraction of hemicellulose by alkaline (Green Liquor) pretreatment produces a neutral-pH extract containing hemicellulose-derived oligomers. A near-term option for use of this extract is to hydrolyze the oligomers to fermentable monomer sugars. Chips of mixed northern hardwoods were cooked in a rocking digester at 160 °C for 110 min in Green Liquor at a concentration of 3% Na2O equivalent salts on dry wood. The mass of wood extracted into the Green Liquor extract was approximately 11.4% of the debarked wood mass, which resulted in a dilute solution of oligomeric hemicelluloses sugars. The concentration of the extract was increased through partial evaporation prior to hydrolysis. Dilute sulfuric acid hydrolysis was applied at conditions ranging from 100 to 160 °C, 2% to 6% (w/v) H2SO4, and 2- to 258-min residence time. The maximum fermentable sugar concentration achieved from evaporated extract was 10.7 g/L, representing 90.7% of the maximum possible yield. Application of the biomass pretreatment severity function to the hydrolysis results proved to offer a relatively poor prediction of temperature and reaction time interaction. The combined severity function, which incorporates reaction time, temperature, and acid concentration, did prove to provide a useful means of trading off the combined effects of these three variables on total sugar yields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
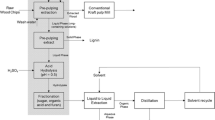
The biorefinery is similar in concept to the petroleum refinery, except that it is based on conversion of biomass feedstocks rather than crude oil. Biorefineries in theory would use multiple forms of biomass to produce a flexible mix of products, including fuels, power, heat, chemicals, and materials. In a biorefinery, biomass would be converted into high-value chemical products and fuels (both gas and liquid). The forest products industry is one of a few national scale industries that have the necessary skill set and infrastructure available to process sufficient biomass for the rapid short-term development and commercialization of biofuel and biochemical technologies. On an annual basis, the US pulp and paper industry sustainably collects and processes approximately 108 million tons of wood for the production of pulp and paper [1, 2]. Extractives from the pulping process provide approximately 700 million liters of turpentine and tall oil annually that could be employed for biodiesel applications [1, 3].
Xylan-based hemicelluloses occur in relatively large amounts in hardwoods (15–30%) and lesser amounts in softwoods (5–10%) [4]. Hemicelluloses normally have monomeric substituents or, in some cases, branch oligomer chains, and vary in composition and structure. In the case of hardwood, the hemicelluloses consist of linear chains of β-1–4 glycosidic-bonded xylose monomers with an average degree of polymerization of 100–200 [5]. Hemicellulose is a byproduct which is not optimally used by Kraft pulp mills today. It is often burned in boilers as part of the black liquor headed for the recovery boiler; however, hemicelluloses have relatively low heating value. Extraction of hemicellulose sugars prior to the pulping operation, if done to a moderate extent, could provide new product opportunities while maintaining production of Kraft pulps. Pre-pulping extraction reduces the mass of dissolved wood components but can be done so as to have little effect on the yield of solid fibers in the final pulp product [6]. The extent of hemicellulose extraction can be tempered by using Green Liquor, the partially recovered (un-causticized) pulping liquor, as the extraction solvent. The salts present in Green Liquor provide a buffering action that prevents the pH from dropping too low, which would result in excessive extraction and reduction in pulp qualities. Pre-pulping extraction releases sugars that are mostly oligosaccharides. Acetic acid is also released in substantial quantities. Acetyl esters normally present on hemicellulose are partly cleaved, leaving some esters still attached to the sugars [7, 8].
Fermentation of the extract to desired products such as ethanol requires that the oligomers be hydrolyzed to their monomer constituents. This hydrolysis can be accomplished through acid- or enzyme-catalyzed hydrolysis. Acid hydrolysis of hemicelluloses has been well studied and is predicted to be effective when applied to Green Liquor extracts of hardwoods. What is not well understood is how much acid will be necessary for optimal hydrolysis of the oligomers, given the highly buffered nature of the Green Liquor extract. Raising acid levels to acidify a strongly buffered solution will increase costs for acid and also costs for ultimate neutralization and subsequent waste (gypsum) disposal. The purpose of this study was to gain a more accurate understanding of the quantities of acid required for effective hydrolysis and the reactivity trade-offs with reaction time and temperature that will enable overall process optimization.
Materials and Methods
Raw Material
Mixed northeast hardwood chips were used throughout this study and were supplied by the Red-Shield Kraft Pulp Mill in Old Town, ME, USA. The chips consisted of roughly 50% maple, with the remainder being mostly beech and poplar. The chips were screened to an average 7/8–5/8 in (22.6∼16.0 mm) using a mechanically vibrated horizontal screen. The screened wood chips were air dried and then used directly in Green Liquor chemical extraction studies. A fraction of the wood chips was ground to an average size of 30∼40 mesh (0.595∼0.420 mm) using a laboratory knife mill for determination of total solids/moisture and carbohydrate content analysis. The composition of this material was analyzed according to the NREL Chemical Analysis and Testing Standard Procedures [9] and is shown in Table 1.
Green Liquor
Green Liquor is the partially recovered form of Kraft liquor. It is obtained after combustion of the black liquor in the recovery boiler by dissolving the smelt from the recovery boiler (Na2S, Na2CO3, and any impurities) in water. In this study, pure chemicals (Na2S: sodium sulfide hydrate, 65% extra pure; Na2CO3: sodium carbonate monohydrate, 99.5% extra pure; Na2SO4: sodium sulfate anhydrous, 99.2% extra pure; and NaOH: sodium hydroxide pellets, 98.5% extra pure; Fisher, Pittsburgh, PA, USA) were used. The composition of the Green Liquor used in the study is listed in Table 2.
Extraction
A hemicellulose extraction was performed using a 20-L rocking digester at the University of Maine Process Development Center. A stainless steel basket in the digester was loaded with 2 kg of air-dried chips of dimension 16 × 22.6 × 4 mm. The residual moisture in the air-dried wood was accounted for in the determination of the amount of Green Liquor to be added, which gave a final ratio of 4 L liquor per kilogram oven dry wood: 0.55 L of Green Liquor (Table 2) was blended with 6.35 L of water and added to 3.1 kg of 36.1% moisture wood. This system was slowly rocked at (2 rpm) and heated up to 160 °C, held for 110 min, and then cooled yielding an H-factor 800. The H factor is an integration of temperature-dependent reaction rate over time, as shown in Eq. 1 [10]. The equation is
in which t is given in minutes and T in degrees Kelvin. Samples were withdrawn from the digester, then analyzed and prepared for subsequent hydrolysis experiments.
Evaporation
A rotary evaporator (IKA® RV 06-ML, Werke GmbH and Co. KG, Germany) was used to concentrate approximately 0.5 L of hemicellulose extraction liquor taken from a single batch from the rocking digester. Samples were removed from the evaporator at roughly 40-min intervals. The evaporator was operated at 150 rpm with a bath temperature setting of 30 °C. Analyses of sugar and solid content were conducted according to NREL Chemical Analysis and Testing Standard Procedures No. 002 and 012 [8], respectively. Liquor concentrated to 5.78% solids was then used for most of the subsequent hydrolysis experiments.
Secondary Acid Hydrolysis
The hydrolysis experiments were performed using sealed tubular reactors. A schematic of a reaction vessel is shown in Fig. 1. The vessels were constructed out of 15-cm long, 1-cm diameter 316 stainless steel tubing, capped at either end with Swagelok fittings, giving an internal volume of 12 cm3. The acid hydrolysis was conducted with 10 mL hemicellulose extract under conditions ranging from 100 to 160 °C, 2% to 6% H2SO4, and 2- to 258-min residence time. Temperature of the vessels was adjusted by immersion in oil baths filled with Heat Transfer Fluid 550 (Fisher, Pittsburgh, PA, USA). The vessels were initially submerged into oil bath set at 50 °C above the desired reaction temperature for rapid preheating. The vessels were then quickly transferred into a second oil bath set at the desired reaction temperature. The temperature of the vessel contents was monitored by a thermocouple (KQXL-18G-12, Omega Eng. Inc., Stamford, CT, USA) inserted into the reactor. After finishing the reaction, the vessel was quenched in an ice bath for 10 min.
Determination of initial carbohydrate concentrations available was also performed by acid hydrolysis, using NREL standard methods No. 002 [9], under conditions of 120 °C, 4% H2SO4, and 60-min residence time. For these determinations, correction for sugars degraded during hydrolysis was made in order to generate accurate compositional analysis of the starting materials. In the subsequent hydrolysis tests, for which the goal was to determine optimal yields of sugars, no corrections were made for degradation of sugars during hydrolysis.
High Performance Liquid Chromatography
The sugar composition of reaction products was quantitatively analyzed by high-performance liquid chromatography (HPLC). The Shimadzu model (LC-10AT Liquid Chromatogram, Shimadzu Corp., Kyoto, Japan) HPLC used for carbohydrates measurement had a Bio-Rad Aminex HPX-87H (300 × 7.8 mm) and a Cation H micro-guard cartridge (30 × 4.6 mm) (Bio-Rad Laboratories Inc., Hercules, CA, USA). The column was maintained at 60 °C and 5 mM H2SO4 was used as eluent at a flow rate of 0.6 mL/min. All of the sugar peaks were detected by a RI detector and UV absorption (280 nm) and were identified and quantified by comparison to retention times of authentic standards. The Bio-Rad Aminex HPX-87H analytical column allows the concurrent analysis for sugars, small chain organic acids, and sugar degradation products, though it does not distinguish between the sugars xylose, mannose, and galactose, which are therefore reported as the aggregate XMG. A previous study has shown that quantification of total sugars in Green Liquor extracts is as effective using the HPX-87H column as other methods that are capable of differentiating all of the sugars present [11].
Results and Discussion
Extraction
The concentrations of carbohydrates released from mixed hardwood chips following Green Liquor extraction are shown in Table 3. Under the extraction conditions used approximately 11.4% of the mass of the wood was extracted. The concentration of Green Liquor used in this study (3% Na2O equivalent on wood) was chosen because it had been previously demonstrated to preserve the quantity and quality of the Kraft pulp that could be produced with the wood solids remaining after extraction [12]. Higher concentrations of Green Liquor have been shown to result in lower carbohydrate concentrations in the liquor, while lower Green Liquor concentrations tend to yield more sugars but degraded pulp quality and quantity [13].
Concentration
Also shown in Table 3 are the concentrations of carbohydrates after being concentrated by evaporation. Hemicellulose extract used for the hydrolysis testing (below) in this study was the partially evaporated extract that contained 0.6 g/L glucan, 9.5 g/L xylan, and 1.7 g/L arabinan, which make up the total carbohydrate content of 11.8 g/L. From the data collected, there is some evidence of preferential evaporation of the more volatile components. The ratios of glucan–XMG–arabinan carbohydrates in this extract were 0.06:1:0.18. The magnitude of these ratios implies that the XMG component is predominantly derived from arabinoglucuronoxylan. Small amounts of glucose are likely the result of hydrolysis of galactoglucomannan [14]. In Table 3, a subtle effect of preferential evaporation of the acetyl groups over carbohydrates is noted, with the ratio of acetyl to total carbohydrates diminishing from 0.93 to 0.83 between the dilute and most concentrated extracts.
Dilute-Acid Secondary Hydrolysis
Comparisons of sugar yields obtained from various acid hydrolysis conditions and processes were simplified with the use of the severity factor R o, which like the H factor used in pulping, combines time and temperature into one variable [15, 16]. The reaction ordinate is given by
in which t is the residence time in minutes and T is the reaction temperature in degrees Celsius. Using this concept, the acid hydrolysis temperature and reaction time conditions are expressed as R o , as shown in Table 4. The experimental design included three ranges of severity: low (R o = 59 to 64), medium (R o = 234 to 256) and high (R o = 935 to 993).
Dilute-Acid Secondary Hydrolysis: Effects of Reaction Severity
Table 4 shows the acid hydrolysis conditions and soluble sugar concentrations of hemicellulose extract after hydrolysis experiments. The effects of temperature (ranging from 100 to 160 °C) on dilute H2SO4 (2, 4, and 6%, w/v) hydrolysis of hemicellulose extract for various reaction times (ranging from 2 to 256 min) were evaluated. The data in Fig. 2 indicate that the sugar, acetic acid, and furfural yields are not completely predicted by the severity of the reaction, since different temperatures at the same reaction severity result in different concentrations of hydrolysis products. This deviation from the predictions of the severity function is particularly apparent with the degradation of sugars to furfural, as can be seen in Fig. 2a, c. It appears that the severity function underpredicts the effects of temperature: as temperature rises, reaction rates appear to increase more than is compensated for by the time–temperature equivalence of the severity function. This result is consistent with previous work on the applicability of the severity function for the prediction of accumulation of degradation products [17]. The maximum yield of xylose of 9.1 g/L was found under conditions of medium severity with 4% H2SO4 (w/v) for 16 min at 140 °C (Fig. 2a). Acetic acid formation is also shown in Fig. 2b. The acetate formation appears to peak at a temperature between 130 and 140 °C for most conditions tested. The flatter curves for the accumulation of acetic acid indicate a more effective prediction by the severity function. Furfural, the product of decomposition of xylose, increased with both temperature and high severity factor (Fig. 2c). This clearly indicates that pentose sugars (xylose and arabinose) derived from hemicellulose were further degraded. However, no HMF was detected after hydrolysis of the extract. The main reason for this may be the small quantities of glucose and glucan that appear in the Green Liquor extract. The data on these minor constituents are, however, subject to little errors because their quantities are small.
Figure 3 plots the same data as Fig. 2, but with time on the ordinate axis. For each combination of temperature and acid concentration several reaction times were studied ranging from 2 to 256 min. As shown in Fig. 3a, a two-level factorial design was used to study the effects of R o and residence time on maximum percent potential xylose in extract for prehydrolysis northeast mixed hardwood. At medium severity and 4% added H2SO4 (pH = 0.85), a maximum of 6.8 g/L of the acetic acid and 9.1 g/L of the potential xylose was found in the secondary hydrolyzate. The maximum occurred at an isothermal reaction time of 16 min. Since shorter reaction times are considered to be impractical, these reaction conditions are thought to be near optimum for secondary sulfuric acid hydrolysis of Green Liquor pre-pulping extract. The results for furfural and acetic acid in extract at the near optimum conditions are plotted as function of residence time in Fig. 3b, c. Here we see that at the maximum, 8.3 g/L of the potential furfural was detected for high severity and 4% added H2SO4 (pH = 0.85) at residence time of 16 min, which implies that most of the xylose fraction has been lost through the hydrolysis (Fig. 3b). Going to higher severity might somewhat reduce both the xylan remaining in the extract and the loss. Tested reaction times were selected such that the longest reaction time was sufficiently long to result in a decreased xylose yield, ensuring that a maximum yield had been at least bracketed by each experimental condition of temperature and pH.
Dilute-Acid Secondary Hydrolysis Results: Effects of Acid Concentration
Since high acid conditions can lead to degradation of sugars to furans, we have evaluated the effects of various acid doses on dilute H2SO4 hydrolysis at the low, medium, and high ranges of the severity factor. Using the 5.87% (w/v, consistency) extracts, an acid dose of 4% (w/v) at medium severity gave the maximum yield of 9.1 g/L XMG, which represents 96% of the available XMG. Overall, about 91% of hemicellulose was converted to monomeric sugars (10.2 g/L, total amount of XMG and arabinose) and only 50% of the glucan (3 g/L) was converted to glucose (Table 4). Interestingly, for the higher and lower acid concentrations tested, the optimal sugar concentrations were achieved at the lower and higher severities respectively, while the optimal sugar yields overall were achieved at medium severity and medium acid concentration. In the lower and higher acid concentrations, the maximum yields of total sugars were 9.4 g/L and 9.7 g/L with 2% H2SO4 for 64 min at 140 °C and with 6% H2SO4 for 8 min at 130 °C, respectively. In addition, little furfural was produced for the runs that resulted in maximum carbohydrates at each acid concentration (run nos. 5, 8, and 18).
The combined severity function has been proposed to consolidate the effects of acid concentration and severity into one variable and is defined as [17]
For which CS is the combined severity factor and R o the severity as defined by Eq. 2 above. Data from Table 4 were translated into product yields relative to the maximum possible sugar concentrations and plotted versus combined severity factor in Fig. 4. It can be seen that the data fall nicely along a continuum of the combined severity factor and that the optimum conditions appear to be in the vicinity of CS = 1.7.
Conclusion
Under conditions optimized to preserve yield and quality of Kraft pulp, the mass of wood extracted into the Green Liquor extract was approximately 11.4% of the debarked wood mass, which results in a dilute solution of oligomeric hemicelluloses sugars. To increase the concentration of sugars achievable, the extract was partially evaporated prior to hydrolysis. Dilute sulfuric acid hydrolysis was investigated as a method to convert the extracted oligomers to fermentable monomeric sugars. The maximum fermentable sugar concentration achieved from partially evaporated extract was 10.7 g/L, representing 90.7% of the maximum possible yield. Application of the biomass pretreatment severity function to the hydrolysis results proved to offer a relatively poor prediction of temperature and reaction time interaction. The combined severity function, which incorporates reaction time, temperature, and acid concentration, did prove to provide a useful means of trading off the combined effects of these three variables on total sugar yields.
References
Ragauskas, A. J., Nagy, M., Kim, D. H., Eckert, C. A., Hallett, J. P., & Liotta, C. L. (2006). Industrial Biotechnology, 2, 55–65.
Hekkert, M. P., Joosten, L. A. J., & Worrell, E. (2001). Resources, Conservation and Recycling, 30, 29–48.
Mabee, W. (2006). Report: Economic, Environmental and Social Benefits of 2nd Generation Biofuel in Canada.
Biermann, C. J. (1996). Handbook of Pulping and Papermaking (2nd ed., p. 34). New York: Academic.
Bailey, J. E., & Ollis, D. F. (1986). Biochemical engineering fundamentals (2nd ed.). New York: McGraw-Hill.
van Heiningen, A. (2006). Pulp Paper Canada, 107(6), 38–43.
Kenealy, C., Horn, E., Davis, M., Swaney, R., & Houtman, C. (2007). Holzforschung, 61, 230–235.
Kenealy, W. R., Houtman, C. J., Laplaza, J., Jeffries, T. W., & Horn, E. G. (2007). A.C.S. Symposium Series, 954, 392–408.
NREL (2004). Chemical Analysis and Testing Laboratory Analytical Procedures (CAT). Golden: National Renewable Energy Laboratory.
Vroom, K. E. (1957). Pulp and Paper Magazine of Canada, 58(3), 228–231.
Um, B-H, & van Walsum, G. P. (2009) Mass Balance on Pre-Pulping Extract of Northeast Mixed Hardwood using High Performance Liquid Chromatography and High Performance Anion Exchange Chromatography. Submitted to Bioresource technology.
Mao, H., Genco, J., van Heiningen, A., & Pendse, H. (2008). J. Biobased Material Bioenergy, 2, 1–9.
Walton, S., van Walsum, G. P., & van Heiningen A., (2009). In preparation.
Jacobs, A., & Dahlman, O. (2001). Bio-macromolecules, 2, 894–905.
Tengborg, C., Stenberg, K., Galbe, M., Zacchi, G., Larsson, S., Palmqvist, E., et al. (1998). Applied Biochemistry and Biotechnology, 70–72, 3–15.
Chum, H. L., Johnson, D. K., Black, S. K., & Overend, R. P. (1990). Applied Biochemistry and Biotechnology, 24/25, 1–14.
Chen, S. F., Du, B., Chambliss, K. C., & van Walsum, G. P. (2007). Applied Biochemistry and Biotechnology, 98(6), 1135–1145.
Acknowledgments
The authors thank Dr. Andriaan vanHeiningen for his many thought-provoking comments and the technical assistance and Red-Shield Kraft Pulp Mill Company (Old Town, ME, USA) for providing northeast mixed hardwood chips. This work was funded by the National Science Foundation (EPSCoR, Contract #: 0554545).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Um, BH., van Walsum, G.P. Acid Hydrolysis of Hemicellulose in Green Liquor Pre-Pulping Extract of Mixed Northern Hardwoods. Appl Biochem Biotechnol 153, 127–138 (2009). https://doi.org/10.1007/s12010-009-8561-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8561-8