Abstract
Biosorption of three divalent metals, viz., lead, copper, and cadmium in ternary aqueous mixtures was studied using Phanerochaete chrysosporium in batch shake flasks. The mixtures were prepared containing the metals at their either varying optimum or equal initial concentration combinations in aqueous solution of pH optimum to each of the metals. Following were the optimum initial concentration ranges of the metals in mixture: lead, 60–100 mg/L; copper, 20–60 mg/L; and cadmium, 5–15 mg/L. And, for varying these optimum concentration levels of the metals, a 23 full factorial design of experiments was employed. The results revealed that an increase in lead and cadmium concentrations helped in their better biosorption by the fungus, but an increase in initial copper concentration slightly diminished its removal. Statistical analysis of the results in the form of analysis of variance and Student t test gave a clear interpretation on the roles of both the individual metals and their interactions in the uptake of metals from mixture. Compared to the uptake of metals when presented individually, lead biosorption in mixture was found to be enhanced to a degree as high as 99%; on the other hand, copper and cadmium removals from mixtures were inhibited to the extent of 100% and 98%, respectively. However, this extent of inhibition or enhancement in the metal removals compared to the individual removals was less in mixtures containing all equal concentrations of the metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The need for controlling heavy metal discharge into the natural environment is very well understood owing to the serious environmental hazard posed by these metals. Biosorption, which is based on the ability of different kinds of microbial biomass to bind heavy metals, has been well established and identified as potential alternative to the conventional metal removal technologies [1–3]. Biosorption may involve a combination of mechanisms such as ion exchange, microprecipitation, electrostatic interaction, complexation, etc., and a number of functional charged groups have been identified to play a vital role in binding the metals. Among these charged groups, carboxylate, hydroxyl, phosphate, amine, amide, etc. are present in microbial polymers [4, 5]. The metal chemistry, nature of binding, and affinity towards binding sites also determine the extent to which metal gets bound to the biomass. Investigations on biosorption of metals using bacterial biomass have been carried out but are highly limited in the literature [6–8]; on the other hand, fungal biomass has been extensively studied for sorption of heavy metals from aqueous solution [9, 10]. And, both the dead and live fungal biomass have shown to be equally potent in biosorbing heavy metals [2]. The major advantages of these fungal biomass is mainly due to their good metal uptake capacities, low cost in preparation, and possibility of regenerating used biomass for reuse [11]. Fungal species of Rhizopus arrhizus and Aspergillus niger have been most extensively studied for heavy metals biosorption [12–16]. And, comparatively, only few studies have been done with the white rot fungus Phanerochaete chrysosporium, which has attracted considerable attention because of its ability to degrade a variety of toxic organic pollutants owing to its extracellular peroxidase enzymes. In fact, our own earlier study demonstrated good results on the biosorption of lead, copper, and cadmium by the fungus but from individual metal solutions [17]. However, studies dealing with biosorption of such metals from mixtures using this potential biosorbent are nil. This is necessary because wastewaters often contain more than one type of metal, which may interfere in the removal and/or recovery of the metals in mixture [18]. Also, it is known that metals often interact to give rise to effects that are synergistic, antagonistic, or noninteractive, and these results, although crucial, are not reflected in single metal studies [19]. The present study was directed towards investigating the biosorption of lead, copper, and cadmium in ternary aqueous mixtures by the white rot fungus P. chrysosporium. And, for one part of the study containing metals at optimum initial concentration combinations, factorial design of experiments, a statistical design technique, was applied.
A statistically designed experiment is usually a systematically tailored series of experiments employing which meaningful results are obtained by changing the input variables in a designed fashion. It refers to a scientific approach of planning the experiments so that the results can be analyzed statistically leading to valid, meaningful, and objective conclusions. Also, statistical methodology plays significant role in analyzing the data where there is a chance of experimental errors [20]. Among the statistical design techniques, the full factorial 2k design is particularly useful in the early stages of experimental work, when there is a likelihood of investigating individual and interaction effects of many factors on the design response. It also provides the smallest number of runs with which ‘k’ factors can be studied in a complete factorial design. Because there are only two levels for each factor, which are usually denoted as low (−) and high (+), it is assumed that the response is approximately linear over the range of the factor levels chosen. More specific details on this design technique and statistical analyses of its results can be found elsewhere in Montgomery (2004) [20].
Materials and Methods
Chemicals and Culture Media
Metal salts of Pb(NO3)2, CuSO4⋅5H2O, and 3CdSO4⋅7H2O, other chemicals, and reagents used in the study were of analytical grade, obtained from s.d. Fine Chemicals, India. The media components, dextrose and soya peptone, used to cultivate the fungal biomass was procured from Himedia Laboratories, India.
Microorganism and Culture Conditions
P. chrysosporium ATCC 24725, a white rot fungus used in this biosorption study, was cultivated at 30–32 °C in liquid media containing dextrose 15 g/L and soya peptone 3 g/L, pH 5.6, with agitation on a rotary shaker at 150 rpm. The fungus was maintained on malt agar slants and subcultured every month and stored in a refrigerator (4 °C).
Biosorption of Metals in Ternary Metal Mixture
All biosorption experiments in the study were performed using 20 g/L (wet weight basis), an equivalent dry weight of 1 g/L, of resting biomass of P. chrysosporium. The biomass, grown in liquid culture media for 3 days, was harvested by centrifugation at 10,000×g for 10 min, washed with deionized water, and subsequently used as biosorbent in all the experiments. Each biosorption flask was incubated for an equilibrium time of 30 min in a rotating orbital incubator shaker set at 150 rpm.
Different combinations of previously found optimum initial metal concentrations with pH optimum to each metal were tested to investigate the biosorption potential of the fungus in removing lead, copper, and cadmium from ternary metal mixtures. For varying the concentration levels of the metals, a 23 full factorial design technique was applied: Table 1 presents the concentration combinations of the three metals in the study. As mentioned in Table 1, the concentration ranges of the metals in mixture were: lead, 60–100 mg/L; copper, 20–60 mg/L, and cadmium, 5–15 mg/L. Three replicates at the center point or middle level of each metal were also included in the design thus giving a total of 11 experimental trials at pH optimum to each metal. The values of both optimum pH and initial concentrations chosen for each metal were based on our earlier study using the fungal biosorbent [17]; optimum pH values chosen for lead and copper were 5.8 and 4.6; in case of cadmium, it was set at 5.3. Thus, for carrying out biosorption experiments containing optimum initial concentrations of the metals in mixture, there were three flasks for each experimental combination having solution pH adjusted optimum to each of the metals. Similarly, another set of experiments, in duplicate, was run in biosorption flasks containing equal concentrations of the three metals in mixture, i.e., at 5, 20, 60, and 100 mg/L each. Biomass free liquid samples at the end of sorption equilibrium time were obtained by centrifugation and analyzed for residual metal concentration. The results of metal removal in the study were expressed as amount of metal uptake, calculated as per the following equation:
where q is the metal uptake (milligrams per gram of the biosorbent), V is the volume of metal solution (liter), C i and C f are the initial and final metal concentrations in solution (milligrams per liter) and m is the dry weight of the biosorbent used in experiments (grams).
Statistical analyses of the results, in the form of analysis of variance (ANOVA) and Student t tests to interpret the individual and interaction effects between the metals on each other removals by the fungus in mixture were performed using the statistical software MINITAB (ver. 12.2, PA, USA).
Metal Analysis
Concentrations of lead, copper, and cadmium in the biomass free samples were estimated using SOLAAR 969 model Atomic Absorption Spectrophotometer (UNICAM, UK) at their respective absorbance maximum of 283.3, 324.8, and 228.8 nm [21]. All analyses were done in the linear range of concentration and the accuracy of analyses was estimated to be ±3%.
Results and Discussion
Biosorption Involving Varying Optimum Initial Concentration Combinations of the Metals in Mixture
As mentioned earlier, the initial concentrations of the metals and pH of the mixtures were kept optimum to the respective metals based on our earlier work dealing with the metals being presented individually to the fungal biomass. The 23 factorial design of experiments was adopted since the metals, and their interactions were expected to play an important role in biosorption in mixture. Table 1, which was earlier referred to present the concentration combinations used in the experiments, also shows the values of metal uptake obtained in the mixture study. Lead removal efficiency in all the experimental runs in this study were superior compared to the observation of Amini et al. [22] where even at optimum condition, the lead removal efficiency was only about 5 mg/g lead of A. niger biomass. However, cadmium removal efficiency in present study was inferior compared to the reported optimal value 6.71 mg/g of Saccharomyces cerevisiae in single metal ion solutions [23]. It can be noted here that the studies by Amini et al. [22] and Ghorbani et al. [23] were not conducted in mixture condition, and the biosorbents used were different.
From our present study, it is clear that lead removals were superior to both copper and cadmium removals, and cadmium removal was the worst in all the experimental runs. These trends on the efficiency of the biosorption system to remove these metals from mixture were consistent when the metals were presented as individual metal solutions at their corresponding optimum concentrations and pH. Very recently, Lu et al. [24] also studied biosorption of lead, copper, and cadmium by Enterobacter sp. J1 in ternary mixture and found that the preference of metal sorption by the bacterium followed the order Pb > Cu > Cd [24]. However, a comparison of these two results from mixture and from individual solutions revealed that while the copper and cadmium removals were severely inhibited, lead removal, on the other hand, was found to be enhanced several times in the mixture. Table 2 illustrates the extent of inhibition in copper and cadmium removals and enhancement in lead removal obtained by comparing the removals from mixture and individual solutions. This table clearly reveals that a maximum extent of the effect, i.e., highest percentage of enhancement in lead removal and inhibition in copper and cadmium removals, was observed when the initial concentrations of the metals were generally at higher levels. For instance, while there was 98.6% enhancement in lead removal, the inhibition in copper and cadmium removal was found to be 89% each at all high concentration levels of the metals. Nonetheless, these effects were also quite significant at other experimental trials. Because the total uptake capacity of the biosorbent in mixture was more compared to that in individual solutions, the presence of multiple binding sites on the biomass cannot be ruled out.
According to the soft and hard principles of metals [18], copper and cadmium belong to the same borderline class of metals and, hence, may compete with each other for the same active sites on the biomass resulting in a diminished uptake of both the metals in mixture compared to their uptakes in individual solutions. Similarly, metals belonging to different classes do not show any inhibition in the uptake of each other [18]. Hence, it could be said that uptake of lead, which belongs to class-b different than copper and cadmium, was not inhibited due to either or both of the two metals in mixture. Therefore, it is quite expected that inhibition in copper or cadmium uptake in mixture occurred due to: (a) competition between the two metals for binding sites on the biosorbent and/or (b) enhanced uptake of lead on the biosorbent surface. Lead, due to its higher atomic weight and ionic radius, can also be more preferentially sorbed than either copper or cadmium or both from such mixtures [25]. This higher capacity of the fungus toward lead uptake compared to the other metals was also found to be true in the individual biosorption experiments. Further, enhancement in lead uptake from mixture can also be attributed to formation of an insoluble lead product resulting in deposition of the metal on the biomass surface: it is well known that lead exists in aqueous solution of pH 5.8 without getting precipitated when presented in the form of nitrate or to a lesser extent, as acetate; the other salts of lead, e.g., sulfates, are less or least soluble [26]. Also, an already known fact, addition of sulfate salt of metal to nitrate salt of another metal in solution leads to formation of sulfate salt of the latter, making it precipitate from solution without any change in pH. The same phenomenon can now be reasoned for the formation of insoluble lead sulfate product in this mixture study where lead was originally added in the form of lead nitrate. These reactions—displacement and formation of insoluble lead sulfate product—could have very well taken place in simple aqueous solution of metal mixture containing no biosorbent, but a significant decrease in lead content from the solution was detected only in samples taken at the end of biosorption experiments involving the fungal biomass. This phenomenon only suggests the active role of cell wall or such surface structures of P. chrysosporium in biosorption mechanism, commonly referred to as the microprecipitation, for removing lead from mixture. Muter et al. [27] have shown a similar enhancement in Cr (VI) removal due to microprecipitation by biomass of Candida utilis in presence of a variety of metals, i.e., either cadmium, lead, or copper in binary mixtures; the insoluble microprecipitate formed was reported to be a metal-chromate complex, viz., cadmium chromate, lead chromate, or copper chromate when cadmium, lead, or copper was respectively presented along with Cr (VI) in solution to the yeast biomass [27].
Statistical Analysis and Interpretation of the Results
To validate the roles played by the different metals and their interactions on their removals, statistical analysis of the results in the form of ANOVA and Student t test was carried out for each metal case. The ANOVA was used to investigate and model the relationship between metal removal by biosorption in mixture and the individual metals and their interactions.
Tables 3, 4, and 5 illustrate the results of ANOVA of removal of the metals in the study. From Table 3 on ANOVA of lead removal from the mixture, it is clear that all the effects—individual (main), two-way, and three-way interactions—were highly significant at more than 99% confidence level (P < 0.01). On the other hand, in case of copper removal, the ANOVA results shown in Table 4 revealed only the individual or main effects of the metals to be highly significant (P = 0.019); all other effects seemed not important on copper removal from the mixture. Similar to lead, for cadmium removal (Table 5), all the effects were also highly significant; however, P values were slightly more between 0.05 and 0.03, i.e., between 95% and 97% confidence levels. The ANOVA tables for each metal also displays an error term, estimated from the results of replicates performed at center point or middle level of initial optimum concentrations of each metal in the mixture. And from these estimates, error in the experiments was found to be less than 1%. Moreover, accuracy and precision of the models, in the form of determination coefficient (R 2), adjusted R 2, adeq. precision, standard deviation (SD), coefficient of variation (CV), and predicted residual error sum of squares (PRESS) and shown in the ANOVA Tables 3, 4, and 5, suggest that the models were highly efficient in predicting the experimental metal removal data. However, among the three models, the model for copper was found slightly less accurate than the respective models for lead and cadmium.
To further understand which of the individual metals of copper, lead, and cadmium and their interactions played a crucial role in biosorption by the fungus in mixture, Student t test was performed. While the estimated coefficients of individual and interaction terms presented in Table 6 described the removal of the metals from mixture, the associated t and P values in the table were used to indicate significance of these coefficient terms. The model equations relating the observed metal removals (q) with their initial concentrations are presented in the Eqs. 2–4 for lead, copper, and cadmium, respectively.
The predicted values of the metals due to these regression model equations are also mentioned in Table 1, which suggests that both the experimental and model predicted metal removal values from each experimental run matched closely well with each other.
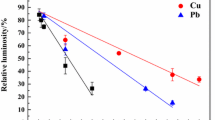
On individual effects of the metals, a term that is synonymous with main or linear effects, coefficient values of both lead and cadmium showed very high significance (P < 0.001) on lead removal by the fungus, thereby, also revealing that the individual effect of copper is weaker than cadmium. All other coefficient values of both two-way and three-way interaction effects of metals, including those with copper, showed high significance on lead removal. However, the t value for the three-way interaction effect was negative, which was only slightly significant than the all-positive two-way interactions. On copper removal from mixture (Table 6), all the coefficients of two-way interaction effects, particularly those obtained from lead–cadmium and copper–cadmium interactions, were significant with P values less than 0.02. Although the coefficient of interaction between lead and copper was less significant, its t value was found to be negative. All other effects, including its own individual effect, did not show any importance on copper removal, as found from the P values of coefficients for these effects (Table 6). The estimated coefficients and their effects on copper removal reveals that copper biosorption by the fungus in mixture is not strongly influenced, positively or negatively, by changes in initial optimum concentration levels of the metals, including its own. But, compared to copper removal from its individual solution, a large inhibition in its removal from mixture is observed, suggesting a major role played by the biomass or similar factors than the chemistry due to the heavy metals on copper removal from mixture, which, however, requires further investigations to confirm. Contrary to the aforementioned findings on lead and copper removals from mixture, the estimated coefficients and their significance on cadmium removal manifested a distinct behavior of inhibition from copper: both the coefficients of individual effect due to copper and its interaction with cadmium were highly significant at more than 99% confidence levels with large negative t values (Table 6). The coefficient of effects due to lead, both individual and its interaction with copper, did not show any significance despite the t values being positive for these two terms. Nonetheless, coefficient of interaction between cadmium and lead was highly significant with relatively a large positive t value suggesting that lead in no way affected cadmium biosorption by the fungus. The main effects plots for the biosorption of Pb, Cu, and Cd achieved from the different experimental conditions are shown in Figs. 1, 2, and 3, respectively. It can be interpreted from these plots that an increase in the concentrations of the individual metals, except for copper, from low to high levels increased their own removals; but for copper removal, all the three metals showed negative main effect. Moreover, an increase in the concentration of copper from low to high level inhibited removal of cadmium but did not have any affect on the lead removal. The interaction plots (Figs. 4, 5, and 6) indicate that all interactions between any two of the metals were positive in case of lead removal (Fig. 4). On the other hand, interaction between lead and copper for copper removal (Fig. 5) or interaction between copper and cadmium for cadmium removal (Fig. 6) were negatively correlated. These types of observations on main and interaction effects between variables on a similar bioprocess have also been made recently by Jo et al. [28] using such main and interaction plots.
Biosorption Involving Equal Concentration Combinations of the Metals in Mixture
Uptake of metals by the fungus in mixture containing equal concentrations of the metals were determined to verify our previous observations on the effects of simultaneously varying initial optimum concentration of a metal over others in mixture. Quite expectedly, inhibition in copper and cadmium removals and enhancement in lead removal occurred in these experiments when comparing their uptake values from individual solutions. Moreover, the extent of these effects (inhibition in copper and cadmium removals and enhancement in lead removal), presented in Table 7, also became pronounced with simultaneous but an equal increase in concentrations of the metals. But, the extent of both these effects was lesser than in the previous experiments with varying optimum initial concentrations of the metals in mixture. Hence, it could be surmised that concentration-dependent mechanism governs the extent of inhibition or synergism in biosorption of metals by P. chrysosporium in mixture. But, because the exact role of individual and interaction between the different metals on their removals in these experiments could not be ascertained, biosorption experiments containing any two of the three metals in mixture are currently underway.
Overall, it could be said that initial concentration of a metal, in relation with other metals, in mixture plays a critical role in its uptake by biosorption using the live resting fungal biomass of P. chrysosporium. It also appears that, from a real wastewater treatment perspective, complex individual and interaction effects due to metals on their removals by biosorption in mixtures can better be obtained by statistically designed experiments than simple extrapolation of results from individual biosorption studies.
Conclusion
Compared to the uptake of lead, copper, and cadmium by P. chrysosporium in individual solutions, biosorption of the three divalent metals in ternary aqueous mixtures, containing either varying optimum initial concentrations or equal concentrations of the metals, revealed enhancement in lead removal and inhibition in copper and cadmium removals by the fungus. Statistical planning of experiments and analysis of the obtained results gave a clear interpretation on the roles of individual metals and their interactions on the biosorption of metals by P. chrysosporium in mixtures containing different optimum initial concentrations of the metals. The effect of lead initial concentration on its removal from mixture was much higher than those due to variation in the levels of copper and cadmium. In the case of copper uptake, increasing the levels of both lead and cadmium seemed inhibitory, and even an increase in initial copper concentration could not improve its removal, probably due to a higher capacity of the biosorbent to microprecipitate lead than binding copper at higher initial copper concentration in the mixture. In biosorbing cadmium, inhibition was mainly due to copper—a divalent metal belonging to the same class, and an increase in cadmium initial concentration, however, improved its biosorption.
The observed negative interaction between copper and cadmium on their removals were attributed due to class behavior of the metals and direct competition between the two for uptake sites on the biomass. On the other hand, high values of lead biosorption seem to arise from both its direct cell surface binding and microporecipitation mechanisms. In biosorption involving mixtures with equal concentrations of the metals, the extent of inhibition (in copper and cadmium removals) and enhancement (in lead removal) was low. Thus, the extent of the effects—inhibition or enhancement—in biosorption of a metal by the fungus largely depended on its initial concentration relative to others in mixture.
References
Chong, K. H., & Volesky, B. (1995). Biotechnology and Bioengineering, 47, 451–460. doi:10.1002/bit.260470406.
Kapoor, A., & Viraraghavan, T. (1995). Bioresource Technology, 53, 195–206. doi:10.1016/0960-8524(95)00072-1.
Tsezos, M., Remoudaki, E., & Angelatou, V. (1996). International Biodeterioration & Biodegradation, 38, 19–29. doi:10.1016/S0964-8305(96)00011-X.
Brady, J. M., & Tobin, J. M. (1995). Enzyme and Microbial Technology, 17, 791–796. doi:10.1016/0141-0229(95)00142-R.
Cabral, J. P. S. (1992). Microbios, 71, 47–53.
Ansari, M. I., & Malik, A. (2007). Bioresource Technology, 98, 3149–3153. doi:10.1016/j.biortech.2006.10.008.
Congeevaram, S., Dhanarani, S., Park, J., Dexilin, M., & Thamaraiselvi, K. (2007). Journal of Hazardous Materials, 146, 270–277. doi:10.1016/j.jhazmat.2006.12.017.
Leung, W. C., Chua, H., & Lo, W. (2001). Applied Biochemistry and Biotechnology, 91–93, 171–184. doi:10.1385/ABAB:91-93:1-9:171.
Murugesan, G. S., Sathishkumar, M., & Swaminathan, K. (2006). Bioresource Technology, 97, 483–487. doi:10.1016/j.biortech.2005.03.008.
Melgar, M. J., Alonso, J., & García, M. A. (2007). The Science of the Total Environment, 385, 12–19. doi:10.1016/j.scitotenv.2007.07.011.
Płaza, G., Łukasik, W., & Ulfig, K. (1996). Sorption of cadmium by filamentous soil fungi. Acta Microbiologica Polonica, 45, 193–201.
Aksu, Z., & Karabayır, G. (2008). Bioresource Technology, 99, 7730–7741. doi:10.1016/j.biortech.2008.01.056.
Preetha, B., & Viruthagiri, T. (2007). Separation and Purification Technology, 57, 126–133. doi:10.1016/j.seppur.2007.03.015.
Naja, G., Mustin, C., Berthelin, J., & Volesky, B. (2005). Journal of Colloid and Interface Science, 292, 537–543. doi:10.1016/j.jcis.2005.05.098.
Mukhopadhyay, M. (2008). Colloids and Surfaces A: Physicochemical and Engineering Aspects, in press.
Mungasavalli, D. P., Viraraghavan, T., & Jin, Y. C. (2007). Colloids and Surfaces A: Physicochemical and Engineering Aspects, 301, 214–223. doi:10.1016/j.colsurfa.2006.12.060.
Gopal, M., Pakshirajan, K., & Swaminathan, T. (2002). Applied Biochemistry and Biotechnology, 102(1–3), 227–237. doi:10.1385/ABAB:102-103:1-6:227.
Puranik, P. R., & Paknikar, K. M. (1999). Bioresource Technology, 70, 269–276. doi:10.1016/S0960-8524(99)00037-1.
Ting, Y. P., & Teo, W. K. (1994). Bioresource Technology, 50, 113–117. doi:10.1016/0960-8524(94)90062-0.
Montgomery, D. C. (2004). Design and analysis of experiments (6th ed.). New York: Wiley.
Xie, F., Lin, X., Wu, X., & Xie, Z. (2008). Talanta, 74, 836–843. doi:10.1016/j.talanta.2007.07.018.
Amini, M., Younesi, H., Bahramifar, N., Lorestani, A. A. Z., Ghorbani, F., & Daneshi, A. (2008). Journal of Hazardous Materials, 154, 694–702. doi:10.1016/j.jhazmat.2007.10.114.
Ghorbani, F., Younesi, H., Ghasempouri, S. M., Zinatizadeh, A. A., Amini, M., & Daneshi, A. (2008). Chemical Engineering Journal, in press.
Lu, W. B., Kao, W. C., Shi, J. J., & Chang, J. S. (2008). Journal of Hazardous Materials, 153, 372–381. doi:10.1016/j.jhazmat.2007.08.059.
Yetis, U., Ozcengiz, G., Dilek, F. B., Ergen, N., Erbay, A., & Dolek, A. (1999). Water Science and Technology, 38, 323–330. doi:10.1016/S0273-1223(98)00515-0.
Phillips, C. S. G., & Williams, R. J. P. (1965). Inorganic chemistry, Vol. I. New York: Oxford University Press.
Muter, O., Lubinya, L., Miller, D., Grigorjeva, L., Ventinya, E., & Rapoport, A. (2002). Process Biochemistry, 38, 123–131. doi:10.1016/S0032-9592(02)00065-1.
Jo, M. S., Rene, E. R., Kim, S. H., & Park, H. S. (2008). World Journal of Microbiology & Biotechnology, 24, 73–78. doi:10.1007/s11274-007-9441-4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pakshirajan, K., Swaminathan, T. Biosorption of Lead, Copper, and Cadmium by Phanerochaete chrysosporium in Ternary Metal Mixtures: Statistical Analysis of Individual and Interaction Effects. Appl Biochem Biotechnol 158, 457–469 (2009). https://doi.org/10.1007/s12010-008-8374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8374-1