Abstract
Citric acid (CA) production has been conducted through a careful strain selection, physical–chemical optimization and mutation. The aim of this work was to optimize the physical–chemical conditions of CA production by solid-state fermentation (SSF) using the Aspergillus niger LPB BC strain, which was isolated in our laboratory. The parental and mutant strain showed a good production of CA using citric pulp (CP) as a substrate. The physical–chemical parameters were optimized and the best production was reached at 65% moisture, 30 °C and pH 5.5. The influence of the addition of commercial and alternative sugars, nitrogen sources, salts, and alcohols was also studied. The best results (445.4 g of CA/kg of CP) were obtained with sugarcane molasses and 4% methanol (v/w). The mutagenesis induction of LPB BC was performed with UV irradiation. Eleven mutant strains were tested in SSF where two mutants showed a higher CA production when compared to the parental strain. A. niger LPB B3 produced 537.6 g of CA/kg of CP on the sixth day of fermentation, while A. niger LPB B6 produced 616.5 g of CA/kg of CP on the fourth day of fermentation, representing a 19.5% and 37% gain, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Citric acid (CA) is one of the most frequently manufactured products from fermentation in the world due to its lower toxicity—among other characteristics—when compared to other acidulates used by the pharmaceutical and food industries. In 2004, the worldwide production of CA was approximately 1.4 million tons, according to the Business Communications Co. (BCC)’s recent studies on fermentation [1]. Moreover, due to its large application and low price, the CA consumption is expected to grow significantly until 2009, and this raises the need for industries to search for new technological alternatives and for cost reduction in CA production [2].
CA has a variety of applications. Seventy per cent of it is used in the food and beverage industries, 12% in the pharmaceutical industry, and 18% in other industries. In the food industry, it is used as an acidulate due to its lower toxicity and high solubility [3]. This property has led to an increase in its use in the cleaning process of special boilers and installations. In some cases, phosphate is replaced by citrate in detergents in order to increase its power. In this case, it is used not only for cleaning metal, but also in domestic detergents. Due to its easy biodegradability, the use of CA expanded and replaced the polyphosphates.
CA is often produced by fermentation using low cost raw materials. The composition of these products varies according to their origin, conservation, and obtaining methods. A great variety of substrates can be used in CA production by solid-state fermentation (SSF) such as some by-products and agro-industrial residues. There are countless possibilities in establishing industrial activities directed to the improvement and/or reprocessing of bioresidues such as sugarcane bagasse, cassava bagasse, and CP [4], [5], [6], [7], which can cause serious environmental problems.
CP is a by-product of the juice industry obtained from the liquid and solid residues treatment remainders of orange juice extraction. Among these residues are rinds, seeds, and orange pulps, representing 50% of the fruit weight. Since these citric residues are rich in carbohydrates and other nutrients, they are viable substrates to CA production by SSF. Its annual exportation is around a million tons. It is largely used in animal feed supplements, especially those for cattle [8].
The industrial CA production is performed using A. niger, due to its higher capacity to accumulate acid when compared to other microorganisms [9], [10], [11]. The main advantages of the use of A. niger are: its easy manipulation, its ability to ferment a great variety of raw materials, the low cost of its fermentation, and its capacity to obtain high yields of CA [9].
Mutations are abrupt and so are the hereditary modifications in the genetic material. The organisms containing the DNA are not static molecules, and their bases are frequently exposed to natural or artificial agents that can cause modifications in their structure or in chemical composition [12], [13]. The increase in CA productivity has been achieved using mutation and strains selection. Strains with certain characteristics—such as enhanced CA production and increased fermentation rate—have been previously selected after submitting the genetic material to physical or chemical mutagenic agents [13], [14], [15], [16]. The most frequently used method is induction by UV irradiation. Although the UV rays do not have much energy and, consequently, they do not induce ionization directly, the unicellular organisms are potent mutagenic agents, since UV rays are absorbed by purine and pyrimidines, making them reactive and inducing mutation [12], [13]. Therefore, the mutations induced by UV can randomly provide a strain with a higher capacity of CA production when compared to the control strain CA production.
The aim of the present work was to optimize physical and chemical parameters of CA production by SSF. CP was used as an alternative substrate/support in order to obtain a CA concentrate product to be used in animal feed. A mutation of A. niger LPB BC strain through UV rays irradiation was performed in order to find a mutant strain with a higher CA accumulation.
Materials and Methods
Substrate Preparation for CA Production
CP was kindly made available by Cargill Agrícola S.A, São Paulo—SP; and it was already dried and pelletized. Pellets were previously crushed in a grinder to obtain a suitable particle size between 0.8–2.00 mm.
Microorganism
A. niger LPB BC strain was isolated from sugarcane bagasse in the Biotechnology Processes Laboratory (UFPR). One gram of sugarcane bagasse was homogenized in 10 ml of sterile distilled water, agitated in shaker at 120 rpm, 28 °C for 15 min. Successive dilutions were done, and 0.2 ml of each was inoculated in a Petri dish and covered with 20 ml of PDA medium (Pour-Plate). All Petri dishes were kept at 28 °C for 7 days. Isolate colonies were then transferred successively to a new plate until total isolation. One of them was identified morphologically as Aspergillus niger and named A. niger LPB BC. The strain was then maintained in a glass test tube with inclined PDA (Potato Dextrose Agar) medium, from which the microorganisms were replicated. Each strain was incubated for growth for 6 days at 28 °C and kept at 4 °C for a maximum of 2 months.
Spores Suspension
The spores were produced in 250 mL Erlenmeyer flasks with 50 mL of PDA medium. The culture medium was sterilized at 121 °C for 15 min. After cooling (45–55 °C), the medium was inoculated in the spore suspension. In order to obtain this suspension, an extraction of the spores was performed from an inclined glass tube with PDA medium, where 5 mL of sterile distilled water, one drop of Tween 80 and previously sterilized glass pearls had already been added. Each Erlenmeyer with 50 mL of PDA was inoculated with 0.2 ml of this suspension and incubated for 7 days without agitation and forced aeration (aerated by diffusion).
The spores were recovered (under non-sterile conditions) from the medium surface using 30 mL of sterile distilled water, containing one drop of the 0.01% Tween 80 solution, glass pearls and one stirring bar, agitated during 15 min on a magnetic agitator at 600 rpm. The suspension obtained was stored at 4 °C for at least 7 days.
Successive spore suspension dilutions were made for spore counting in a Neubauer chamber using an optical microscope. Based on the counting results, the amount of spore suspension that should be added to the fermentation medium was calculated so as to contain 107 spores/g of substrate.
Solid-state Fermentation for CA Production
Parental and mutant strains of A. niger LPB BC were tested in SSF with CP as substrate using the following initial physical–chemical conditions: 75% initial humidity, obtained by adding a solution containing KH2PO4 (1g/L), ZnSO4 (0.2 g/L) and methanol (4% w/v), pH 6.5 in 250 ml Erlenmeyer flasks. The fermentation was conducted at 30 °C for 4 or 5 days. After optimizing the physical–chemical variables of the process, some of these conditions were then changed.
Experimental Design
Statistical experiments and analysis were carried out using the software package STATISTICA 7.1 (StatSoft, Tulsa, OK, USA).
Optimization of Physical–Chemical Variables of CA Production
pH and Initial Humidity
A 32–0 full factorial design leading to nine sets of experiments, performed in duplicate, was used to find optimal conditions for the pH and initial moisture variables, and also to show the influence of these variables on CA production. Uncoded variables are given in Table 1. The results were adjusted to the following quadratic model (Eq. 01):
Ten grams of CP—with particle size between 0.8 and 2 mm—were distributed in 250 mL Erlenmeyer flasks. The substrate was impregnated with the nutritive solution KH2PO4—1 g/L, ZnSO4·7H2O—0.2 g/L and 4% methanol (v/w) in order to have initial moisture of 65%, 75%, or 80%. pH was adjusted to 5.5, 6.0, or 7.0, using NaOH and HCl 0.1 N. Afterwards, the substrate was inoculated in a concentration of 107 spores/g of dry CP and homogenized for subsequent incubation at 30 °C during 96 h.
Effect of Salt Addition
The Plackett–Burman design was the statistical approach chosen for this optimization study. It allows to investigate up to N – 1 variables with N experiments. Plackett–Burman designs are efficient for screening purposes when one knows which components should be present in the medium [17]. All the experiments are carried out according to a design matrix, which is based on the number of variables to be studied. A seven factor Plackett–Burman design, resulting in eight runs, performed in duplicate, was used to determine the most significant salts in CA production. The following salts were used with their respective concentrations: NaCl—1 g/L, KH2PO4—0.7709 g/L, MgSO4·7H2O—0.18 g/L, FeSO4·7H2O—0.0105 g/L, ZnSO4·7H2O—0.1542 g/L, CuSO4·5H2O—0.0005 g/L. The choice of salts for the experiment was made according to the literature [18], [19], [20], [21], [22], [23]. All the values (replicates) were used in statistical evaluations.
Fermentation was carried out at 30 °C for 4 days at an initial pH 5.5, 65% initial humidity, with the addition of 4% methanol (v/w) without carbon sources.
Effect of Carbon Sources
Experiments were carried out using the previous optimized conditions: pH 5.5, 65% moisture, 4% methanol (v/w) and particle size between 0.8 and 2 mm. Different commercial carbon sources such as glucose, fructose (30, 60, and 120 g/L) and sucrose (27, 54, 108, and 216 g/L) were tested, regarding their concentration and the molecular weights. After analyzing the preliminary results, other concentrations of glucose (60, 120, and 240 g/L) and sucrose (54, 108, and 216 g/L) were tested. From those results, cheaper carbon sources were tested: sugarcane molasses and soy molasses, both diluted so as to have a total sugar concentration of 108 g/L.
Effect of Nitrogen Sources
The nitrogen sources were chosen based on the optimization of CA production made by Vandenberghe [22], where 2.93 g/L of urea was used. Thus, the tests were carried out with the addition of the following nitrogen sources: NH4Cl, NH4NO3, (NH4)2SO4 and urea in the corresponding concentrations of 1.8 g of N/L. Fermentation conditions were the same as presented before.
Effect of Lower Alcohols
Methanol and ethanol were tested in different concentrations such as 2, 4, 6, and 8% (v/w) without the addition of carbon sources. After these tests, methanol was defined as the best low alcohol source. Further tests were conducted with 4, 5, 6, and 8% of methanol, using pH 5.5, 65% initial moisture, and the addition of sugarcane molasses (108 g/L). Fermentation was carried out for 4 days at 30 °C.
Mutation of A. niger BC Induced by UV
Spores of A. niger LPB BC strain were collected from the surface of a test tube containing PDA medium. With the aid of a platinum grip, spores were harvest in a previously prepared and sterilized solution containing Tween 80. This suspension was named “mother suspension”.
UV Irradiation
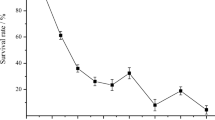
The spore suspension was transferred from the glass tube to a Petri dish. An UV lamp was used in a dark environment in order to hinder the repair mechanisms that could invert the mutagenic effects [12]. The radiation was diffused above the suspension for 2 min to obtain 5% strain survival. For the establishment of the irradiation time (2 min) and the estimation of 5% survival of the microorganism, a curve of survival percentage versus UV exposition time (min) was previously built (data not shown). In the 5% survival zone, there was a large concentration of mutants.
In a dark environment, the spore suspension treated with UV light was diluted until achieving a 10−3 dilution in order to obtain 30 colonies per plate. One ml of the dilution was inoculated in three Petri dishes with MM and two Petri dishes with CM, and spread using a Drygalski grip.
All Petri dishes containing the “mother” suspension and those with the suspension treated with UV irradiation were incubated at 28 °C for 48 h. They were inverted to avoid contamination by spore dissemination. The plates containing the spores treated with UV light were placed into closed metal tubes to protect them from light.
Preparation of Culture Mediums
The culture mediums, the minimal (MM) and complete (CM) mediums consisted of a mixture of components which are essential for microorganism growth. These mediums were prepared in the laboratory with dehydrated nutrients.
Minimal Medium (MM)
The MM has the following formulation: NaNO3 6.0 g; KH2PO4 1.5 g; KCl 0.5 g; MgSO4·7H2O 0.5 g; FeSO4 0.01 g; ZnSO4 0.01 g; glucose 10.0 g; agar 15.0 g and the volume completed to 1,000 ml with distilled water.
Complete Medium (CM)
The CM used presents a formulation similar to that of the MM with the addition of the following ingredients: peptone 2.0 g; yeast extract 2.0 g; hydrolyzed casein 1.5 g; vitamins solution 1.0 ml; agar 15.0 g; and the volume completed to 1,000 ml with distilled water.
Foster Medium
The Foster medium was prepared with glucose 5 g, peptone 1 g, KH2PO4 1 g, MgSO4 0.5 g, agar 15 g, 65 ml of bromocresol green (BCG) solution (0.5 g of bromocresol green in 7 ml of NaOH 0.1 N completed to a volume of 130 ml and stored in a dark flask) and completed to 1,000 ml with distilled water. The pH was adjusted to 5.0 and the medium was autoclaved at 121 °C for 15 min. The medium was transferred to sterilized Petri dishes (15 ml). After the medium had cooled and solidified, the spores were inoculated on its surface.
Tests with Strains Obtained From UV Irradiation in Foster Medium
The obtained colonies were transferred to ten Petri dishes containing Foster media. Each plate contained one control colony and eight treatment colonies. The colonies were separated in such a way that five plates containing Foster medium received the colonies grown in MM, and five plates received the colonies grown in MC. All plates were incubated at 28 °C for 48 h.
In the Foster medium plates, the colonies and the developed yellow halos were measured. The Foster media presented an initial green coloration—due the presence of the bromocresol green (BCG) solution—used as an indicator of pH variation. The Foster medium coloration changed from green to yellow through acid liberation in that medium.
Kinetics of CA Production by SSF Using the Mutant Strains
A seven-day kinetics was performed in SSF at 30 °C with the A. niger strains and it showed good CA production results using the same optimized conditions of the original A. niger BC strain (pH 5.5, addition of aqueous solution of sugar cane molasses with 108 g/l of total sugar and 4% methanol (v/w) obtaining a 65% initial moisture). This medium was autoclaved at 121 °C for 15 min in 250 mL Erlenmeyer flasks and then inoculated with the A. niger spores suspension. Samples of fermented CP were removed and analyzed daily.
Analytical Methods
CA was extracted from a solution prepared with 5 g of the fermented material macerated with 50 mL of water and homogenized for 15 min with a magnetic agitator. This mixture was then filtered in a vacuum pump with Whatman no. 1 filter paper. The filtrate was centrifuged under 4,500 rpm for 20 min. pH was determined by using a digital pH meter. The supernatant was then diluted (1:4) with Milli-Q water and filtered through a Millipore membrane of 0.45 μm and stored in an Eppendorf. Samples were subjected to high performance liquid chromatograph (HPLC) analysis using a Shimadzu LC-10AD. A 60 °C temperature and 5 mM H2SO4 as the mobile phase at a flow-rate of 0.6 mL/min were used. Citric acid was detected in the column eluate by differential refractometer (Shimadzu RID-10A).
Results and Discussion
Optimization Steps with the Original Strain A. niger LPB BC
Effect of Initial pH and Moisture on CA Production
The initial pH and initial moisture influence on CA production is presented in Table 1. CA production was favored by low levels of initial humidity (65%). However, pH did not have a significant effect at p ≤ 0.05 on CA synthesis, which represents an operational advantage.
However, the initial moisture of the medium influenced CA production showing an increase tendency with a decrease in moisture content, according to the contour plot in Fig. 1. Generally, fungi prefer low pH levels for a good growth; in this work the natural pH of the substrate, which was 5.42 ± 0.075 [8] (without adjustment or buffering) was the best for fungi development and CA production. Otherwise, low moisture content causes reduction in the solubility of substrate nutrients, although it can limit biomass production, which is the main condition for CA accumulation.
The results showed that variations on pH did not present significant effect on CA production using CP in SSF; however, the initial moisture proved to be an important factor. In fact, CA production using the strain A. niger LPB BC with CP was more effective when lower levels of initial humidity were used. Therefore, the following conditions were selected: pH 5.5 and 65% initial moisture. Low levels of initial humidity facilitate gaseous exchanges in the medium, which is enough to guarantee the diffusion of nutrients in the fermentation medium. Besides, those conditions improved CA production-reaching 388.5 g of CA/kg dry CP—and are suitable from an economic and operational point of view because pH adjustment was not necessary—whereas pH 5.5 was near to the natural pH found in the dried CP (pH 5.42)—and less water will be spent in the process to adjust the initial moisture without production losses.

where X stands for pH values and Y stands for moisture values.
Regression analysis was performed to fit the response function with the experimental data. The statistical significance of the second-order model equation was checked and the determination coefficient (R 2) of the model was calculated to be 0.91, indicating that 91% variability in the response could be explained by the model. The Lack-of-Fit tests did not result in a significant p-value, indicating that the model is sufficiently accurate to predict the factor responses within the ranges studied (Table 2).
Salt Addition
The results of the salt addition tests to the medium were submitted to statistical analysis, where they had been plotted on Pareto diagram (Fig. 2) with p < 0.05 (95% reliability). The value obtained for R 2 was 0.76, which has been considered satisfactory due to the solid-state fermentations nature.
Unexpectedly, none of the salts studied were found above the significance line. These results suggest that none of the studied salts were significant for CA production by SSF using the CP. This could be explained by the CP’s composition which certainly disposes the necessary micronutrients to CA synthesis by the A. niger LPB BC strain.
Effect of Different Carbon Sources on CA Production
The best results of CA production were observed with glucose 120 g/L (427.7 g of CA/kg of CP) and with sucrose at 108 g/L (405.6 g of CA/kg of CP). The addition of fructose at 120 g/L led to a production of 371.9 g CA/kg dry CP. Further assays were conducted using only glucose (60, 120, and 240 g/L) and sucrose (54, 108, and 216 g/L). The increase in sucrose concentration (Table 3) had a positive effect on CA production, which reached 476.1 g/kg dry CP, representing a 21.2% gain. The control, without addition of carbon sources, reached only 372.2 g CA/kg dry CP. It was suggested that the mycelial growth and the strain have an extracellular invertase linked to mycelium, and this breaks the sucrose producing energy exactly at the level in which the increase in CA production is observed.
These results were very significant and confirm that CA can be produced from purified carbohydrates or from crude carbohydrate sources such as sugar cane molasses, beet molasses, crude sucrose, and starch hydrolyzates, representing a lower production cost. Most of these sources present sugars as mono, bi, and oligosaccharides, among other impurities [9], [22]. As these crude sources have bigger sucrose concentration in its formulation, they can stimulate a significant increase in CA production. The use of molasses could be possible in the process of CA production by SSF using CP as substrate/support. Thus, tests with two crude carbon sources, sugarcane molasses, and soy molasses were carried out.
The concentration of 108 g/L in total sugars was used for sugarcane molasses and soy molasses. There was a decrease in CA production with the use of soy molasses (277.9 g of CA/kg of CP) when compared to the control without additional carbon source (363.7g of CA/kg of CP). Soy molasses present higher protein content, which balances the medium regarding carbon and nitrogen composition and, consequently, favors fungus growth. Growth limitation is essential for CA accumulation [1], [22]. However, the addition of sugarcane molasses significantly increased the production (420.3 g of CA/kg of CP). Sugarcane molasses is poor in nitrogen content when compared to soybean molasses. Assays with several sugarcane molasses concentrations were carried out in order to find out the best concentration to be added to the CP in order to increase CA production. A concentration of 445.4 g of CA/kg of dry CP was achieved with 108 g/L of sugarcane molasses, as shown in Fig. 3. Therefore, this condition was used for further studies.
Effect of Nitrogen Sources on CA Production
The high need of nitrogen and phosphorus by some microorganisms is not ideal in SSF due to the low diffusion rate of these components that occurs in low water activities processes [9], [24], [25]. In this study the nitrogen source showing the best CA production was the NH4Cl (334.6 ± 0.636 g of CA/kg of CP), although this production was lower than the one obtained without the addition nitrogen (377.6 ± 1.202 g of CA/kg of CP). Thus, it was not necessary to add supplemental nitrogen sources to the fermentation medium, and this represents a great economical advantage of this process.
Alcohols
The addition of low alcohol decreases the inhibition caused by heavy metals that can be present in the fermentation medium. Besides, it facilitates CA permeability through the A. niger membrane.
Tests were made with the addition of three different concentrations of methanol and ethanol (2, 4, and 6%) without sugarcane molasses addition, at pH 5.5, 65% initial moisture, 4 days of fermentation at 30 °C. It was observed that 6% methanol contributed to the best CA production (438.4 g/kg of dried CP). Thus, new tests were carried out with different methanol concentration (4, 5, 6, and 8%) using the best sugarcane molasses concentration (108 g/L) at pH 5.5, 65% moisture, during 4 days at 30 °C.
CA production (418.3 and 425.1 g/kg of CP, respectively) was higher with 5% and 6% of methanol, without sugarcane molasses addition. However, when sugarcane molasses at 108 g/L of total sugars was added, CA production was 457.9 g/kg of CP with the addition of only 4% of methanol. With 8% of methanol, CA production was not significant, with or without sugarcane molasses addition, proving that this methanol concentration inhibits CA accumulation (Table 4). Thus, 4% of methanol with sugarcane molasses (108 g/L of total sugar) addition was chosen for CA production by SSF using CP as substrate.
Kinetics of CA Production with the Optimized Conditions
Seven-day kinetics was carried out in duplicate with the optimized conditions: pH 5.5, 65% initial moisture obtained with a solution containing sugarcane molasses (108 g/L of total sugars) and 4% of methanol and 30 °C. The results are presented in Table 5.
A pronounced decrease in the pH of the medium—from 5.5 to around 2.70—was observed on the second day of fermentation justified by the beginning of CA accumulation. The fourth day of fermentation presented the best CA production (441.05 g/kg of dry CP), when pH values had a little increase and stabilized around 3, due to the decrease of acid in the medium.
In the study developed by Castro et al. [26], higher biomass productions were observed when higher water activity values were used. The water activity during the kinetics of CA production by the strain A. niger LPB BC remained with values above 0.94 during the whole fermentative process, which is very relevant for microorganism development.
Figure 4 shows the strain adaptation stage to the fermentation medium in the first 24 h, when the total sugars consumption and the CA production begin. Mycelia formation was observed in 48 h, which was marked by the consumption of sugars in the medium. Thus, the highest CA production occurred in 96 h (441.05 g of CA/kg of dry CP) when all the necessary sugars were available to metabolism and CA production. Sugars were then consumed and its concentration stabilized at 3% of total sugars. This low concentration of sugars was then used for its maintenance and could lead to CA consumption. This fact was observed in the 17.2% decrease in the amount of CA after 144 h of fermentation.
Tests with Strains Obtained from UV Irradiation in Foster Medium
Halo Formation in Foster Medium
The halo formation resulted from the acid production in Foster medium, which was verified through the observation of a yellow halo formation (initially green), due to the glucose degradation present in the medium and, consequently, the fall of pH. The obtained results can be observed in Fig. 5.
Seventy-two strains were obtained. The qualitative results were analyzed according to the relation between the diameter of halo formation (DH) and the diameter of colony (DC) of fungus growth (data not shown).
Out of forty strains, replicated in CM, twenty treated strains presented the formation of a yellow halo, which evidenced acid production, representing 52.5% of the total. However, only one of them presented a DH/DC relation (1.87) higher than that presented by the control (1.70). This could mean that this strain probably presented a better capacity to produce acid when compared to the original strain. Out of the forty colonies seeded in the Foster media derived from MM, 23 (71.88%) showed a halo formation with a yellow coloration evidencing acid formation. Ten [10] treated colonies presented a DH/DC relation higher than the DH/DC relation of the control strain (data not shown).
According to Azevedo [27], a wild microorganism can grow in a CM, where all the nutrients are provided, or in a MM, where only a carbon source, a nitrogen source, some minerals and small quantities of nutritional supplements are present. A fungus that shows an auxotrophic mutation can only grow in CM, because it does not produce all nutrients necessary to its survival in MM. So the A. niger strains obtained from the UV irradiation that also grew in MM may not present auxotrophism, which could involve a metabolic route of the production of desired substances to ensure its survival. Consequently, the mutant A. niger strains could have suffered some biochemical modifications that induced an increase in the production of secondary metabolic [falta um substantivo?], such as citric acid (CA).
After mutation induction by UV of A. niger LPB BC strain, qualitative and quantitative tests were done to quantify CA production by ten mutant strains obtained from this study.
CA Production in SSF by Mutant A. niger Strains
Ten mutant strains that presented the best results in the Foster medium were tested in SSF using the initial fermentation (before optimization of physical and chemical conditions). These strains were called, A. niger LPB A4, A. niger LPB B3, A. niger LPB B4, A. niger LPB B6, A. niger LPB B7, A. niger LPB B8, A. niger LPB D1, A. niger LPB D3, A. niger LPB D4, A. niger LPB D7. Fermentation results on the 4th and 5th days of fermentation are represented in Fig. 6.
Five mutant strains (LPB B3, LPB B6, LPB B8, LPB D3, and LPB D7) showed better results in CA production than the control A. niger LPB BC strain. Two months after the mutation induced by UV irradiation, two strains were chosen: A. niger LPB B3 and A. niger LPB B6, which showed the best results among all mutant strains.
Kinetic of CA Production in SSF by Mutants Strains
A seven-day kinetics was performed with the mutant strains LPB B3 and LPB B6, using previously optimized conditions (addition of aqueous solution of sugarcane molasses with 108 g/l of total sugars and 4% methanol (v/w), obtaining 65% initial moisture, pH 5.5 and 30 °C temperature). The results can be observed in Fig. 7.
A. niger LPB B6 and LPB B3 showed very promising results, achieving a 537.6 g of CA/kg of CP production on the sixth day of fermentation, and 616.5 g of AC/kg of CP on the fourth day of fermentation, respectively. A productivity of 6.42 g CA/kg h was attained by LPB B3 against 4.69 g CA/kg h by the parental strain.
Conclusions
With the physical–chemical parameters optimization of the fermentation using A. niger LPB BC, a 17.5% increase in CA production was obtained with which the production passed from 383.5 g of CA/kg of dry CP to 450 g of CA/kg of dry CP. This production was reached with the best conditions of fermentation: pH of 5.5, 65% initial moisture obtained with the addition of sugar molasses (108 g/L of total sugars) and 4% methanol (v/w) at 30 °C in Erlenmeyer flasks for 4 days.
A. niger LPB BC strain suffered a mutation induced by UV in an attempt to obtain a mutant that could produce a higher amount of CA than the control strain. Two mutant strains were obtained: A. niger LPB B3 and A. niger LPB B6. These mutant strains were chosen to be used in an SSF kinetics using CP as substrate with the same optimized conditions adopted for A. niger LPB BC.
The results obtained were promising for both mutant strains: A. niger LPB B3 produced 537.6 g of CA/kg of CP on the sixth day of fermentation, whereas A. niger LPB B6 produced 616.5 g of CA/kg of CP on the fourth day of fermentation. The production of CA was much higher when compared to the production obtained by the parental strain A. niger LPB BC, which was around 450 g of CA/kg of CP.
A material containing CA in a concentration above 300 g of CA/kg of dry matter is considered suitable for its direct application in animal feed, for example, or extraction. The developed process must be transferred to a semi-pilot scale in which the produced CA may be used in animal feed. These results can certainly contribute to the Brazilian agro-industries. First of all, the process utilizes a residue from the orange juice industry to produce a commercial product. Second, the optimization of physical–chemical conditions of the process and the mutagenesis induced by UV can be useful and so can be the simple methods to increase the efficiency of producing biotechnological products and adding value to different Brazilian agricultural residues and by-products.
References
Soccol, C. R., Vandenberghe, L. P. S., Rodrigues, C., & Pandey, A. F. (2006). Food Tech Biotechnol, 45, 141–150.
Vandenberghe, L. P. S., Soccol, C. R., Pandey, A., & Lebeault, J. M. (2000). Bioresource Technology, 74, 175–178. doi:10.1016/S0960-8524(99)00107-8.
Kapoor, K. K., Chaudhary, K., & Tauro, P. (1982). In: G. Reed (Ed.), Prescott and Dunn’s industrial microbiology. 4ed. Westport, Conn: AVI.
Kolicheski, M. B. (1995). Master of Science Thesis, Federal University of Paraná, Curitiba—PR, Brazil
Soccol, C. R. (1996). Journal of Scientific and Industrial Research, 55, 358–364.
Pandey, A., Soccol, C. R., Nigam, P., Soccol, V. T., Vandenberghe, L. P. S., & Mohan, R. (2000). Bioresource Technology, 74, 81–87. doi:10.1016/S0960-8524(99)00143-1.
Soccol, C. R., & Vandenberghe, L. P. S. (2003). Biochemical Engineering Journal, 13, 205–218. doi:10.1016/S1369-703X(02)00133-X.
Rodrigues, C. (2006). Master of Science Thesis, Federal University of Paraná, Curitiba-PR, Brazil.
Yokoya, F. (1992). Fermentação cítrica, Fundação Tropical de Pesquisas e Tecnologia “André Tosello”, Campinas, São Paulo, Brazil.
Pazouki, M., Felse, P. A., Sinha, J., & Panda, T. (2000). Bioprocess Engineering, 22, 353–361. doi:10.1007/PL00009115.
Crolla, A., & Kennedy, K. J. (2001). Journal of Biotechnology, 89, 27–40. doi:10.1016/S0168-1656(01)00278-4.
Zaha, A. (2003). Biologia molecular básica (3rd ed.). Porto Alegre–RS, Brazil: Mercado Aberto.
Griffiths, A. J. F., Wesller, S. R., Lewontin, R. C., Gelbart, W. M., Suzuki, D. T., & Miller, J. H. (2006). Introdução à genética (8th ed.). Rio de Janeiro–RJ, Brazil: Guanabara Koogan.
Ikram-ul-Haq, , Khurshid, S., Ali, S., Ashraf, H., Qadeer, M. A., & Rajoka, M. I. (2001). World Journal of Microbiology and Biotechnology, 17, 35–37. doi:10.1023/A:1016625130070.
Lotfy, W. A., Ghanem, K. M., & El-Helow, E. R. (2007). Bioresource Technology, 98, 3464–3469. doi:10.1016/j.biortech.2006.11.007.
Röhr, M., Kubicek, C. P., & Komínek, J. (1983). In: Reed, G., Rehm, H. J. (ed.), Biotechnology. Chemie, Weiheim. vol. 3
Ooijkaas, L. P., Weber, F. J., Buitelaar, R. M., Tramper, J., & Rinzema, A. (2000). Trends in Biotechnology, 18, 356–360. doi:10.1016/S0167-7799(00)01466-9.
Aboud-Zeid, A., & Ashy, M. A. (1984). Agricultural Wastes, 9, 51–76. doi:10.1016/0141-4607(84)90075-1.
Shankaranand, V. S., & Lonsane, B. K. (1994). Process Biochemistry, 29, 29–37. doi:10.1016/0032-9592(94)80056-1.
Lima, V. L. A. G., Stamford, T. L. M., & Salgueiro, A. A. (1995). Arquivos de Biologia e Tecnologia, 38, 773–783.
Pintado, J., Torrado, A., González, M. P., & Murado, M. A. (1998). Enzyme and Microbial Technology, 23, 149–156. doi:10.1016/S0141-0229(98)00042-8.
Vandenberghe, L. (2000). PhD Thesis. Université de Technologie de Compiègne, Compiègne, FR.
Prado, F. C., Vandenberghe, L. P. S., Lisboa, C., Paca, J., Pandey, A., & Soccol, C. R. (2004). Engineering in Life Sciences, 4(2). doi:10.1002/elsc.200420020
Kubicek, C. P., & Röhr, M. (1986). Critical Reviews in Biotechnology, 3, 331–373. doi:10.3109/07388558509150788.
Grewal, H. S., & Kalra, K. L. (1995). Biotechnology Advances, 13, 209–234. doi:10.1016/0734-9750(95)00002-8.
Castro, M. F. P. M., Gragagnolo, N., & Valentini, S. R. T. (2002). Brazilian Journal of Microbiology, 33, 22–26. doi:10.1590/S1517-83822002000100004.
Azevedo, J. L. (1998). Genética de Microrganismos. Editora UFG, Goiânia, GO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, C., de Souza Vandenberghe, L.P., Teodoro, J. et al. Improvement on Citric Acid Production in Solid-state Fermentation by Aspergillus niger LPB BC Mutant Using Citric Pulp. Appl Biochem Biotechnol 158, 72–87 (2009). https://doi.org/10.1007/s12010-008-8370-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8370-5