Abstract
Hyoscyamine biosynthesis in Datura stramonium hairy roots with different ploidy levels was investigated. For the first time, we report that hairy roots undergo endoreduplication and therefore consist mainly of cells with doupled sets of chromosomes of primary plant tissues, used for Agrobacterium transformation. The alkaloid profiles of hairy roots obtained from diploid and tetraploid plants were similar in terms of the major compounds, but they differed significantly with respect to the minor compounds (here defined as those that accounted for <1% of the total ion current of the alkaloid mixture in gas chromatography–mass spectrometric analyses). Significant differences in the effects of the main nutrients on the growth of the hairy roots obtained from diploid and tetraploid plants and their hyoscyamine contents were observed. The maximal yield of hyoscyamine (177 mg/L) was obtained when hairy roots from tetraploid plants were cultivated in Murashige–Skoog nutrient medium supplemented with 6% sucrose. Time courses of utilization of the main nutrients in the medium during cultivation of D. stramonium hairy root cultures are also presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyoscyamine is an important drug that acts mainly on the parasympathetic nervous system [1]. It is extracted from plants belonging to several genera and species of the family Solanaceae, where it is synthesized in the roots [2]. Various types of in vitro systems of plant materials from this family have also been established for the biotechnological production of hyoscyamine [2–5].
Industrial application of hairy root cultures requires the development of techniques for selection of highly productive lines. Good model systems for experimental studies for this purpose are hairy root cultures obtained from Datura stramonium because they grow rapidly and are genetically stable [6]. D. stramonium hairy root cultures have been widely investigated with respect to the biosynthesis and accumulation of tropane alkaloids. In contrast, there is little information on the relationship between genome size (ploidy level) and the biosynthesis of secondary metabolites by genetically transformed root cultures [4]. It is well known that polyploidy is frequently accompanied by conspicuous changes in morphology and secondary metabolism [7–9]. The ploidy manipulation could be useful in increasing the production of valuable phytopharmaceuticals in plants [9, 10], as well as in plant in vitro systems [11]. It seems that the increased genetic variability is a prospective approach for the selection of precious hairy root lines, as well as for the investigation of the relationship between the ploidy level and the biosynthesis of secondary metabolites by hairy root cultures.
In this study, alkaloid accumulation, flow cytometry analyses, as well as the relationships between growth and hyoscyamine production by D. stramonium hairy root cultures with different ploidy levels, in parallel to the utilization of the main nutritive compounds from the medium, are presented.
Material and Methods
Plant Material
The initial diploid cytotype (2x = 24) of D. stramonium L., collected from natural habitats in the vicinity of Lovech, Bulgaria, was polyploidized with colchicine solution following the method of Pundir et al. [12]. An autotetraploid line (4x = 48) was obtained as reported by Berkov et al. [5].
Bacteria
Agrobacterium rhizogenes ATCC 15834 was used. The bacteria were grown on YEB [8 g/L Nutrient broth (Sigma), 15 g/L yeast extract (Merck) and 0.5 g/L MgSO4] medium and were subcultured at monthly intervals. Before transformation, the bacteria were cultivated in liquid YEB in flasks (100 mL) with 25-mL medium on a shaker (220 rpm) at 26 °C for 16 h. The obtained bacterial suspension was transferred to a sterile centrifuge tube and was centrifuged at 5,000 rpm for 30 min. The biomass of Agrobacterium was suspended in Murashige–Skoog (MS) hormone-free nutrient medium (Duchefa Biochemie BV, the Netherlands), supplemented with 30 g/L sucrose, resulting in a final bacterial optical density (600 nm) between 0.4 and 0.6. The bacterial suspension obtained was used for the establishment of hairy roots.
Establishment of Hairy Root Cultures
Leaves from 6–8-week-old diploid and tetraploid D. stramonium plants were surface-sterilized for 15 min with sodium hypochlorite (2% available chlorine) and two to three drops Tween 20, thoroughly rinsed with sterile distilled water, and dried on sterile filter paper. The sterile leaves were infected with Agrobacterium suspension following the method of direct infection [5]. The appearance of hairy roots was observed 4 weeks later. The root segments (20 mm long) were cut off, transferred to the hormone-free MS medium, supplemented with 30 g/L sucrose and 5.5 g/L “Plant” agar (Duchefa Biochemie BV, the Netherlands), cultured at 26 °C in darkness, and subcultured at 21-day intervals. The first three subcultivations were carried out with the addition of 0.25 g/L Claforan (Hoechst Ltd., Istanbul Turkey).
Culture Conditions
Characteristics of D. stramonium hairy root cultures with different ploidy levels cultivated under submerged conditions
To assess the characteristics of hairy root cultures with different ploidy levels cultivated under submerged conditions, portions of the fresh hairy root cultures (approximately 1 g) obtained from the diploid and autotetraploid plants were grown, under the conditions described above, for 14 days on MS medium (supplemented with 5.5 g/L “Plant” agar and 30 g/L sucrose) followed by 14 days in liquid MS medium (supplemented with 30 g/L sucrose). They were then transferred to 50 mL MS medium (again supplemented with 30 g/L sucrose) in 300-mL Erlenmeyer flasks and cultivated on a shaker (110 rpm), in darkness, at 26 °C.
The effect of sucrose
The cultivation was performed in MS nutrient medium, supplemented with different amounts of sucrose (20, 30, and 40 g/L for diploid hairy roots and 20, 30, 40, 50, 60, and 70 g/L for tetraploid hairy roots, respectively), under the conditions described above.
The effect of phosphate ions
The cultivation was performed in MS nutrient medium, supplemented with 0.085, 0.170, 0.255, and 0.340 g/L KH2PO4, under the conditions described above.
The effect of nitrate ions
The cultivation was performed in MS nutrient medium, supplemented with 1.9 g/L KNO3, 2.85 g/L KNO3, and 3.8 g/L KNO3, under the conditions described above.
The effect of ammonium ions
The cultivation was performed in MS nutrient medium, supplemented with different amounts of NH4NO3, KNO3, and (NH4)2SO4, as follows: 0,412 g/L NH4NO3 and 3.47 g/L KNO3; 0.825 g/L NH4NO3 and 2.95 g/L KNO3; 1.65 g/L NH4NO3, 1.9 g/L KNO3, and 1 g/L (NH4)2SO4, under the conditions described above.
Extraction on Nuclear DNA
For DNA content determination of nuclei from plants and hairy roots, the nuclei were extracted and stained using a high-resolution kit (Partec CyStain PI, Partec GmbH, Münster, Germany) according to the manufacturer’s instructions.
Alkaloid Extraction and Quantification of Hyoscyamine
Hairy root cultures were dried at 50 °C and powdered in a mortar and pestle. Samples (200 mg) were extracted with methanol (400 μL), left for 5 min, and then extracted with 3% sulfuric acid (4.00 mL) in an ultrasonic bath for 45 min. The extracts were centrifuged at 4,000 rpm, for 10 min, and an aliquot (2.00 mL) of the supernatant was transferred into a test tube, basified with 25% ammonium hydroxide (400 μL), and applied to a Merck (Darmstadt, Germany) Extrelut® 3 column. The alkaloids retained on the column were eluted with dichloromethane (2 × 10 mL) which was dried over anhydrous sodium sulfate (1 g). After solvent removal with a stream of air from a 24-port airstream evaporator, the residues were dissolved in methanol (200 μL) for quantification. Aliquots (100 μL) of the extracts were diluted in methanol (400 μL). The hyoscyamine was quantified exactly as described in Berkov and Pavlov [13].
Analysis
Dry weight
The growth of the hairy roots was monitored by measurement of the dry biomass and the accumulated dry biomass (ADB) calculated on this base, respectively [14, 15].
Alkaloid identification
The alkaloids were identified by GC–MS analyses performed on a Hewlett Packard (HP; Palo Alto, CA, USA) 6890 + MSD 5973 equipment operating in the EI mode at 70 eV. An HP-5 MS column (30 m × 0.25 mm i.d.; 0.25 μm) was employed with a 30-min temperature program of 80–280 °C at 10 °C/min followed by a 10-min hold at 280 °C. The injector temperature was 280 °C; the flow rate of the carrier gas (helium) was 0.8 mL/min; the split ratio was 1:20. The MS spectrum of hyoscyamine standard (Fluka) was recorded and compared with those found in the samples. The identification of the rest alkaloids was performed by comparing the measured data with those of authentic compounds according to the National Institute of Standardization and Technology (NIST) 98 database (NIST Mass Spectral Database, National Institute of Standardization and Technology, Gaithersburg, MD, USA) or with literature data as specified in Table 1.
Flow cytometry
The measurements were performed with a CyFlow SL (CyTecs, GmbH, Görlitz, Germany) equipped with a 20-mW 488-nm solid-state laser. The propidium iodide fluorescence was detected through a 630-nm high-pass filter. The signal intensity of each peak and the number of events in the regions was determined by FlowMax software (Partec). Measurement of nuclear DNA content was carried out during different stages of growth with at least three replications per sample. To determine the standard peak position of 2 and 4 °C nuclei, the peak of young leaves of diploid and tetraploid D. stramonium were analyzed. The data were plotted on semilogarithmic scale, so that the peaks of 2 to 16 °C could be detected at identical gain settings. Doublets were discriminated by pulse processing.
pH, sugars, nitrate, ammonium, and phosphate ions
pH of the medium was measured by a pH–cond. meter (INOLAB, WTW, Germany). Sucrose, glucose, and fructose contents in the culture medium were determined by means of an enzyme test combination (R-Biopharma, Germany). Nitrate, ammonium, and phosphate ions in the culture medium were determined by chemical test combinations Spectroquant© (Merck, Germany).
The data presented are the averages from three independent experiments, which were repeated twice and expressed as the means with standard deviations (±SD). The results for the influence of macronutrients concentrations on hyoscyamine production were analyzed by one-way analysis of variance and Tukey honestly significant difference test with α = 0.05.
Results and Discussion
The yield of the target metabolite(s) is the most important variable to consider during the development of plant in vitro system-based biotechnological techniques. In most cases, these yields are low and unsatisfactory. Therefore, integrated approaches for their improvement have to be developed, including genetic engineering, selection of highly productive lines, and optimization of variables such as the nutrient medium and bioreactor environmental conditions [16, 17]. The scope for improving production by ploidy manipulation (in parallel with the selection of highly productive lines) should be investigated during early stages of the optimization process, but its potential importance is usually underestimated or completely ignored.
Hairy Roots Establishment and Line Selection
After Agrobacterium transformation of young leaves of the diploid and tetraploid D. stramonium plants, 20 hairy root lines were obtained from diploid plants and 20 from tetraploid plants. After 6 months culturing on agar MS medium, with a subcultivation period of 21 days, the ten fastest-growing lines obtained from diploid plants and the ten fastest-growing tetraploids were selected for further analyses of hyoscyamine accumulation, in which the diploid and tetraploid hairy roots accumulated 1.3–2.1 mg/g DB and 1.0–1.9 mg/g DB hyoscyamine, respectively. The most highly productive diploid and tetraploid D. stramonium hairy root cultures were selected for further investigations of their growth and hyoscyamine accumulation characteristics. They were maintained for more than 2 years on the agar medium and subcultivated every 21 days. During this time, the selected cultures showed stable growth and morphological characteristics, including profuse branching with many lateral branches. Both the tips and other parts of the hairy roots obtained from the tetraploid plants were consistently thicker than those of the diploid-derived cultures, but no other morphological differences were observed.
Flow Cytometry Analyses
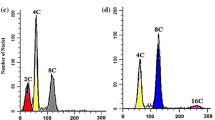
In contrast to undifferentiated plant cells grown in vitro, which tend to be highly unstable genetically [18], it is widely accepted that hairy root cultures are genetically stable and possess identical chromosome numbers to cells of the primary plants used for transformation with Agrobacterium transformation [6]. This is the main reason for the stability of their secondary metabolite production during long periods of subcultivation [6, 17, 19]. To confirm the ploidy levels of the hairy root cultures obtained in this study, flow cytometry analyses were performed (Fig. 1). Unexpectedly, in the hairy roots obtained from the D. stramonium diploid plants, a small fraction (24.9%) of the nuclei examined had 2-C DNA contents, corresponding to the diploid state of the genome in the G1 phase, there was a substantially higher fraction of 4-C nuclei (64.0%), and a smaller fraction (11.1%) of 8-C nuclei, indicating that certain cells underwent a cycle of endoreduplication. An analogous pattern was observed in the tetraploid hairy roots. Here, a large fraction of 8-C (57.2%) signals, and smaller fractions of 4-C (33.1%) and 16-C (9.7%) signals were detected, indicating a similar degree of endoreduplication to that apparently observed in the cultures obtained from the diploid tissue. In contrast, our investigations of the leaf tissues of the primary plants showed them to be stable diploids and tetraploids, respectively (Fig. 1a,b). The diploid leaves possessed 91.5% 2-C level nuclei and 8.5% 4 C. The tetraploid D. stramonium leaves possessed 91.4% 4-C level nuclei and 8.6% 8 C. The 4-C signals from the sample of 2-C leaves and the 8-C signals from the 4-C sample presumably originated from replicating cells. However, no large deviations from this pattern could be observed in the different stages of growth. It should be emphasized that the genetic stability of the hairy root cultures was high, since no significant variations in the DNA content distributions were observed over approximately 3 months. This corresponds well with the conclusions of Baíza et al. [6] that D. stramonium hairy roots are genetically very stable. However, our investigations conflict with the conclusions of the cited authors concerning the chromosome number of hairy root cells. The endopolyploidization we observed in the hairy root cells is probably due to events in cell differentiation [20, 21].
Characteristics of Diploid and Tetraploid D. stramonium Hairy Roots Cultivated Under Submerged Conditions
The hairy root cultures readily adapted to submerged conditions of cultivation. They grew rapidly and by the end of the culture cycle they filled the entire medium volume. The highest biomass accumulation was reached on day 15 of cultivation (0.41 and 0.38 g ADB per flask for the hairy roots obtained from diploid and tetraploid plants, respectively; Fig. 2a). The pH of the medium decreased from 5.8 to 4.9 during the cultivation of both types of hairy root cultures (Fig. 2b). These profiles are not typical for plant in vitro systems [14]. However, the decrease in the pH of the medium was strongly inversely correlated with the increase in dry biomass (r > 0.95 in both cases; Figs. 2 and 3), thus the pH of the medium provides a potentially valuable indicator for online monitoring of the biomass of the cultures, which could not be directly measured if these hairy root lines were cultivated in bioreactors. We have previously developed online control algorithms based on similar inverse relationships between conductivity and biomass accumulation in various plant in vitro systems, including cell suspensions, hairy roots, and shoots [15, 17, 22, 23].
As expected, observed changes in the hyoscyamine contents of the D. stramonium hairy root cultures indicate that it was most strongly produced during the late exponential and stationary growth phases (between the 9th and 18th days; Fig. 4). The maximum amounts of hyoscyamine detected in the hairy roots obtained from the diploid and tetraploid plants were 66.7 and 52.9 mg/L, respectively (Fig. 4). It is important to emphasize that the productivity of both hairy root lines was high, 3.7 mg/l per day for diploid and 2.9 mg/l per day for tetraploid cultures.
In our preliminary investigations [5] of unselected diploid and tetraploid D. stramonium hairy roots, we observed significant differences in alkaloid patterns. In the current study, the alkaloid patterns of the selected D. stramonium hairy root cultures were investigated in detail at various stages of their growth cycle by GC–MS, and the detected compounds are presented in Table 1 (see the tables’ legend and “Materials and Methods” for details regarding their identification). Since direct precursor of hyoscyamine–littorine [compound 22] [24] coeluted with its positional isomer hyoscyamine [compound 23], we have referred to these compounds collectively as hyoscyamine. The occurrence of compound 22 in the peak of compound 23 was confirmed by the presence of a small ion at m/z 142 [25, 26]. The alkaloid profiles of the hairy roots obtained from diploid and tetraploid plants were found to be similar in terms of major components but to differ significantly with respect to the minor components, defined here as those accounting for <1% of the total MS ion current of the alkaloid mixture (Table 1). The results show that the diploid hairy roots had similar alkaloid patterns during the early stages of their development to those observed in all studied developmental stages of the tetraploid hairy root cultures. However, the diploid hairy roots contained twice as many detectable alkaloids as tetraploid hairy roots in stationary phase. For instance, on the 15th day of the cultivation (in stationary phase), 22 alkaloids were detected in the diploid hairy roots and ten alkaloids in the tetraploid D. stramonium hairy roots. It should be noted that the hyoscyamine contents, as proportions of the total alkaloid contents, of both types of hairy root cultures remained fairly constant during their cultivation, although there was a significant apparent drop in the proportion of hyoscyamine in the stationary phase of the diploid cultures, which can be attributed to the appearance of a number of minor compounds that were not detected in the earlier stages of development. In contrast, the proportion of hyoscyamine in the tetraploid hairy roots was highest in stationary phase. Hence, it could be valuable to obtain precise estimates of the potential advantages of manipulating the ploidy of transformed root cultures to optimize hyoscyamine production. This could be done after optimizing the macronutrient contents of the medium, for which the first step is to investigate their utilization during the growth cycle, as described below.
It is well known that sucrose is the most energetically advantageous of the carbohydrates used as carbon sources in the cultivation of plant in vitro systems, especially for the biosynthesis of secondary metabolites [27]. Therefore, the utilization of sucrose during the cultivation of our D. stramonium hairy roots cultures was investigated (Fig. 5). The time course of sucrose hydrolysis to glucose and fructose followed that of biomass accumulation, and at the end of the exponential growth phase no sucrose was detected in the culture medium. The sucrose seems to have been inverted extracellularly [28] and the consumption of the glucose and fructose thereby produced followed a parallel pattern to its hydrolysis (Fig. 5). By the 18th day of cultivation, the carbon sources were completely exhausted in the media of both types of cultures. In order to facilitate further optimization procedures, it is important to determine the relationships between utilization of the carbon source and hyoscyamine accumulation. The period of intensive hyoscyamine accumulation (stationary growth phase; Fig. 4) coincided with the period of intensive utilization of glucose and fructose. At this time, no nonhydrolyzed sucrose was detected in the culture medium of either culture types, and the maximal amounts of hyoscyamine were accumulated on the 18th day of cultivation (Fig. 4), after the carbon sources were completely exhausted from the medium (Fig. 5).
Significant differences in nitrogen utilization by D. stramonium hairy root cultures obtained from diploid and tetraploid plants were observed (Fig. 6). Diploid hairy root cultures utilized both nitrate and ammonium ions uniformly until the 11th day of cultivation (the end of the exponential phase of growth). After that, their concentrations remained constant until the end of the cultivation (at approximately 1,300 and 100 mg/L, respectively; Fig. 6a). Thus, the stationary growth phase of D. stramonium hairy root cultures obtained from diploid plants began, and their hyoscyamine contents peaked, after uptake of nitrogen ions had ceased. However, in the tetraploid hairy root system (Fig. 6b), utilization of the ammonium and nitrate ions continued until the end of the growth cycle. In this case, the final contents of nitrate and ammonium ions in the medium were significantly lower (approximately 800 and 50 mg/L, respectively; Fig. 6b). Hence, the ploidy level of the culture affects the uptake of nitrogen by the investigated hairy root systems.
The time course of the uptake of phosphate ions was similar in both types of hairy root cultures, following in both cases the time course of biomass accumulation, and at the end of the growth cycles approximately 10 mg/L of phosphate ions remained in the media.
Influence of The Concentrations of Macronutrients on Hyoscyamine Accumulation by Diploid and Tetraploid D. stramonium Hairy Roots
To further assess the biosynthetic potential of the investigated D. stramonium hairy root cultures with different ploidy levels, the influence of the concentration of the macronutrients (sucrose, nitrate ions, ammonium ions, and phosphate ions) on their growth and hyoscyamine accumulation were investigated. The accumulated biomass and hyoscyamine contents both increased with increases in the initial sucrose concentration in the nutrient medium used to cultivate the hairy roots obtained from tetraploid plants, reaching 1.09 g per flask and 177 mg/L, respectively (Fig. 7b,d). In contrast, variations within the tested range of sucrose concentrations in the nutrient medium did not appear to significantly affect the biomass and hyoscyamine accumulation of the hairy roots obtained from diploid plants. These are interesting and potentially important findings since they show there are clear physiological differences between the investigated hairy root cultures.
Influence of sucrose concentrations on biomass and hyoscyamine accumulation by D. stramonium hairy root cultures obtained from diploid (a—biomass; c—hyoscyamine) and tetraploid (b—biomass; d—hyoscyamine). Bars represent standard deviations. Data obtained for the hairy roots are significantly different at P < 0.05
Bensadek et al. [4] suggested that ammonium ions play an important role during the first days of the hairy root cultures, while nitrate influences later events, like the biosynthesis of tropane alkaloids. They also noted that ammonium ions had a strong influence on biomass accumulation, while nitrate clear influence the alkaloid content. However, our results (Figs. 8 and 9) showed that neither reduction nor increase of concentrations of the nitrogen ions stimulated biomass or hyoscyamine accumulation. At this point, it should be noted that the relevance of the nitrogen source for secondary metabolism is a specificity of the species. The role of the nitrogen source in secondary metabolism has not been thoroughly clarified [27, 29]. However, nitrogen must be included in the scheme for further optional optimization of the nutrient medium for hyoscyamine production by D. stramonium hairy root cultures with different ploidy levels as a component of the carbon-to-nitrogen ratio.
Influence of ammonium ion concentrations on biomass and hyoscyamine accumulation by D. stramonium hairy root cultures obtained from diploid (a—biomass; c—hyoscyamine) and tetraploid (b—biomass; d—hyoscyamine). Bars represent standard deviations. Data obtained for the hairy roots are significantly different at P < 0.05
Influence of nitrate ion concentrations on biomass and hyoscyamine accumulation by D. stramonium hairy root cultures obtained from diploid (a—biomass; c—hyoscyamine) and tetraploid (b—biomass; d—hyoscyamine). Bars represent standard deviations. Data obtained for the hairy roots are significantly different at P < 0.05
The phosphate ion concentration in the medium strongly influenced the biomass accumulation by D. stramonium hairy roots obtained from tetraploid plants (Fig. 10b). The maximum amounts of biomass (0.85 g per flask) accumulated when they were cultivated in the medium with 0.63 mM phosphate ions; 2.4-fold more than the 0.35 g/L obtained when they were cultivated in the control, MS medium, which contains 1.25 mM phosphate ions. In contrast, variations in the concentration of phosphate ions had minor effects on the biomass accumulation of hairy roots obtained from diploid plants (Fig. 10a). Similar differences in the effects of phosphate concentrations on hyoscyamine production by the investigated hairy root systems were also observed (Fig. 10c,d). The amount (95 mg/L) of hyoscyamine accumulated by hairy roots obtained from tetraploid plants was maximal when the phosphate concentration in the medium was 0.63 mM, the lowest concentration tested (Fig. 10d). However, both reduction and increase of concentrations of the phosphate ions in the nutrient medium had negative effect on the hyoscyamine accumulation in D. stramonium hairy roots obtained from diploid plants (Fig. 10c). These results show that the phosphate ion requirements of the investigated hairy root cultures were significantly different and the optimal levels of phosphate ions depended strongly on the genome size.
Influence of phosphate ion concentrations on biomass and hyoscyamine accumulation by D. stramonium hairy root cultures obtained from diploid (a—biomass; c—hyoscyamine) and tetraploid (b—biomass; d—hyoscyamine). Bars represent standard deviations. Data obtained for the hairy roots are significantly different at P < 0.05
Conclusion
Flow cytometry analyses revealed that the hairy roots of D. stramonium produced in this study were genetically stable, but they had undergone endoreduplication and thus consisted mainly of cells with twice as many sets of chromosomes as the D. stramonium leaves they were obtained from. To our knowledge, this is the first time differences between the genome sizes of hairy roots and the primary plant material, used for Agrobacterium transformation, have been observed. The significant differences observed in alkaloid profiles during the growth cycle, in the utilization of macronutrients, and the influence of the main macronutrients on the biomass and hyoscyamine production by the investigated D. stramonium hairy root cultures show that an integrated approach, in which the relationships between genome size (ploidy level) and the other variables are thoroughly assessed, should be applied in order to optimize hyoscyamine production. The data obtained provide a good basis for further optimization of the nutrient medium for hyoscyamine production by D. stramonium hairy roots with different ploidy levels and the development of algorithms for selecting high alkaloid-producing lines.
References
Matsuda, J., Okabe, S., Hashimoto, T., & Yamada, Y. (1991). Journal of Biological Chemistry, 266, 9460–9464 Medline.
Pitta-Alvarez, S. I., Spolansky, T. C., & Giulietti, A. M. (2000). Enzyme and Microbial Technology, 26, 252–258 DOI 10.1016/S0141-0229(99)00137-4, Medline.
Zabetakis, I., Edwards, R., & O’Hagan, D. (1999). Phytochemistry, 50, 53–56. DOI 10.1016/S0031-9422(98)00490-7.
Bensaddek, L., Gillet, F., Sausedo, J., & Fliniaux, M.–A. (2001). Journal of Biotechnology, 85, 35–40 DOI 10.1016/S0168-1656(00)00372-2, Medline.
Berkov, S., Pavlov, A., Kovatcheva, P., Stanimirova, P., & Philipov, S. (2003). Zeitschrift für Naturforschung, 58c, 42–46.
Baiza, A., Quiroz-Moreno, A., Ruiz, J., & Loyola-Vargas, V. (1999). Plant Cell, Tissue and Organ Culture, 59, 9–17. DOI 10.1023/A:1006398727508.
Stebbins, G. L. (1971). Chromosomal evolution in higher plants. London: Edward Arnold.
Berkov, S., & Philipov, S. (2003). Pharmaceutical Biology, 40, 617–621.
Lavania, U. C. (2005). Plant Genetic Resources, 3, 170–177.
Dhawan, O. P., & Lavania, U. C. (1996). Euphytica, 87, 81–89. DOI 10.1007/BF00021879.
De Jesus-Gonzalez, L., & Weathers, P. J. (2003). Plant Cell Reports, 21, 809–813 Medline.
Pundir, R. P. S., Rao, N. K., & van der Maesen, L. J. G. (1983). Theoretical and Applied Genetic, 65, 119–122. DOI 10.1007/BF00264878.
Berkov, S., & Pavlov, A. (2004). Phytochemical Analysis, 15, 141–145 DOI 10.1002/pca.756, Medline.
Dixon, R. A. (1985). In R. A. Dixon (Ed.), Plant cell culture—A practical approach: Isolation and maintenance of callus and cell suspension cultures pp. 1–20. Oxford: IRL.
Pavlov, A., Georgiev, V., & Ilieva, M. (2005). Process Biochemistry, 40, 1531–1533. DOI 10.1016/j.procbio.2004.01.001.
Sung, L.-S., & Huang, S.-Y. (2000). Biotechnology Progress, 16, 1135–1140 DOI 10.1021/bp000062t, Medline.
Georgiev, M., Pavlov, A., & Bley, Th. (2007). Applied Microbiology and Biotechnology, 74, 1175–1185 DOI 10.1007/s00253-007-0856-5, Medline.
Bayliss, M. W. (1980). International Review of Cytology, 1117, 113–144.
Payne, J., Hamill, J. D., Robins, R. J., & Rhodes, M. J. C. (1987). Planta Medica, 53, 474–478 DOI 10.1055/s-2006–962776, Medline.
Kudo, N., & Kimura, Y. (2002). Plant Biotechnology, 19, 45–52.
Peter, C. L. J., & Ruhu, Q. I. (2008). Trends In Plant Science, 13, 121–127 Medline.
Pavlov, A., & Bley, Th. (2006). Process Biochemistry, 41, 848–852. DOI 10.1016/j.procbio.2005.10.026.
Pavlov, A., Berkov, S., Courot, E., Gocheva, T., Tuneva, D., Pandova, B., & Georgiev, M. (2007). Process Biochemistry, 42, 734–739. DOI 10.1016/j.procbio.2006.12.006.
Patterson, S., & O'Hagan, D. (2002). Phytochemistry, 61, 323–329. DOI 10.1016/S0031-9422(02)00200-5.
Witte, L., Müller, K., & Alfermann, H.-A. (1987). Planta Medica, 52, 192–197.
Ionkova, I., Witte, L., & Alfermann, H. A. (1994). Planta Medica, 60, 382–384 DOI 10.1055/s-2006–959509, Medline.
Ilieva, M., & Pavlov, A. (1997). Applied Microbiology and Biotechnology, 47, 683–688. DOI 10.1007/s002530050995.
Shin, K. S., Chakrabarty, D., Ko, J. Y., Han, S. S., & Paek, K. Y. (2003). Plant Growth Regulation, 39, 187–193. DOI 10.1023/A:1022525308389.
Su, W. W. (1995). Applied Biochemistry and Biotechnology, 50, 189–229. DOI 10.1007/BF02783455.
Robins, R. G., Parr, A. G., Payne, J., Walton, N. J., & Rhodes, M. G. C. (1990). Planta, 181, 414–422. DOI 10.1007/BF00195896.
Parr, A., Payne, J., Eagles, J., Chapman, R., Robins, R., & Rhodes, M. (1990). Phytochemistry, 29, 2545–2550. DOI 10.1016/0031-9422(90)85185-I.
Acknowledgement
This research was supported by a Marie Curie Fellowship of the European Community program “Development Host Fellowships” under contract number HPMD-CT-2001-00092, DAAD, references number D/05/01303, and the Bulgarian Science Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlov, A., Berkov, S., Weber, J. et al. Hyoscyamine Biosynthesis in Datura stramonium Hairy Root In Vitro Systems with Different Ploidy Levels. Appl Biochem Biotechnol 157, 210–225 (2009). https://doi.org/10.1007/s12010-008-8264-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8264-6