Abstract
Lipase from Brevibacillus agri 52 was found stable up to 90% diethylenglycol (DEG), glycerol (GLY), and 1,2 propanediol (1,2 PRO) at 37 °C for 1 h and the stability was reduced only approximately 20% after 12 h incubation, but in 40% dimethylsulfoxide (DMSO), lipase activity was stable only for 1 h. Inhibition of the biocatalysts with dimethylformamide (DMF) was detected at 20% solvent concentration. In water immiscible systems, the stability of lipase in n-hexane, n-tetradecane and n-heptane resembles the water activity, but in the presence of isobutanol, 1-hexanol, and butylbutirate, the stability was significantly reduced. Lipase 52 precipitates in the presence of 50% acetone or ethanol/water mixtures, but enzymatic activity was partially recovered by adding 20% GLY, DEG, 1,2 PRO, or DMSO to the reaction mixture. Furthermore, by increasing DEG in 70% DMF/DEG mixtures, the lipase activity was protected. Encapsulation of lipase in pectin gels cross-linked with calcium ions brings three to four times more enzymatic activity in 70% water miscible organic solvents compared to aqueous systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of biocatalyst outside from their biological environments enables their catalytic ability and delicate specificity to be extended toward synthetic chemistries using new substrates in novel reaction systems and non-natural unknown products. Even though the promise of new and exciting uses for biocatalyst is wide open, reduced reaction rates, short long-term stability, and reuse in reactors appear as barriers for the implementation of non-aqueous biocatalysis on biotechnological processes [1].
Hydrolases, like lipases, are gaining more attention in the biotechnology field because of enantioselectivity, substrate specificity, and physicochemical properties [2]. Lipases are commonly used in many fields ranging from detergents to food and pharmaceutical and chemical industries with more than billion dollar market. However, the little diversity of commercially available lipases are restricting potential uses for synthesis in organic solvents. Most of commercial lipases are produced from fungi or yeast cells and a few from Gram-negative bacteria [3]. Additionally, little information is available on lipases produced by Gram-positive bacteria, particularly from Bacilli, one of the most relevant extracellular industrial enzyme producers of the microbial world [4]. Recently, Bacillus licheniformis S-86 showed esterase activity in 50% dimethylsulfoxide (DMSO), ethylene glycol, glycerol (GLY), methanol, and propylene glycol [5]. In addition, lipase synthesized by the hyperthermophile Pyrobaculum calidifontis VA1 was found active for 1 h at 80% of acetonitrile, ethanol, dimethylformamide (DMF), DMSO, methanol, and 2-propanol [6].
However, operational and environmental parameters of the biocatalysts have been reported to modify relevant properties of the enzymes for biotechnological purposes like the enantio-, prochiral-, and regio-selectivity. In some cases, the physicochemical properties and composition of the organic media used as the reaction media determine the efficiency of the enzymatic conversion and product of the reaction [7].
Enzyme immobilization is a feasible industrial alternative with the advantages of enhancing enzyme half-life, improving downstream processing of products, and reusing the bioreactor in many cycles. Immobilization of the biocatalysts in matrixes can be developed using two major strategies, either by entrapment or by adsorption. Entrapped biocatalysts have the benefit of higher protection of the protein structure and biological activity compared to the adsorption method. A successful example of lipase entrapment developed in inorganic matrix based on sol-gel chemically modified silica was reported [8]. However, this technique is time-consuming because it involves the chemical modification of the matrix to be protein-friendly and also requires further processing of the sol-gel support and waste treatment of the reagents. On the other side, natural polymers offer significant advantages to encapsulate active proteins instead of chemical- modified matrixes including low cost of equipment, less expensive waste treatments, using soft techniques for cross-linking, and tailorability of molecular structure. Pectin, a heteropolysaccharide, has the advantages of gelling in presence of divalent cations, is biodegradable, non-toxic, and can be obtained from higher terrestrial plants.
Pectin is a heteropolysaccharide extracted from plant cell walls and used in many industrial applications as thickener or gelling agent. It chemically contains α-d-galacturonic acid as backbone linked through α-1,4-glycosidic linkages [9]. Some of the carboxylic groups of galacturonic acid are methylated, and depending on natural sources, pectins may have different side chains of arabinose, galactan, arabinogalactan, glucose, mannose, and xylose [10]. Commercial pectins in aqueous solution with esterification degree between 30% and 35% form gels in the presence of divalent cations. In addition, the strength of the gel increases with the decrease of temperature and pH and increase in ion concentration like calcium [11].
In a previous screening procedure, a wild-type bacterium characterized as Brevibacillus agri 52 was able to synthesize an extracellular lipase resistant to DMSO (Baigorí, personal communication). Considering the relevance of solvent-resistant enzymes for biotransformations, lipase 52 was used as model to explore the enzyme stability in binary and ternary miscible and immiscible water–organic solvents systems and encapsulated in pectin gels in the presence of organic solvents.
Materials and Methods
Microorganisms, Culture Conditions, and Extract Preparation
The initial assay to detect lipolytic activity was done on tributyrin agar plates containing per liter of distilled water, 5 g peptone, 3 g yeast extract, 1% (w/v) tributyrin, and 15 g agar. The plates were grown overnight at 37 °C, and the lipolytic activity was indicated by a clear halo around the colonies. Then, the cells were grown on Luria–Bertani (LB) agar medium during 16 h at 37 °C and picked from the agar plate and pre-cultured in 10 ml of LB medium for 4 h at 37 °C with absorbance at 560 nm (A 560) between 8.60 and 9.00. Those cells were then used to inoculated 150 ml of fresh LB medium during 19 h at 37 °C (early stationary phase) in a rotary shaker at 120 rpm. The resulting culture was centrifuged (Sorvall RC5C, Du Pont) at 8,000×g for 15 min (4 °C), and the cell-free supernatant was maintained at −20 °C until use and used as lipase extract.
Enzyme Assay
Lipase hydrolytic activity was measured spectrophotometrically at 405 nm with p-nitrophenyl palmitate (p-NPP) as substrate at 37 °C in 50 mM Tris–HCl buffer (pH = 7.1), 0.4% (w/v) Triton X-100 and 0.1% (w/v) arabic gum [12]. One unit of enzyme activity was defined as the amount of enzyme that releases 1 μmol of p-NPP per minute.
Protein Determination
Protein concentration was determined with Coomassie brilliant blue G-250 using bovine serum albumin (Fraction V) as standard [13].
Effect of Temperature, pH, and Ionic Strength on Enzymatic Activity
Activity assay were done at temperature between 30°C and 80°C in 50 mM Tris–HCl buffer (pH 7.1).
The effect of pH on the enzyme activity was tested at 50 °C in the range of pH 6.0–10.6, using the following buffers: 50 mM 2-(N-morpholino)ethanesulfonic acid (pH = 6.0), 50 mM Tris–HCl, (pH = 7.1, 8.0 and 8.9), and 50 mM glycine–NaOH (pH = 9.6 and 10.6).
The optimal ionic strength was determined in 50 mM Tris–HCl buffer (pH = 8.9) in 25 to 500 mM of NaCl at 50 °C.
In all cases, the enzymatic activity was estimated using the p-NPP method.
Stability Assays in Water-Miscible Solvents
Supernatant containing lipase activity was diluted properly with the organic solvent system and incubated at 37 °C for 1 h. Residual activity was assayed using the p-NPP method.
A proper dilution of lipase extract was incubated at 37 °C in presence of each immiscible organic solvent with constant magnetic stirring. After 1 h, the residual lipase activity was measured with the method mentioned above.
Effect of Water-Miscible Solvents on Lipase Stability
Lipase extract was incubated at different concentrations of each organic solvent during 1 and 12 h at 37 °C. After that time, the residual lipase activity was measured.
The organic solvents tested were acetone, DMF, DMSO, diethylenglycol (DEG), ethanol, glycerol (GLY), and 1,2-propanediol (1,2 PRO).
Effect of Mixed Solvents on Lipase Stability
The extract containing lipase activity was incubated in mixed solvents for 1 and 12 h at 37 °C. After that time, the residual lipase activity was measured by p-NPP method described above.
Preparation of Pectin Hydrogel Beads and Enzyme Activity
Citrus pectin powder was dissolved in water with agitation and mixed with the same volume of buffer 50 mM Tris–HCl buffer (pH = 8.9). The pectin solution (1.5%) was drop-wise into 0.25 M CaCl2 containing glycerol with gentle agitation at room temperature. The gel beads formed were filtrated and incubated 1 h with acetone at 4 °C. The beads were maintained at the same temperature after solvent evaporation.
Enzyme-loaded pectin hydrogel beads were prepared suspending the crude extract containing lipase activity in pectin solution in the ratio 1:1 (v/v). The solution containing lipase activity was drop-wise into 0.25 M CaCl2 containing glycerol with gentle agitation at room temperature. The beads formed were treated in the same manner as blank pectin hydrogel beads.
Lipase activity in enzyme-loaded pectin hydrogel beads was determined, incubating 50 mg of beads in 50 mM Tris–HCl buffer, pH = 8.9 during 1 h at 37 °C. Then, the beads were centrifuged, and the supernatant was removed. New buffer and 100 μl of 1 mM p-NPP in acetone were added. After 30 min of incubation at 37 °C with gentle agitation, the beads were centrifuged again, and the supernatant was read against the blank in a spectrophotometer at 405 nm.
Lipase stability in enzyme-loaded pectin hydrogel beads was determined, incubating 50 mg of beads in each solvent at 37 °C during 1 h. After this time, the supernatant was removed, and the residual activity was measured described above.
Reagents
All reagents were of analytical or microbiological grade from Sigma (St. Louis, MO, USA) or Merck (Darmstad, Germany).
Results and Discussion
To optimize lipase 52 operational parameters, pH, temperature, and ionic strength were determined. The maximum lipase activity in the extract was found in the range of 37°C to 65°C, pH 8.9 and 50 mM NaCl (data not shown). Using the optimum values, lipase 52 activity was determined in the presence of organic solvents with different physicochemical properties. The criteria adopted was to select solvents with wide range of log P, between −2.66 (very hydrophilic solvent like glycerol) to 7.6 (very hydrophobic, e.g. n-tetradecane) [14]. Lipase 52 stability was assayed at 0% to 90% concentrations of water-miscible organic solvents: acetone (log P = −0.23), DEG (log P = −1.98), DMF (log P = −1.038), DMSO (log P = −1.378), ethanol (log P = −0.24), GLY (log P = -2.66), and 1,2 PRO (log P = 0.67). Furthermore, the enzymatic activity was tested in presence of the following immiscible solvents butyl butyrate (log P = 1.78), n-heptane (log P = 4.0), 1-hexanol (log P = 1.8), isobutyl alcohol (log P = 0.79), and n-tetradecane (log P = 7.6).
Lipase 52 stability was tested in water-soluble organic solvents at 1 to 12 h incubation at 37 °C (Figs. 1 and 2). In presence of DEG, GLY, and 1,2 PRO, the lipase stability remained for 1 h and decrease about 20% after 12 h of incubation (Fig. 1A, B). In DMSO, the lipase stability is reduced drastically after the solvent overpass 40% concentration. A more dramatic effect of organic solvent on the reduction of lipase 52 stability was found on DMF: less than 20% and 0% activity remains after 1 and 12 h by incubation at 40% DMF concentration at 37 °C, respectively (Fig. 2). Similarly, in a previous report, lipase from Bacillus megaterium CCOC-P2637 incubated in hydrophilic solvent–water mixtures, e.g., acetone, ethanol, and isopropanol, was activated in the 25% to 50% solvent range, but at higher solvent concentrations, enzyme stability was solvent-dependent [18]. More recently, lipase produced by Serratia marcescens ECU1010 was found very stable in solvent mixtures acetone, ethanol, DMSO, isopropanol, and methanol at 30 °C for 24 h [19]. In agreement with our results, the activity of Streptomyces rimosus R6-554W lipase was reduced to 63% and 4% by incubation in 50% DMSO and DMF, respectively, for 18 h at 25 °C [20]. In the last case, loss of enzyme activity in homogeneous solvent mixtures could be correlated to the decrease of the solvation water-layer from the protein surface, thereby rigidifying the protein structure and/or denaturing the enzyme by solvent–protein interaction.
Another problem associated with some water-miscible organic solvents is the precipitation of proteins forbidding the development of those systems for biotransformation. In our case, the lipase extract became turbid at acetone and ethanol concentrations higher than 50%; under these conditions, the enzymatic activity was not able to be measurable.
In non-miscible water solvents, lipase 52 stability was reduced in all cases after 1 h incubation. The enzyme stability is reduced to about 10% to 15% by incubation in 1-hexanol and isobutanol. However, in the presence of n-hexane and n-tetradecane, the stability of lipase 52 resembles the activity in pure water systems (Fig. 3).
It is well-known that the biocatalyst specificity depends on the properties of the environment, and solvents play a crucial role in enzymes to determine activity and specificity [15]. However, lipase 52 cannot be used in the presence of organic solvents like acetone, ethanol, and DMF because the enzyme was inactivated and/or precipitated. Considering solvents like 1,2 PRO, DEG, and GLY, where lipase 52 activity was maintained for almost 12 h, it could be possible to “rescue” the enzyme activity using organic mixtures among solvents and mimicking the water effect in the reaction mixture [1]. The effects of 20% concentration of “protective” solvents in 50% acetone and ethanol–water mixtures on lipase 52 stability are presented in Fig. 4. In acetone, lipase 52 stability was higher than 70% for 1 h in the presence of 20% concentration of GLY, DEG, DMSO, and 1,2 PRO (Fig. 4A). However, in 50% ethanol, the results can be arranged in two groups (Fig. 4B). At high log P (DMSO and 1,2 propanediol), the enzyme stability was lower than 40% compared to the aqueous system. On the contrary, at lower log P, the lipase 52 stability was maintained higher than 50%. However, the “activity rescue” by solvents of lipase 52 activity was better in acetone compared to ethanol, both with very close log P. However, the presence of OH groups in the ethanol probably increases the protein–solvent interaction by hydrogen bridges changing structural molecular movement of the biocatalysts [16].
In another experiment, stability of lipase 52 was studied in DEG-DMF water system at 1 and 12 h, keeping the organic concentration at 70% in solution but changing the ratio DEG-DMF (Fig. 5). As it can be expected, the lipase stability increases as the DMF concentration decreases and concomitantly with DEG concentration increase. In this case, DEG is acting as protective agent against the deleterious presence of DMF on the enzyme stability.
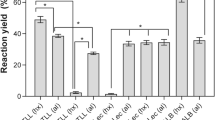
One the other hand, lipase 52 stability in pectin gels cross-linked with calcium was studied to test the water-miscible solvents at 50% concentration at 37 °C used in previous experiments (Fig. 6).
The protective effect of lipase 52 encapsulation was clearly observed in all analyzed systems (Fig. 7). In DMF, the lipase stability is very high compared to water at 1 h of incubation, and also after 12 h of incubation, the enzyme activity resembles the water system value. A very different landscape when soluble lipase stability in DMF is compared with immobilized biocatalyst stability in pectin microspheres (see Fig. 2). In the case of glycerol, lipase stability was approximately constant showing no signs of inactivation, but the enzyme activity was constrained probably because of the additional diffusional barrier of the solvent [17]. Very importantly, the lipase stability was significantly increased between three to four times in DMSO, DEG, and 1,2 PRO by encapsulation in pectin (Fig. 7). In the previous experiments using soluble lipase, the enzymatic activity was stabilized in GLY, DEG, and 1,2 PRO, and up to 40% concentration in DMSO (Fig. 1A, B). However, in the immobilized system, the organic concentration tested was 50%, and the lipase activity was improved many times, as well as in presence of DMF and DMSO (Fig. 7). Similarly, lipase was activated by the presence of hydrophilic support and polar organic solvents providing a sustainable system for biocatalysis: Exposure of Baacillus coagulans lipase immobilized on Nylon-6 in presence of 2-propanol (log P = 0.28) increased the enzyme activity by interfacial effect [21]. A possible explanation is that the support might trap and prevent the disruption of the enzyme–water essential to maintain the three-dimensional structure of the enzyme for catalysis, as the polar solvents tend to strip water from the enzyme molecule [21]. Additionally, conformational changes around a tryptophan residue on lipase by polar miscible solvents were observed by fluorescence studies and correlated to changes of the biocatalytic activity [22]. These structural changes could be interpreted, as the change of the lid position that covers the lipase active site allow partially “unlocking” or “opening” the catalytic center of the enzyme and consequently favoring the contact with the substrate.
In conclusion, the strategy of using two water-soluble organic solvents to stabilize enzyme activity in systems where the activity was negligible or totally inhibited combined with enzyme encapsulation using biopolymers open an alternative way for the development of new biotransformation systems that can be easily scale up with the advantage of recycling the biocatalyst.
References
Vulfson, E. N., Halling, P. J., & Holland, H. L. (2001). Enzymes in non-aqueous solvents. Totowa, NJ: Humana.
Jaeger, K. E., & Reetz, M. T. (1998). Tibtech, 16, 396–403.
Pandey, A., Benjamin, S., Soccol, C. R., Nigam, P., Krieger, N., & Soccol, V. T. (1999). Biotechnology and Applied Biochemistry, 29, 119–131.
Haba, E., Breco, O., Ferrer, C., Marqués, A., Busquets, M., & Manresa, A. (2000). Biotechnology and Applied Biochemistry, 26, 40–44.
Torres, S., & Castro, G. R. (2004). Food Technology and Biotechnology, 42, 271–277.
Hotta, Y., Ezaki, S., Atomi, H., & Imanaka, T. (2002). Applied and Environmental Microbiologys, 68, 3925–393.
Carrea, G., & Riva, S. (2000). Angewandte Chemie. International Edition in English, 39, 2226–2254.
Reetz, M. T. (1997). Advanced Materials, 9, 943–954.
Van Buren, J. P. (1991). Function of pectin in plant tissue structure and firmness. In H. W. Reginald (Ed.), The chemistry and technology of pectin (pp. 3–4). New York: Academic.
Ryden, P., & Selvendram, R. R. (1990). Carbohydrate Research, 195, 257–272.
Lootens, D., Capel, F., Durand, D., Nicolai, T., Boulenguer, P., & Langendorff, V. (2003). Food Hydrocolloid, 17, 237–244.
Gupta, N., Rathi, P., & Gupta, R. (2002). Analytical Biochemistry, 311, 98–99.
Bradford, M. M. (1976). Analytical Biochemistry, 78, 248–254.
Laane, C., Boeren, S., Vos, K., & Veeger, C. (1987). Biotechnology and Bioengineering, 30, 81–87.
Hudson, E. P., Eppler, R. K., & Clark, D. S. (2005). Current Opinion in Biotechnology, 16, 637–643.
Ferst, A. (1999). Structure and mechanism in protein science. New York: Freeman.
Castro, G. R., & Knubovets, T. (2003). Critical Reviews in Biotechnology, 23, 195–231.
Lima, V. M. G., Krieger, N., Mitchell, D. A., Baratti, J. C., De Felippis, I., & Fontana, J. D. (2004). Journal of Molecular Catalysis. B, Enzymatic, 31, 53–61.
Zhao, L. -L., Xu, J. -H., Zhao, J., Pan, J., & Wang, Z. -L. (2008). Process Biochemistry, 43, 626–633.
Leščic, I., Vukelic, B., Majeric-Elenkov, M., Saenger, W., & Abramic, M. (2001). Enzyme and Microbial Technology, 29, 548–553.
Pahujani, S., Kanwar, S. S., Chauhan, G., & Gupta, R. (2008). Bioresource Technology, 99, 2566–2570.
Tsuzuki, W., Ue, A., & Nagao, A. (2003). Bioscience, Biotechnology, and Biochemistry, 67, 1660–1666.
Acknowledgments
The authors would like to thank M.D. Baigorí for helpful discussions and providing for the strain. The authors gratefully acknowledge the financial support from Pew Charitable Trust (USA), CONICET (PIP 6203/06, Argentina) and ANPCyT (BID no. 1728/ OC-AR, Argentina).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costas, L., Bosio, V.E., Pandey, A. et al. Effects of Organic Solvents on Immobilized Lipase in Pectin Microspheres. Appl Biochem Biotechnol 151, 578–586 (2008). https://doi.org/10.1007/s12010-008-8233-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8233-0