Abstract
Pseudomonas aeruginosa PACL strain, isolated from oil-contaminated soil taken from a lagoon, was used to investigate the efficiency and magnitude of biosurfactant production, using different waste frying soybean oils, by submerged fermentation in stirred tank reactors of 6 and 10 l capacities. A complete factorial experimental design was used, with the goal of optimizing the aeration rate (0.5, 1.0, and 1.5 vvm) and agitation speed (300, 550, and 800 rpm). Aeration was identified as the primary variable affecting the process, with a maximum rhamnose concentration occurring at an aeration rate of 0.5 vvm. At optimum levels, a maximum rhamnose concentration of 3.3 g/l, an emulsification index of 100%, and a minimum surface tension of 26.0 dynes/cm were achieved. Under these conditions, the biosurfactant production derived from using a mixture of waste frying soybean oil (WFSO) as a carbon source was compared to production when non-used soybean oil (NUSO), or waste soybean oils used to fry specific foods, were used. NUSO produced the highest level of rhamnolipids, although the waste soybean oils also resulted in biosurfactant production of 75–90% of the maximum value. Under ideal conditions, the kinetic behavior and the modeling of the rhamnose production, nutrient consumption, and cellular growth were established. The resulting model predicted data points that corresponded well to the empirical information.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several properties peculiar to surfactants make them valuable for numerous industrial applications [1]. While often thought of as synthetic in origin, molecules with surfactant properties are also produced by bacteria and fungi [2]. In fact, microbiologically produced surfactants, called biosurfactants, offer some advantages over their synthetic equivalents, such as selectivity and efficiency at extreme values of pH, salinity, and temperature [3–5].
In recent years, interest in the potential applications of biosurfactants has increased significantly, particularly because of their biodegradability and very low environmental toxicity [1].
Also, as a product of microbial metabolism, it is possible to produce a commercially viable biosurfactant using low-cost raw materials or high-pollutant wastes. The use of waste materials to actually generate a usable product helps address another crucial problem, that is, waste disposal. Because of the escalating costs of waste treatment and disposal, as well as increasing societal interest in reducing consumption and waste production, a decrease in both is highly desirable and beneficial.
The economy is other great challenge in biotechnological, especially for the biosurfactants production. Successful biosurfactant production depends on the development of low-cost processes and raw material which should not be 10% to 30% of the cost of the final product [6].
In turn, the type and the quantity of the biosurfactant produced depends not only on the particular microorganism but also on the culture conditions, including carbon and nitrogen sources, temperature, aeration, and other factors [7].
The glycolipids are the best known microbial surfactants and, among these, the rhamnolipids are the most studied due to their proven capacity for reducing the surface and interfacial tensions, which makes possible the formation of stable oil–water emulsions [8]. Several species of Pseudomonas are capable of producing large quantities of rhamnolipids from different substrates [9–11].
In the last decade, efforts have been focused on minimizing biosurfactant-producing costs in order to guarantee their commercial use. The promising future of these compounds depends specifically upon the use of abundant and low-cost raw materials and the optimization of the operational fermentation conditions in order to achieve high yields.
Annually, worldwide vegetable oil production is approximately 3 million tons [12]. Most of the production processes generate enormous amounts of waste, which are available for potential use in subsequent bioprocesses. Previously we defined the optimum nutritional conditions for the production of rhamnolipids by an isolated Pseudomonas aeruginosa strain using soybean residual oil [13].
The purpose of the present work was to determine, by means of a statistically based experimental design, the extent to which aeration and agitation influence biosurfactant synthesis, with the goal of improving the fermentation process.
Materials and Methods
Microorganism
The P. aeruginosa strain PACL used in this study was isolated from a soil sample collected from a gasoline/diesel oil-contaminated lagoon from Rio das Pedras Farm, Uberlândia (Minas Gerais, Brazil).
Identification of the Pseudomonas strain was completed at the Laboratório de Enterobactérias do Instituto Oswaldo Cruz (Rio de Janeiro, Brazil), following traditional procedures based on bacteria cytomorphology, biochemistry, and physiology. The culture was maintained on bacto nutrient broth (BD cod. 234000) supplied by BD (Becton Dickinson and Company, USA) at 4°C, using monthly subcultures.
Media and Growth Conditions
Growth of the bacterial culture was performed on medium proposed by Santos et al. [14], consisting of (g/l): NH4NO3 1.7; Na2HPO4 7.0; KH2PO4 3.0; MgSO4.7H2O 0.2; yeast extract 5.0; and glucose 10.0.
Biosurfactant production assays were conducted on the same mineral medium used for microbial growth, with the addition of 5.625 g/l NH4NO3, 22.0 g/l of waste fried soybean oil (WFSO), and 11.5 g/l residual brewery yeast (RBY) in substitution of glucose and yeast extract. These concentrations were based on data from a previous study where those parameters had been optimized [13].
The WFSO employed throughout this study was a mixture of soybean oils, collected from several snack bars, which had been used to fry several different foods, including potatoes, rissoles, pastries, meat balls, etc. In WFSO, the soy used for production of the oil was obtained from tropical and sub-tropical climates and provided in several lots by different providers.
For comparison, some experiments were also conducted with non-used (fresh) soybean oil (NUSO) and soybean oil used to fry different types of food separately, that is: meat ball (MFSO), potato (POFSO), and pastries (PAFSO). The MFSO, POFSO, and PAFSO soy oils were prepared in a laboratory with the use of two soy oils. The first one was made by using soy cultivated in the central area of the country where the climate is tropical. The other one was made by using soy cultivated in the area with subtropical climate. In the cases mentioned previously, the oils were reused five times for each food type. In the experimental run using the oil NUSO, the two soy oils was used as previously mentioned.
The RBY, consisting of 100% inactivated, dried cells of Saccharomyces cerevisiae, was supplied by a local brewery. The product composition was: moisture 8.0%, protein 40.0%, fibrous matter 3.0%, mineral matter 8.0%, and aflatoxin 50 ppb. All media were autoclaved at 121°C for 15 min after adjusting the pH to 7.0 with 0.1 N HCl.
The inoculum was prepared by adding three loopfuls of cells from the stock culture to a 500 ml Erlenmeyer flask containing 100 ml of the growth medium. The inoculated medium was incubated at 30 ± 1°C for 24 h on a rotary shaker (New Brunswick, USA) at 170 rpm.
Production of Biosurfactant in Bioreactor
A complete factorial experimental design was used to optimize the levels of aeration (0.5, 1.0, and 1.5 vvm) and agitation (300, 550, and 800 rpm). For each aeration rate/agitation speed combination that was tested, the oxygen-related mass transfer coefficient (kLa), was calculated following the method of Rainer [15].
The factorial experimental design were completed in a 6.0 l Biostat B. Fermentor (B. Braun Biotech International, USA) containing 3.3 l of production medium. The inoculum was added in order to establish an initial concentration of 0.3 g/l, exponential growth-phase cells.
The kinetic study was performed in a 6.0 l and 10.0 l Biostat B. Fermentor (B. Braun Biotech International, USA).
During a 48 h period, the temperature and pH were held at 30 ± 1°C and 7.0, respectively, and samples of foam were continuously withdrawn with the aid of a foam collector, located within the reactor, immediately above the broth surface.
Kinetic Study
These experiments were completed in triplicate in a 6.0 l stirred tank reactor with 3.3 l of medium for 96 h. Temperature was maintained at 30 ± 1°C; aeration and agitation were set at 0.5 vvm and 555 rpm, respectively.
In order to compare the kinetic study results to the biosurfactant production results, the experiments were conducted under the same conditions of inoculum, carbon and nitrogen sources, agitation, aeration, and kLa in the 10 l reactor.
A 5.5 l of medium was used in the 10.0 l fermentor aiming to keep the same ratio medium height/diameter as the 6.0 l fermentor. Both fermentors have cylindrical shape with dimensions given as follows. The 6.0 l fermentor has a height of 345 mm and a diameter of 160 mm and the 10.0 l fermentor has a height of 470 mm and 190 mm of diameter.
A parameter identification technique was conducted under controlled conditions by using a nonlinear, multiple replies regression algorithm [16].
The integration of the set of differential equations, for the calculation of the parameters used to adjust the experimental data, was performed with the aid of the fourth-order algorithm of Runge–Kutta [17].
Parametric identification is indicated when there is good agreement between the experimental values and those supplied by the model. The relative average error of the parameters in the nonlinear regression was calculated, together with the addition of the quadratic of the residues.
Kinetic Model Construction
The models express growth and product formation as a function of only biomass and evolution over time. Changes in biomass and rhamnolipid production were represented by Eqs. 1 and 2, respectively. Temporal variations in nutrient levels (nitrate, phosphorus, and total Kjeldahl nitrogen) were defined by Eqs. 3, 4, and 5, respectively.
Where X is the concentration of biomass (g X/l); Ram, the rhamnolipid concentration (g Ram/l); \({\text{NO}}_3^{ - } \), the nitrate concentration (g \({\text{NO}}_3^{ - } \)/l); Pt, the total concentration of phosphorus (g Pt/l); and Nt, the total nitrogen concentration (g Nt/l).
In Eqs. 1–5, the terms μ (1/h), λ (g Ram/g biomass), α (g \({\text{NO}}_3^{ - } \)/g biomass), β (g Pt/g biomass), and γ (g Nt/g biomass) represent the specific growth rate, the inverse of the yield coefficient that relates to the rhamnolipids production associated to the cells growth, and the inverse of yield coefficients that relate to the consumption of the three nutrients for cellular growth (nitrate, total phosphorus, and total Kjeldahl nitrogen), respectively.
Cellular Growth Modeling
The specific growth rate (μ) was expressed as a function of biomass only by using the logistic equation described by Verhulst (1844) and Pearl and Reed (1920) in Bailey and Ollis [18] (Eq. 6).
Where μ max is the maximum specific growth rate (1/h) and X* is the maximum obtained cell concentration.
Modeling of Other Functions
The model describing the formation of rhamnose and consumption of nutrients was given by Eqs. 2, 3, 4, and 5 of Luedeking et al. [19].
Analytical Methods
Total Kjeldahl nitrogen was determined by a titulometric method after acid digestion with sulfuric acid, catalyzed with copper sulfate and potassium [20]. Total phosphorus was measured by the digestion of percloric acid in a reaction with ammonium molybdate and ascorbic acid [21]. Nitrate determination was completed using an adapted colorimetric method with nitrate precipitation [22]. The rhamnose concentration was determined according to a method described by Rahman [23].
Surface tension was measured at 25°C using a Tensiometer (Fisher Scientific, USA, model 21), which was previously calibrated with surveyor weights. A decrease in surface tension was used as a qualitative measurement of surfactant concentration and a quantitative indicator of efficiency.
The biosurfactant emulsification index (EI) was determined according to Cooper and Goldenberg [24]. Cell-free culture samples and kerosene, at a ratio of 4:6, were vigorously mixed for 2 min using a vortex (Phoenix, Brazil, model AP-56) and left undisturbed for 24 h at room temperature. EI 24 is the percent of the height of the emulsified layer (cm), relative to the total height of the liquid column, determined at the 24 h time point.
Cell growth was determined by measuring the optical density of samples, using a UV–visible spectrophotometer (20 Genesis, BR) at 540 nm. Cell concentration was determined by dry weight by filtering through a 0.45 μm previously weighted Millipore membrane [25].
Results and Discussion
Biosurfactant Production
The effect of the aeration to agitation ratio on the biosurfactant production by P. aeruginosa PACL in WFSO was evaluated by monitoring changes in rhamnose concentration, superficial tension, and the EI on culture supernatants after 48 h of processing time.
Nine experiments were conducted using the complete factorial experimental design (Table 1). Experiment 2 resulted in the highest level of rhamnose synthesis (3.3 g/l), that is, the maximum biosurfactant production of any of the experiments.
In the conditions of experiment 2, the minimum surface tension achieved (26.0 dynes/cm) is one more indication that the aeration to agitation ratio of 0.5 vvm:550 rpm favored rhamnolipid production from NUSO. For the majority of experiments, the produced biosurfactant had intense emulsifying properties, with the capability of forming complete kerosene in water emulsions that were stable for at least 24 h. Under these conditions, cell growth also reached its maximum, around 4.0 g/l.
Haba et al. [12] selected the strain P. aeruginosa 47T2 NCIB 40044, from 36 screened strains, for its capacity to produce 2.7 g/l of rhamnose in a nonaerated culture with WFSO. Previous studies found that this strain produced only 6.4 g/l of rhamnose through cultivation in waste olive oil with an aeration rate of 1.0 vvm [26].
Less rhamnose production (1.41 g/l) had been achieved using the P. aeruginosa LBI strain in a bioreactor culture utilizing fresh soybean oil as the carbon source, with an aeration rate of 2.5 vvm [27].
According to Rahaman et al. [23], cultivation of the P. aeruginosa GS9-119 strain in soybean and sunflower oils for 228 h resulted in maximum rhamnolipid productions of only 1.75 and 1.66 g/l, respectively. In this case, the respective surface tensions of culture medium were 28 and 30 dynes/cm. Although low, those results were somewhat better than what was obtained when the strain was cultured in glycerol, which yielded values of only 1.06 g/l and 29.1 dynes/cm.
Of the variables evaluated in the present work, aeration was the most important. The maximum rhamnose concentration and EI, and minimum surface tension values were reached at an aeration rate of 0.5 vvm, independent of the agitation speed, inside of the studied range (300 to 800 rpm).
Despite this, the final biomass concentrations did not significantly vary with the aeration to agitation ratio. Not surprisingly, there was a direct relationship between rhamnose synthesis and the EI. Greater biosurfactant concentration in the culture medium was related to greater emulsion capacity.
Emulsification is one of the most interesting of the tensoactive parameters relative to practical application of the product [28]. Because of its importance, it is normally evaluated separately for each biosurfactant produced. In the food industry, these compounds are used in the processing of raw materials. Cream, butter, mayonnaise, sausage, and ice cream, among others, are examples of the use of emulsions in processed foods [4]. The emulsion formation is also important in the paper industry, favoring a consistent and desirable texture, as well as in phase dispersion [29].
In experiment 9, which was conducted using the most vigorous agitation and aeration conditions, resulted in the lowest output of rhamnose (Table 1). This may have been due to a substantial loss of liquid related to elevated aeration and solution agitation, which intensified foam formation. Within the first 24 h period, approximately 50% of the medium volume had been removed from the bioreactor, which probably affected biosurfactant formation.
Similar results were reported by Santos [14] using the P. aeruginosa PA1 strain. In that study, an air bubbling system failed to control foam formation.
As expected, the increase in agitation and aeration caused an increase in the value of kLa (Table 1). It is apparent that the kLa values were related neither to the cell growth or biosurfactant synthesis. Indeed, while rhamnolipid concentration showed substantial variability with aeration to agitation ratios, biomass did not. Maximum cell growth and biosurfactant production was achieved with a kLa of 10.2 h−1.
Oxygen often limits the performance of aerobic bioprocesses, due to the low oxygen solubility in the aqueous media. However, since Pseudomonas species are capable of anaerobic respiration using nitrate, low oxygen concentrations may actually improve rhamnolipid synthesis. It is likely that a high degree of foaming actually limits the aeration rate of the medium.
Statistical Analysis
The operational conditions—aeration rate (X 1) and agitation speed (X 2)—that maximize biosurfactant production, were determined according to the complete factorial experimental design at three levels [30].
Statistical analyses were completed using Statistica 5.0 Software (Statsoft Inc, USA). The parameters and the significance levels of the model variables are described in Table 2. The adjusted empirical model obtained for the rhamnose synthesis, containing only the significant parameters (p ≤ 0.05) determined using a Student’s t test, is represented by Eq. 7.
From Eq. 7, a quadratic coefficient of multiple correlation of 0.9 was calculated, that is, 90% of the data variability had been explained by the equation. Also, this analysis verified that of the variables analyzed, aeration rate had the greatest influence on the production of rhamnose. The negative sign of X 1 (see Eq. 7) indicates that a change from level +1 to level −1 results in an increase in rhamnose synthesis. This response is supported by the response surface obtained through Eq. 7 (Fig. 1), where it is apparent that there is an optimal region for rhamnose production, comprised of given levels of aeration (0.5 to 0.55 vvm) and agitation (400 to 700 rpm).
The complete equation of rhamnose production, as a function of the variables X 1 (agitation) and X 2 (aeration), and an algorithm of optimization, developed with Maple VIII, Release 4 software (Waterloo Maple Inc., CAN), were used to determine the ideal point for aeration (vvm) and agitation (rpm). The resulting values were: X 1 = 0.5 and X 2 = 555.
As the ideal aeration rate was the lowest studied, it was necessary to determine if an even lower value would promote higher rhamnose production. Thus, experiments were initiated with aeration rates of 0.25 and 0.35 vvm at 555 rpm (Table 3). The results indicated that the greatest production of rhamnose was achieved at the previously identified aeration rate of 0.5 vvm.
Comparison of Biosurfactant Production using Different Carbon Sources
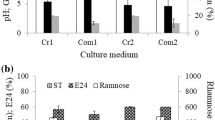
Adopting the optimized aeration to agitation operational conditions, the production of rhamnolipid was investigated using different waste soybean oils (WFSO, MFSO, POFSO, and PAFSO) and non-used soybean oils (NUSO). The type of food fried in the soybean oil did not influence biosurfactant production significantly (Table 4).
The highest rhamnose amount was achieved when non-used (fresh) soybean oil was employed. However, the use of waste frying soybean oils (WFSO, MFSO, POFSO, and PAFSO) also favored biosurfactant production corresponding to 75–90% of the maximum value.
According to Damy and Jorge [31], Nawar [32], Waltking and Wessels [33], and Tyagi and Vasishtha [34], during the fry process, the oil interacts with the air, the water, and other components of the food that it is being fried, originating a great diversity of chemical reactions, such as hydrolyzation, oxidation, and polymerization of the triacylglycerol molecule. The products obtained from the lack of an appropriate control of the fry process, affect the nutritional quality once oils and fats heated up to high temperatures can suffer oxidation and to produce substances potentially toxicant. Those physical-chemistry modification affected the efficiency of the fermentation process probably.
Kinetic and Modeling Of Rhamnose Production, Cell Growth, and Consumption of Nutrients
As shown in Fig. 2, intense cell growth was observed through 48 h of fermentation, at which time the stationary phase was achieved, corresponding to an experimental biosurfactant production (Yp/x) of 0.8 g rhamnose/g cells; the model-calculated Yp/x was 0.73 g rhamnose/g cells. At 54 h, the isolated strain reached maximum growth and rhamnose synthesis levels of 5.04 and 3.34 g/l, respectively. Between the hours 12 and 48, high nitrogen and phosphorus consumption was evident, which apparently influenced biomass production and biosurfactant synthesis. It has been reported that these nutrients stimulate microbial growth [14]. By contrast, an older study demonstrated that bacterial growth and surfactant accumulation were not modified by the addition of phosphate and magnesium [26]. The presence of phosphorous, therefore, seems to be unnecessary, or at least not a limiting factor, for the fermentation process.
Kinetics of growth (filled square), nitrate (filled diamond), phosphorus (filled triangle), total Kjeldahl nitrogen (filled star), and rhamnose (filled circle) during Pseudomonas aeruginosa PACL cultivation from waste frying soybean oil in stirred tank reactor (6 l) at 0.5 vvm and 555 rpm. The symbols represent the experimental results and the lines correspond to the data determined by the kinetic model described by Eqs. 1 to 6
Kinetic study results indicate the biosurfactant produced by P. aeruginosa PACL is a primary metabolite. In contrast, Benincasa et al. [27] reported that initially, biosurfactant production follows an exponential growth phase but, when microbial growth ceases and a stationary phase is achieved, rhamnose synthesis continues, which suggests biotensoactive production half-associated with microbial growth. It is important to note, however, that their observations were made over 80 h of fermentation.
Furthermore, an interruption of cellular growth and biosurfactant formation occurred, even though the medium, and therefore a nitrogen source, was still present in significant quantities. At the end of the process, nitrate consumption of approximately 65% was detected. The process interruption, therefore, may be attributed to a limitation on the carbon needed for bacterial metabolism. Usually, the carbon to nitrogen ratio is important to the product yield of bioactive molecules [5, 10, 14, 24, 35]. The nitrogen source, in association with carbon availability, favors the maintenance of the necessary enzymatic machinery for the synthesis of rhamnose [10]. Also, the end of the fermentation process may be associated with the formation of toxic by-products or to the solubility of toxic constituents in the waste oil through biosurfactant accumulation in the medium.
The model consisted of Eqs. 1 to 6—one algebraic equation and five distinguishing equations. Parameter values and constants are presented in Table 5. The terms of maintenance and product, not associated with cell growth, were rejected in the equations that describe rhamnose production (Eq. 2) and nitrate (Eq. 3) and phosphorus (Eq. 4) consumption, since adjustment of the model parameters indicated they were not significant, and demonstrated that the biosurfactant production process, using the microbial strain and used soybean oil as a carbon source, was associated with the cellular growth. The resultant model is only valid for the cell and nutrient concentrations established in this study. The parametric adjustment demonstrated a good correlation between the experimental values and those supplied by the model. The relative errors were less than 6% for the model elements (Table 5).
Yield values determined with the model (Y mod), and those determined experimentally (Y exp), were similar (Table 6). From the parameters obtained by model adjustment, defined by Eqs. 1 to 6, it was possible to validate the experimental data for the prediction of rhamnose production under operational conditions. In addition, the model also has value in the validation of other experimental conditions, as for example, different substrates and nutrient concentrations. The parameters described by the model for Eqs. 1 to 6, obtained in the 6 l reactor were used to predict the experimental values that would be obtained from the 10 l reactor. The accuracy of those predictions validates the model as representative of the experimental data (Fig. 3).
Kinetics of growth (filled square), nitrate (filled diamond), phosphorus (filled triangle), total Kjeldahl nitrogen (filled star), and rhamnose (filled circle) during Pseudomonas aeruginosa PACL cultivation from waste frying soybean oil in stirred tank reactor (10 l) at 0.5 vvm and 555 rpm. The symbols represent the experimental results and the lines correspond to the data determined by the kinetic model described by Eqs. 1 to 6
Similar 48 h (reaction time for optimization experiments) biomass and rhamnose production values were obtained for both reactors (see Figs. 2 and 3 and Table 1). As both experiments had been carried under the same conditions, with only volume being different, it can be concluded that the parameters obtained by the adjustment had suitably described the experiment carried out on a bigger scale.
It is also important to note, despite that change in reactor scale, that there was no modification of the values of kLa. The value of kLa (10.2 h−1) for the maximum biosurfactant production in the 6 l reactor (Table 1) was essentially the same as for the larger scale (10.4 h−1).
Conclusions
The results obtained from these studies demonstrate that the new isolated strain has the potential to produce biosurfactant from waste frying soybean oil at low aeration rates. Under optimal conditions (0.5 vvm and 555 rpm), this process resulted in 3.3 g/l of rhamnose, 26.0 dynes/cm for surface tension and 100% of the EI. These values are greater than most of the comparable data identified majority cited in the literature. The concentration of rhamnose was somewhat higher when fresh, non-used soybean oil was employed as a carbon source. The model obtained has proven very effective in describing the kinetic behavior for rhamnose production and phosphorus, nitrate, and total nitrogen consumption, as a function of cellular growth.
References
Makkar, R. S., & Cameotra, S. S. (2002). Applied Microbiology and Biotechnology, 58, 428–34.
Banat, I. M., Samarah, N., Murad, M., Roene, R., & Banerju, S. (1991). Applied Microbiology and Biotechnology, 7, 80–88.
Lin, S. C., Sharma, M. M., & Georgiou, G. (1996). Biotechnology, 9, 138–145.
Nitschke, M., & Pastore, G. M. (2002). New Journal of Chemistry, 25, 772–776.
Reis, F. A. S. L., Servulo, E. F. C., & França, F. P. (2004). Applied Microbiology and Biotechnology, 115, 899–912.
Cameotra, S. S., & Makkar, R. S. (1998). Applied Microbiology and Biotechnology, 50, 520–529.
Francy, D. S., Thomas, J. M., Raymond, R. L., & Ward, C. H. (1991). Journal of Industrial Microbiology & Biotechnology, 8, 237–246.
Mulligan, C. N. (2004). Environmental Pollution, 133, 183–198.
Syldatk, C., Lang, S., & Wagner, F. (1985). Zeitschrift für Naturforsch, 46, 51–60.
Bodour, A. A., Drees, K. P., & Maier, M. R. (2003). Applied and Environmental Microbiology, 69, 3280–3287.
Ochsner, A. U., Hembach, T., & Fiechter, A. (1996). Advances in Biochemical Engineering, Biotechnology, 53, 89–119.
Haba, E., Espuny, M. J., Buquets, M., & Manresa, A. (2000). Journal of Applied Microbiology, 88, 379–387.
De Lima, C. J. B., De França, F. P., Sérvulo, E. F. C., Resende, M. M., & Cardoso, V. L. (2007). Applied Biochemistry and Biotechnology, 136–140, 463–470.
Santos, A. S., Sampaio, A. P. W., Vasquez, G. S., Santa Anna, L. M., Pereira, N., & Freire, D. M. G. (2002). Applied Biochemistry and Biotechnology, 98–100, 1025–1035.
Rainer, B. W. (1990). Chemical and Biochemical Engineering, 4, 185–196.
Marquardt, D. W. (1963). Journal of the Society for Industrial and Applied Mathematics, 11, 431–441.
Gill, S. (1951). Proceedings of the Cambridge Philological Society, 47, 96–108.
Bailey, J. E., & Ollis, D. F. (1986). Biochemical engineering fundamentals (Paperback) (p. 4042nd ed.). Singapore: McGraw-Hill.
Luedeking, R., & Piret, E. L. (1959). Journal of Biochemical and Microbiological Technology and Engineering, 1, 393–412.
APHA. American Public Health Association. (1989). Standard Methods for the Examination of Water and Wastewater. 17th ed (pp. 123). Washington DC.
APHA. American Public Health Association. (1989). Standard Methods for the Examination of Water and Wastewater. 17th ed (pp.151). Washington DC.
Normas Analíticas do Instituto Adolfo Lutz (1985). Métodos Químicos e Físicos para Análises de Alimentos, 1, 317–319.
Rahman, K. S. M., Rahaman, T. J., Mcclean, S., Marchant, R., & Banat, I. M. (2002). Biotechnology Progress, 18, 1277–1281.
Cooper, D. G., & Goldenberg, B. G. (1987). Applied and Environmental Microbiology, 42, 224–229.
Reis, F. A. S. L., Sérvulo, E. F. C., & De França, F. (2004). Applied and Environmental Microbiology, 899(912), 113–116.
Mercadé, M. E., Manresa, A., Robert, M., Espuny, M. J., Andrés, C., & Guinea, J. (1993). Bioresource Technology, 43, 1–6.
Benicasa, M., Contiero, J., Manresa, M. A., & Moraes, I. O. (2002). Journal of Engineering, 54, 283–288.
Rosen, M. J. (1989). Surfactants and interfacial phenomena p. 431. New York, USA: Wiley.
Banat, I., Makkar, R., & Cameotra, S. (2000). Applied Microbiology and Biotechnology, 53, 495–508.
Jeff Wu, C. F., & Hamada, M. (2000). Experiments: Planning, analysis, and parameter design optimization. New York: Wiley.
Tyagi, V. K., & Vasishtha, A. K. (1996). Journal of the American Oil Chemists Society, 73, 499–506.
Waltking, A. E., & Wessels, H. (1981). Journal of the Association Office Analytical Chemists, 64, 1329–1330.
Nawar, W. W. (1996). Food chemistry. New York: M. Dekker. 3, 225–319.
Damy, P. C., & Jorge, N. (2003). Brazilian Journal of Food Technology, 6, 251–257.
Desai, J. D., & Banat, I. M. (1997). Microbiology and Molecular Biology Reviews, 61, 47–64.
Acknowledgements
The authors wish to thank Prof. Dr. Dália dos Prazeres Rodrigues (Curator of Instituto Oswaldo Cruz, RJ, Brazil) for carrying out the bacterial strain identification. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministério da Educação, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Lima, C.J.B., Ribeiro, E.J., Sérvulo, E.F.C. et al. Biosurfactant Production by Pseudomonas aeruginosa Grown in Residual Soybean Oil. Appl Biochem Biotechnol 152, 156–168 (2009). https://doi.org/10.1007/s12010-008-8188-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8188-1