Abstract
The purified α-amylase of Geobacillus thermoleovorans had a molecular mass of 26 kDa with a pI of 5.4, and it was optimally active at 100 °C and pH 8.0. The T 1/2 of α-amylase at 100 °C increased from 3.6 to 5.6 h in the presence of cholic acid. The activation energy and temperature quotient (Q 10) of the enzyme were 84.10 kJ/mol and 1.31, respectively. The activity of the enzyme was enhanced strongly by Co2+ and Fe2+; enhanced slightly by Ba2+, Mn2+, Ni2+, and Mg2+; inhibited strongly by Sn2+, Hg2+, and Pb2+, and inhibited slightly by EDTA, phenyl methyl sulfonyl fluoride, N-ethylmaleimide, and dithiothreitol. The enzyme activity was not affected by Ca2+ and ethylene glycol-bis (β-amino ethyl ether)-N,N,N,N-tetra acetic acid. Among different additives and detergents, polyethylene glycol 8000 and Tween 20, 40, and 80 stabilized the enzyme activity, whereas Triton X-100, glycerol, glycine, dextrin, and sodium dodecyl sulfate inhibited to a varied extent. α-Amylase exhibited activity on several starch substrates and their derivatives. The K m and K cat values (soluble starch) were 1.10 mg/ml and 5.9 × 103 /min, respectively. The enzyme hydrolyzed raw starch of pearl millet (Pennisetum typhoides) efficiently.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
α-Amylase (E.C. 3.2.1.1) hydrolyses starch, glycogen, and related polysaccharides by randomly cleaving internal α-1,4-glucosidic linkages and liberates different types of oligosaccharides [1]. They are produced commercially in bulk from microorganisms, such as Bacillus and Aspergillus, and represent about 25–33% of the world enzyme market that occupies second place after proteases. Their main applications are in the production of high fructose corn syrup, detergents, and baking and ethanol industries [1–3]. Among all industrial enzymes, hydrolytic enzymes account for 85% of the total. The market size was approximately US$1.6 billions in 2002 and has witnessed ∼12% annual growth over the last decade. It is expected that the market will continue to grow fast and reach US$3 billions by 2008 [4]. The global market for starch-processing enzymes is around US$156 million and the cost of the enzymes used in the liquefaction process represented 24% of the total process cost [5]. Therefore, any improvement either in the enzyme purification/thermostability/activity will have a direct impact in the process performance, economics, and feasibility [6].
The species of Bacillus have been widely used for the commercial production of thermostable α-amylases. The most important characteristic of thermophilic organisms is their ability to produce thermostable enzymes with a higher operational stability and a longer shelf life [7]. The α-amylases presently used in starch saccharification require Ca2+ for activity and/or stability and the added Ca2+ must be removed from the product stream by using ion exchangers. The compelling need for a novel α-amylase that does not require Ca2+ has been emphasized [1]. Although the pure enzyme is not needed for industrial applications, it is indispensable for studying structure–function relationships and biochemical and kinetic properties [8].
We have recently shown an extremely thermophilic bacterium Geobacillus thermoleovorans to secrete hyperthermostable, Ca2+-independent, and maltogenic α-amylase [9–12]. In this investigation, α-amylase of G. thermoleovorans was purified to homogeneity and characterized. The concentrated enzyme was tested for its applicability in saccharification of raw pearl millet starch.
Materials and Methods
Microorganism and α-Amylase Production
Geobacillus thermoleovorans NP54 was isolated from a water sample of a hot water spring of the Waimangu Volcanic Valley (New Zealand). The culture is deposited at the Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh (India) [MTCC 4220]. The partial 16S rRNA gene (500 nucleotides) of the strain showed 99% sequence similarity with G. thermoleovorans (based on BLAST from GenBank). The partial 16 S rRNA gene sequence of strain is deposited at the GenBank (AY 330699) and the culture has been maintained as described earlier [11, 13].
The α-amylase was produced by cultivating the bacterial strain in 1,000-ml Erlenmeyer flasks containing 200 ml of glucose-yeast extract-tryptone broth (in grams per liter: glucose 20.0, yeast extract 3.0, tryptone 3.0, MgSO4·7·H2O 1.0, K2HPO4 1.0, and NaCl 1.0 at pH 7) and incubated for 12 h in an incubator shaker at 70 °C and 200 rpm. The culture broth was harvested by centrifuging at 8,000×g for 15 min at 4 °C, and the cell-free supernatant was used as the source of crude extracellular α-amylase.
α-Amylase Assay and Protein Estimation
The saccharogenic α-amylase activity was assayed by determining the reducing sugars liberated from soluble starch according to the modified method of Bernfeld [14], by incubating the reaction mixture containing 0.5 ml of 0.5% of soluble starch prepared in phosphate buffer (pH 8.0) with 0.5 ml of appropriately diluted α-amylase for 10 min at 100 °C, and quantitating the liberated reducing sugars using dinitrosalicylic acid reagent. One saccharogenic α-amylase unit is defined as the amount of enzyme required for the liberation of 1 μmol of reducing sugars as maltose per milliliter per minute under the assay conditions. The specific enzyme activity (U/mg protein) of the enzyme samples was calculated by determining the protein according to Lowry et al. [15] using bovine serum albumin as the standard.
α-Amylase Purification
The crude enzyme filtrate was concentrated by lyophilization, reconstituted in phosphate buffer (pH 8.0), followed by thermal precipitation at 100 °C and pH 8 for 2 h and storage overnight at 4 °C. After centrifugation at 10,000×g for 20 min, the denatured protein precipitate was decanted, and the supernatant was used for further enzyme purification by ion exchange chromatography. The concentrated enzyme sample was loaded on to preequilibrated swollen Q Sepharose (bead size 45–165 μm) (Sigma Chemicals Co., St. Louis, MO, USA) column in 50 mM of Tris–glycine buffer (pH 8.0), and the protein molecules were eluted with a discontinuous gradient of the buffer containing 0.1–0.5 M of NaCl. The eluted protein was collected in 1.0-l fractions, and the protein content of the fractions was determined by measuring absorbance at 280 nm and assayed for α-amylase. The fractions showing enzyme activity were pooled and concentrated by vacuum evaporation. The concentrated and dialyzed enzyme sample was applied on Sephadex G-50 (Pharmacia) column (1.5 × 50 cm, void volume 20 ml) and the eluted protein was collected at 1.0 ml at a flow rate of 1.0 ml/min.
SDS-PAGE and Molecular Mass Determination
The homogeneity of the α-amylase preparation was checked by silver staining on 10% (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) [16]. Molecular weight of the purified enzyme was determined using a SDS-PAGE and native polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide gel). The purified protein (10 μg/ ml) was run along with medium molecular weight protein markers: phosphorylase (97), albumin (66), ovalbumin (45), carbonic anhydrase (30), trypsin inhibitor (20.1), and α-lactalbumin (14.4) (obtained from Pharmacia Biotech). The activity was demonstrated by native PAGE containing 1.0% soluble starch in 10% gel.
Isoelectric Focusing
Isoelectric focusing was carried out on 7.5% PAGE in LKB 2117 multiphor II, using ampholines of 3010 (LKB Sweden). Electrofocusing was done on a prefocused pH 3–10 gradient native 7.5% PAGE (0.35 mm thick) containing 2.4% (w/v) ampholytes by conducting at 2 mA per tube. The gel was stained by Coomassie brilliant blue R-250 and the pI value was determined using Gel Works 1 D Intermediate Software (ampholytes and pI markers were procured from Pharmacia Biotech).
Effect of pH and Temperature on α-Amylase Activity
The starch solution and enzyme dilutions prepared in different buffers of pH ranging between 4 and 10 were used in the reaction mixtures and incubated at 100 °C. For assessing the effect of temperature on enzyme activity, the reaction mixtures were incubated at different temperatures [40–110 °C (oil bath was used for maintaining a temperature of 110 °C)]. The effect of temperature on the rate of reaction was expressed as temperature quotient (Q 10), which is the factor by which the reaction rate increases with rise in the temperature by 10 °C (enzyme activity at 100 °C/enzyme activity at 90 °C).
Effect of Cations, Additives, and Inhibitors on Enzyme Activity
Cations (Ca2+, Co2+, Mn2+, Mg2+, Fe2+, Cu2+, Ba2+, Zn2+, Cd2+, Pb2+, and Hg2+ either as sulfate/chloride salts) were incorporated into the reaction mixtures (1.0 and 5.0 mM) for evaluating their effect on α-amylase activity.
The effect of Fe2+ on enzyme activity was studied by introducing 10 mM of Fe2+ into enzyme sample and its subsequent removal by dialysis against EDTA (10 mM) in 0.1 M phosphate buffer (pH 8.0). Similarly the effect of EDTA on enzyme activity was also studied by introducing 10 mM of EDTA into enzyme sample and its subsequent removal by dialysis against 0.1 M of phosphate buffer (pH 8.0).
The effects of additives [polyethylene glycol (PEG) 8000, dextran, glycine, propylene glycol, and glycerol] were assessed by incorporating these (1 and 2%) into the reaction mixtures. Ionic [sodium dodecyl sulfate (SDS)], nonionic detergents (Tween 20, Tween 80, and Triton X-100), metal chelators (EDTA) (0.1 and 0.5%), ethylene glycol-bis (β-amino ethyl ether)-N,N,N,N-tetra acetic acid (EGTA) (1.0 and 5.0 mM), and inhibitors [β-mercaptoethanol, dithiothreitol, N-ethylmaleimide, and phenyl methyl sulfonyl fluoride (PMSF)] were included in the reaction mixtures.
Action of α-Amylase on Different Substrates
Soluble starch was purchased from Merck India Pvt. Ltd. potato soluble starch, corn starch, α-cyclodextrin, β-cyclodextrin, and amylopectin were procured from Sigma/Sigma-Aldrich, USA, and the flours of wheat starch, rice starch, water chestnut (Trapa bispinosa), and Pennisetum typhoides were from a local market. The efficiency of starch hydrolysis was determined as V max/K m ratio. For determining the catalytic turnover number (K cat) of purified α-amylase, the assays were carried out at various concentrations of enzyme (0.5–1.5 mg/ml) while keeping the substrate concentration (soluble starch) constant.
Thermostability, pH Stability, and Half Life of α-Amylase
Thermostability and half life of α-amylase were determined by incubating 50 ml of the suitably diluted (dilutions were prepared in phosphate buffer at pH 8.0 and at concentration of 10 U/ml) enzyme sample at 100 (with/without cholic acid, 0.03%), at 90 and 80 °C over a period of 12 h, and subsequently assayed at 100 °C at the desired intervals. The pH stability of α-amylase (10 U/ml) was determined by subjecting it to different pH values (0.1 M of acetate buffer for pH 4.0 and 5.0, 0.1 M of phosphate buffer for pH 6.0–8.0, and 0.1 M of Tris–glycine buffer for pH 9.0) over a period of 7 h, and subsequently assayed at 100 °C at the desired intervals.
Effect of Organic Solvents on Enzyme Activity
Different organic solvents were prepared in distilled water and added to the reaction mixture at 10 and 20% concentration to study their effect on enzyme activity (enzyme concentration 10 U/ml at pH 8.0).
α-Amylase Adsorption to Soluble Starch
One milliliter of enzyme sample containing 29 U/ml of pure α-amylase was incubated with the buffer containing soluble starch (100–500 mg/ml) at 80 °C for 20 min. After centrifugation, the residual α-amylase activity of the supernatant was measured and compared with the initial enzyme concentration. Adsorption rate was calculated according to Kumar and Satyanarayana [8].
Activation Energy and Temperature Quotient
Thermal activation plots were then plotted from the regression of log K vs 1/T, and the activation energy (E a) of the purified enzyme was calculated.
Shelf Life of Enzyme
The shelf life of the α-amylase was determined by storing it at room temperature and 4 °C and checking its residual activity at desired time intervals. Thermal activation was performed by incubating the enzyme at 100 °C for 2 h, followed by centrifugation and assay for α-amylase. The enzyme was also lyophilized and its activity was checked.
Starch Saccharification
The slurry of 20 and 30% (w/v) raw starch of pearl millet (P. typhoides) was prepared in phosphate buffer (0.1 M, pH 8.0), gelatinized at 105 °C for 5 min, followed by treatment with α-amylase (5 U/g) for 6 h at 100 °C. The sugars liberated were determined using anthrone reagent [17]. The hydrolysis of raw starch granules was followed under scanning electron microscopy (SEM). The percent starch saccharification was calculated according to Mishra and Maheshwari [18].
All the experiments were carried out in triplicate and their average values are presented.
Results and Discussion
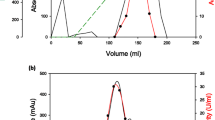
Information on the kinetics, stability, and shelf life of an enzyme is mandatory in determining its applicability in biotechnological industries [8]. Traditionally, α-amylases from various bacterial species have been purified by conventional methods employing ammonium sulfate, ethanol, and acetone precipitation [19, 20]. These methods not only require a prolonged purification but also give a lower enzyme yield. In contrast, thermal precipitation is a rapid procedure that when applied for purification of α-amylase of an extreme thermophile G. thermoleovorans, yielded 12-fold purification in a single step. The α-amylase was purified 54.8-fold from the culture filtrate (Table 1). The purified enzyme appeared homogenous on the basis of the identical protein and activity profiles after the final anionic (Fig. 1a) and gel filtration chromatography (Fig. 1b). The monomeric α-amylase had a molecular mass of 26 kDa (Fig. 2) with a pI of 5.4. The molecular mass of α-amylase of G. thermoleovorans is very close to that of Bacillus licheniformis (28 kDa) [20].
Although enzyme thermostability is an intrinsic property dictated by its primary structure, many external factors, including ions, influence the thermostability and activity. Majority of α-amylases are active and stable at slightly acidic or neutral pH. However, a few alkalophilic α-amylase-producing strains have also been reported [21, 22]. The amylase of Bacillus sp. TS-23 was optimally active at pH 9.0 [23]. The Ca2+-independent α-amylase of G. thermoleovorans was active in a broad pH range with an optimum at 8.0 (Fig. 3a), which is similar to those from Pseudomonas stutzeri and Pseudomonas saccharophilia [24]. α-Amylase of G. thermoleovorans was stable at pH 8.0 for 10 h at 100 °C as reported for that of Bacillus circulans G-6 [25]. Approximately 50% of the residual activity was recorded when the enzyme was exposed to pH 6.0 and 7.0 for 4.5 and 7.5 h, respectively (Fig. 3b).
The enzyme activity enhanced consistently with increase in temperature from 40 to 100 °C, and thereafter it declined (Fig. 4a). Arrhenius plot suggested that beyond transition point (100 °C), V max declined, indicating its inactivation beyond 100 °C (Fig. 4b). The enzyme also exhibited high thermostability. Half life of the enzyme increased from 3.6 to 5.6 h with cholic acid at 100 °C (Fig. 4c). T 1/2 values of the enzyme at 80 and 90 °C were 26.5 and 8.0 h (Fig. 4d). For α-amylase of B. circulans GRS 313, the T 1/2 values at 60 and 70 °C were 80 and 30 min, respectively [26]. An amylase from Bacillus sp. MD 124 was stable only for 60 min at 65 °C [27].
Among the cations tested, α-amylase activity was stimulated strongly by Co2+ and Fe2+, and slightly by Mn2+, Ni2+, Ba2+, and Mg2+. The enzyme activity was inhibited strongly by Sn2+, Pb2+, and Hg2+, and slightly by Zn2+ and Cu2+. Although α-amylase was stimulated by several metal ions, none of them were absolutely required for its activity and stability. Sn2+, Pb2+, and Hg2+ completely inhibited α-amylase of B. circulans GRS 313 [26, 28]. There was 100% inhibition of enzyme activity in the presence of Hg2+, indicating the presence of thiol or carboxyl groups in the enzyme molecule. α-Amylase activity was stimulated by Co2+ and Mg2+ as in B. circulans GRS 313 [26]. Cu2+ has been reported to inhibit the amylase activity of B. circulans [25], Bacillus coagulans [29] and B. licheniformis [26]. The occurrence of Ca2+-independent amylase has been reported in B. circulans [31], Bacillus acidocaldarius [30] and Bacillus brevis [31]. Ba2+ exhibited a positive effect on the enzyme activity as reported in the thermophilic fungus Thermomyces lanuginosus strain ATCC 34626 [32].
All the tested additives except PEG 8000 inhibited α-amylase activity. Among detergents, Tween 20, 40, and 80 stabilized enzyme activity (Table 2). Sodium dodecyl sulfate inhibited α-amylase activity even at lower concentration (0.1%) as observed in amylopullulanase of G. thermoleovorans NP33 [33]. The α-amylase of G. thermoleovorans was sensitive to Triton X-100. The surface active agents might have increased the turnover number of α-amylase by increasing the contact frequency between the active site of the enzyme and substrate by lowering the surface tension of reaction mixture [34]. The enzyme exhibited high tolerance to acetone compared to other organic solvents (Table 3). Thermostable enzymes are known to be resistant to organic solvents and detergents [35]. Nearly 40% inhibition of α-amylase activity of G. thermoleovorans by EDTA was recorded. In Thermus sp., EDTA inhibited α-amylase activity completely [36]. The α-amylase of G. thermoleovorans was inhibited when α-amylase was preincubated with EDTA, but the subsequent removal of EDTA by dialysis restored its activity. The enzyme activity was stimulated by Fe2+ (Table 4). When EDTA and Fe2+ were used together in the reaction mixture, there was no observable effect on the enzyme activity. These observations suggested that EDTA does not appear to act as a chelating agent but it might change conformational structure of α-amylase that led to lowering of the enzyme activity as reported in Haloferax mediterranei [37]. There was no loss in enzyme activity by EGTA, suggesting that Ca2+ is not required for α-amylase activity of G. thermoleovorans. The inhibition of α-amylase of G. thermoleovorans by PMSF suggested the importance of the seryl hydroxyl group in enzyme catalysis. Dithiothreitol inhibited the α-amylase activity, suggesting a possible role of disulfide linkages in maintaining the conformation of G. thermoleovorans α-amylase. The strong inhibition of α-amylase by N-ethylmaleimide revealed the importance of cysteine residues either for activity/binding/retaining the conformation of amylase of G. thermoleovorans. The pI value (5.4) of the α-amylase of G. thermoleovorans was close to that of Streptomyces megasporus strain SD12 [38], and almost double to that of alkaliphilic Bacillus KSM-1378 (9.0) [39]. The activation energy of the purified α-amylase of G. thermoleovorans (84.10 kJ/mol) is higher than that of B. circulans GRS 313 (7.52 kcal/mol) [26] and Mucor sp. (11.13 and 37.5 kcal/mol) [40].
The enzyme exhibited a broad substrate specificity because it acted on all the substrates tested, except the β-cyclodextrin (Table 5). The K m of α-amylase of G. thermoleovorans was lower (Table 6) than those of Aspergillus oryzae EI 212 (3.86 mg/ml) [41], Lipomyces kononenkoae (2.7 mg/ml) [42], Bacillus flavothermus (2.2 mg/ml) [43], and Bacillus thuringiensis (0.37, 14, and 4.3 mM) [44]. The low K m value of α-amylase of G. thermoleovorans is similar to that of B. coagulans [29]. Kinetic linearity was determined by plotting liberation of reducing sugars vs time (results are not shown). A linear response over time was observed for the first 10 min, after which it leveled off as observed in B. coagulans [29]. The α-amylase activity of G. thermoleovorans was observed to be linear in the range between 0.1 and 1.5 mg/ml protein. The K cat of the enzyme, as computed from the kinetic linearity experiment, was 5.9 × 103/min.
When 20 and 30% raw pearl millet starch were treated with α-amylase for 3 h; the sugar yields were 68 and 55.8%, respectively. On extending treatment with α-amylase for 6 h, the sugar yields were 72 and 63%, respectively. These values are higher than those reported by other workers [18]. Scanning electron micrographs of raw pearl millet starch incubated with the enzyme revealed hydrolysis of starch grains (Fig. 5).
The enzyme (75.8%) was adsorbed to soluble-starch. Kanlayakrit et al. [16] and Kumar and Satyanarayana [8] have also reported similar observations for glucoamylases of Rhizomucor pusillus and Thermomucor indicae-seudaticae, respectively. The enzyme retained 100% activity for 6 months at 4 °C and at room temperature. There was no loss of activity when the enzyme was lyophilized as reported for pancreatic α-amylase [45]. The raw starch hydrolyzing ability, its activity on several starch substrates, high thermostability, independent of Ca2+ for its activity/stability, and broader pH range make the α-amylase of G. thermoleovorans a good candidate for industrial application in starch saccharification.
References
Antranikian, G. (1992). In G. Winkelmann (Ed.), Microbial Degredation of Natural Products (pp. 27–56). Germany: Weinheim, VCH.
Vihinen, M., & Mantsala, P. (1989). Critical Reviews in Biochemistry and Molecular Biology, 24, 329–418.
Jensen, B., & Olsen, J. (1999). In B. N. Johri, T. Satyanarayana & J. Olsen, (Eds.), Thermophilic Moulds in Biotechnology (pp. 115–137). Netherlands: Kluwer Academic Publishers.
Pandey, A., & Ramachandran, S. (2005). In A. Pandey, C. Webb, C. R. Soccol, & C. Larroche (Eds.), Enzyme Technology (pp. 1–10). New Delhi: Asiatech Publishers Inc.
Crabb, W. D., & Mitchinson, C. (1997). Trends in Biotechnology, 15, 349–352.
Rivera, M. H., Lopez-Munguia, A., Soberon, X., & Saab-Rincon, G. (2003). Protein Engineering, 16, 505–514.
Niehaus, F., Bertolldo, C., Kahler, M., & Antranikian, G. (1999). Applied Microbiology and Biotechnology, 51, 711–729.
Kumar, S., & Satyanarayana, T. (2003). Biotechnology Progress, 19, 936–944.
Malhotra, R., Noorwez, S. M., & Satyanarayana, T. (2000). Letters in Applied Microbiology, 31, 378–384.
Uma Maheswar Rao, J. L., & Satyanarayana, T. (2003). Letters in Applied Microbiology, 36, 191–196.
Uma Maheswar Rao, J. L., & Satyanarayana, T. (2003). Journal of Applied Microbiology, 95, 712–718.
Uma Maheswar Rao, J. L., & Satyanarayana, T. (2007). Bioresource Technology, 98, 345–352.
Narang, S., & Satyanarayana, T. (2001). Letters in Applied Microbiology, 32, 31–35.
Bernfield, P. (1955). Methods in Enzymology, 1, 149–158.
Lowry, O. W., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Kanlayakrit, W., Ishimatsu, K., Nakkao, M., & Vayashida, S. (1987). Journal of Fermentation Technology, 65, 370–385.
Brink, R. H., Dubach, P., & Lynch, D. L. (1960). Soil Science, 89, 157–166.
Mishra, R., & Maheshwari, R. (1995). Journal of Biosciences, 21, 653–672.
Bose, K., & Das, D. (1996). Indian Journal of Experimental Biology, 34, 1279–1282.
Krishnan, T., & Chandra, A. K. (1983). Applied and Environmental Microbiology, 46, 430–437.
Yamamoto, M., Tanaka, Y., & Horikoshi, K. (1972). Agricultural and Biological Chemistry, 36, 1819–1823.
Fogarty, W. M., & Kelly, C. T. (1979). Progress in Industrial Microbiology, 15, 87–150.
Lin, L. L., Chyau, C. C., & Hsu, W. H. (1998). Biotechnology and Applied Biochemistry, 28, 61–68.
Robyt, J. F., & Ackerman, R. J. (1971). Archives of Biochemistry and Biophysics, 145, 105–114.
Takasaki, Y. (1982). Agricultural and Biological Chemistry, 46, 1539–1547.
Dey, G., Palit, S., Banerjee, R., & Maiti, B. R. (2002). Journal of Industrial Microbiology & Biotechnology, 28, 193–200.
Jana, M., Chattopadhyay, D. J., & Pati, B. R. (1997). Acta Microbiologica et Immunologica Hungarica, 44, 281–289.
Mamo, G., & Gessesse, A. (1999). Enzyme and Microbial Technology, 25, 433–438.
Babu, K. R. (1994). Ph.D. thesis, University of Delhi, Delhi, India.
Kanno, M. A. (1986). Agricultural and Biological Chemistry, 50, 23–31.
Tsvetkov, V. T., & Emanuilova, E. I. (1989). Applied Microbiology and Biotechnology, 31, 246–248.
Quang, D. N., Rezessy-Szabo, J. M., Claeyssens, M., Stals, I., & Hoschke, A. (2002). Enzyme and Microbial Technology, 31, 345–352.
Noorwez, S. M. (2000). Ph.D. thesis, University of Delhi, Delhi, India.
Kapoor, M., Beg, Q. K., Bhushan, B., Dadhich, K. S., & Hoondal, G. S. (2000). Process Biochemistry, 36, 467–473.
Klibanov, A. M. (2001). Nature, 409, 241–246.
Shaw, J. F., Lin, F. P., Chen, S. C., & Chen, H. C. (2003). Botanica Bulletin of Academia Sinica, 36, 195–200.
Perez-Pomares, F., Bautista, V., Ferrer, J., Pir, C., Marhuendra-Egea, F. C., & Bonete, M. J. (2003). Extremophiles: Life Under Extreme Conditions, 7, 299–306.
Dey, S., & Agarwal, S. O. (1999). Indian Journal of Biochemistry & Biophysics, 36, 150–157.
Igarashi, K., Hatada, Y., Hagihara, H., Saeki, K., Takaiwak, M., Uemura, T., et al. (1998). Applied and Environmental Microbiology, 64, 3282–3289.
Mohapatra, B. R., Banerjee, U. C., & Bapuji, M. (1998). Journal of Biotechnology, 60, 113–117.
Kundu, A. K., & Das, S. (1970). Applied Microbiology, 19, 598–603.
Eksteen, J. M., Steyn, A. J. C., Rensburg, P. V., & Otero, R. R. C. (2003). Yeast, 20, 69–78.
Bolton, D. J., Kelly, C. T., & Fogarty, W. M. (1997). Enzyme and Microbial Technology, 20, 340–343.
Rowe, G. E., & Margaritis, A. (2004). Biochemical Engineering Journal, 17, 121–128.
Gubern, G., Canalias, F., Gella, F. J., Colinet, E., Profilis, C., Calam, H., et al. (1996). Clinica Chimica Acta, 252, 145–162.
Acknowledgements
We wish to thank Mr. Pathania and Mr. Girish of All India Institute of Medical Sciences, New Delhi, India, for providing technical assistance in SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uma Maheswar Rao, J.L., Satyanarayana, T. Purification and Characterization of a Hyperthermostable and High Maltogenic α-Amylase of an Extreme Thermophile Geobacillus thermoleovorans . Appl Biochem Biotechnol 142, 179–193 (2007). https://doi.org/10.1007/s12010-007-0017-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-0017-4