Abstract
Background
Cryotherapy has been used to enhance recovery after orthopaedic surgery. Several cooling devices are available but few can guarantee a fixed temperature during a prolonged time and therefore have been criticized. The arrival of new advanced cryotherapy devices made it possible to test the effect of prolonged cooling on rehabilitation after joint replacement.
Questions/purposes
The hypotheses of this randomized controlled trial (RCT) were that advanced cryotherapy devices compared with cold packs result in (1) better postoperative pain control resulting in a lower consumption of narcotics; (2) better early ROM; and (3) less postoperative bleeding and swelling.
Methods
A priori sample size calculation had determined that to detect a difference of 2 points on the VAS, a sample size of 50 subjects per group at followup would be required, given a study power of 80%. One hundred sixteen patients were included and randomly allocated to receive advanced cryotherapy (n = 58) or use of cold packs (n = 58). The primary outcomes for the study were to evaluate pain with the VAS and analgesics consumption. Secondary outcomes were postoperative ROM, swelling, and blood loss. One hundred (50 in each group) patients had complete data available for analysis.
Results
No statistically significant differences in VAS, need for analgesics, nor in secondary outcomes were observed, except for substantially reduced flexion at 6 weeks in the advanced cryotherapy group (114° versus 120°).
Conclusions
Advanced cryotherapy with a continuous temperature for a prolonged period does not deliver expected results of superior early recovery after knee arthroplasty. Greater sample sizes are required to fully determine significant differences between the two techniques for these study parameters. Immobilization of the knee in extension during the prolonged cryotherapy session resulted in lower active flexion at 6 weeks after surgery for the advanced cryotherapy group. Advanced cryotherapy should not be used in fast track knee arthroplasty if the economic cost is higher than the price of cold packs or offers no other concomitant advantages.
Level of Evidence
Level II, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A total knee arthroplasty (TKA) is a major orthopaedic intervention accompanied by tissue damage, inflammatory responses, and pain [2, 8, 11]. Secondary events accompanying a TKA are local swelling and edema, reduced ROM and stiffness, reduced quadriceps strength, and finally sometimes as much as 1.5 L blood loss, with transfusion in 10% to 15% of patients [2, 13, 23]. Despite multimodal pain management and advances in anesthetic techniques, a TKA remains difficult for most patients. Cryotherapy has been appealing in this multimodal concept because it has minimal disadvantages compared with the possible benefits [11, 15].
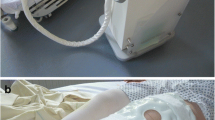
Cryotherapy involves the application of cold to the skin surrounding the injured soft tissues and in joint surgery is supposed to reduce the intraarticular temperature, especially in the knee, which is a superficial joint [18]. It will reduce the local blood flow by vasoconstriction and therefore the local inflammatory reaction, swelling, and heat experience and also will slow the conduction of nerve signals potentially reducing pain transmission [3, 16]. Several cryotherapy options are available including first-generation cold therapy like crushed ice in a plastic bag, cold or gel packs; second-generation cold therapy with circulating ice water with or without compression like a Cryo/Cuff (Aircast, Vista, CA, USA) or GameReady (CoolSystems Inc, Concord, CA, USA); and third-generation advanced computer-assisted devices with continuous controlled cold therapy (cTreatment, Waegener, Beerse, Belgium; and CTM 5000, Ener-C AG, Baar, Switzerland). The advantage of these latter devices would be controlled-temperature modulation with cooling at a specific and continuous temperature (11° C) for a prolonged time. In addition to guaranteeing a steady temperature, these devices also allow a progressive increase of the temperature during the course of treatment to avoid cold-induced vasodilatation, and they are less labor-intensive because there is no need to fill them with cold water or ice [22].
Cryotherapy is the standard of care in some countries and rarely is used in others [4]. Conflicting evidence regarding the value of this treatment from randomized trials may contribute to that practice disparity [2]. Because of this disparity, third-generation cryotherapy providers stated that the lack of clinical evidence was linked to an inappropriate cooling technique and were proposing a value add-in exchange for an important financial cost. One company (www.waegener.com) stated, in their commercial communications, that the devices provided less pain, less swelling, and shorter length of stay without any scientific peer-reviewed data to back up those statements, to the best of our knowledge.
The hypotheses of this randomized controlled trial (RCT) were that advanced cryotherapy devices compared with first-generation cryotherapy (cold packs) result in (1) better postoperative pain control with lower consumption of narcotics; (2) better early ROM; and (3) less postoperative bleeding and swelling.
Patients and Methods
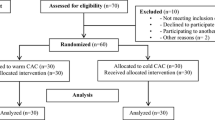
An RCT was performed on patients undergoing primary knee arthroplasty in a university center with a single-surgeon setting to study superiority of an advanced cryotherapy device over cold packs. At least four primary knee arthroplasties a day were performed in one room and patients were equally allocated to both groups. Patients were quasirandomized in blocks per day by the typical order of first case of the day advanced cryotherapy, second case cold packs, third case advanced cryotherapy, and fourth case cold packs again. In what could be considered centralized assignment, the operating room management decided the surgical order for each day and therefore also the randomization of patients. The inclusion criterion was primary knee arthroplasty for osteoarthritis from January 2012 until October 2012 without previous surgery except open meniscectomy. Exclusion criteria were inflammatory disease, infection, neurologic problems, coagulopathy, preoperative anticoagulation, or history of deep vein thrombosis or pulmonary emboli requiring a higher dose of postoperative anticoagulation. During this period, 160 consecutive patients were eligible for enrollment (Fig. 1). One hundred sixteen patients were included in the study on an intent to treat protocol and allocated to one of the two study groups (n = 58). A per-protocol analysis was conducted, including only patients with complete followup (n = 50 in each group). Based on previously performed randomized cryotherapy studies, a Type I error of 5%, and a Type II error of 20% (80% power), a sample size of 50 patients at followup, given an anticipated dropout rate of 10%, was supposed to provide enough statistical power based on a difference in VAS of two points according to previous studies [3, 9, 16, 25].
The staff of the university hospital had greater than 1 year experience with advanced cryotherapy devices and fast track programs before starting the study. All patients consented to participation in the study which was approved by the Ethical Committee of the Saint Luc University Hospital in Brussels, Belgium. The surgeon, physiotherapist, and patients completed the study protocol. Patients undergoing one type of treatment were blinded for the other possible treatment arm but not for their own treatment. The physiotherapist (VB) evaluating functional outcome could not be blinded for organizational reasons. The data were blinded for type of treatment and then put in an Excel file (Microsoft Inc, Redmond, WA, USA) by a resident (BW) not previously involved in the study.
Demographics of the study population did not show any statistical difference between both groups (Table 1). There were 92 varus knees with a hip-knee-ankle (HKA) angle less than 180° and eight valgus knees with a hip-knee-ankle angle greater than 180° (range, 171°–189°).
Surgical Technique and Rehabilitation
All patients underwent minimally invasive knee arthroplasty with a subvastus approach and received a cemented implant while under general anesthesia. A tourniquet at 100 mm Hg above systolic blood pressure was used for a mean of 55 minutes (SD, 9 minutes) until implantation of all components followed by meticulous hemostasis before closure. Preventive multimodal pain treatment with 1 g acetaminophen, 100 mg celecoxib, and local infiltration analgesia with adrenaline were combined to control pain. No lateral releases were performed. No drains were used in either group. Postoperative multimodal pain control was used with four doses of 1 g acetaminophen, two doses of 100 mg celecoxib, and morphine (first 48 hours) or tramadol (after 48 hours) for breakthrough pain. The same rehabilitation program was used for all patients, with full weightbearing without aid the day after surgery and active ROM exercises. No continuous passive motion was used. Enoxoparin was used for all patients for 10 days after surgery. All patients received an identical Robert Jones compressive dressing [9] for 12 hours after surgery that was kept thin to allow cTreatment or cold packs to be applied and penetrate to the skin immediately after surgery.
cTreatment Protocol and Control Group Treatment Protocols
In the cTreatment group, patients received 4 hours of continuous cooling at 11° C (range, 6–15° C) immediately after surgery (starting in the recovery room and continued in their room). The day after surgery the protocol consisted of 2 hours of treatment followed by their standard physiotherapy followed by 2 hours of cTreatment. In the afternoon, this schedule was repeated. During the evening and night, patients were allowed to use the cTreatment because they considered it useful for comfort and pain control and 47 of 50 used it continuously during the night.
In the control group, patients received 15 minutes of cold pack (conserved at −17° C) treatment, with two cold packs anterior and one posterior to the knee, on arrival to the recovery room and again on arrival to the ward. This would be repeated 2 hours and 4 hours after surgery. The following days patients received the same cold pack cryotherapy 15 minutes after their physiotherapy session (11 AM and 3 PM) and during the evening and night whenever they considered it useful for comfort and pain control. No compression was applied to the cold packs.
Fifty patients were available in both groups for analysis after loss to followup of 15 patients and one exclusion from the advanced cryotherapy group. The minimal clinical followup was 6 weeks for data collection and 3 months after surgery for adverse events (Fig. 1).
Prespecified Outcomes Measured
The primary outcome measured in this study was postoperative pain as evaluated by the VAS at rest, during deep active knee flexion, and walking without aid at the same time of the day, and analgesic use measured as morphine and tramadol consumption as equianalgesic amounts. The data were collected on a study protocol by the physiotherapist (VB) and nurses.
Secondary outcomes were knee ROM (measured as degrees of active knee flexion and extension evaluated with the use of a handheld goniometer, at different stages of hospital stay), active straight leg raising, walking without aid, swelling (measured as circumference in millimeters at two fixed points of the knee at the same time of day at different stages postoperatively, 10 cm superior to the patella and 10 cm inferior to the patella and then the average was divided by two and expressed in millimeters), visual hematoma (yes/no), and length of stay (in days). Postoperative blood loss (measured as hemoglobin decrease at Day 4 to include visible and hidden blood loss), transfusion rate (percent of patients and amount of transfused units with a transfusion trigger of 8 g/dL), and reported adverse events were noted on the prepared study document. All these clinical parameters were collected again by the same experienced physiotherapist (VB) at fixed times during the day.
Statistical Analysis
Sample characteristics are presented as numbers, means, SDs, and ranges. The normal distribution of the data was assessed using the Kolmogorov-Smirnov test. The nonnormally distributed data were analyzed using the nonparametric statistical Mann-Whitney test for independent samples and Wilcoxon signed rank test for dependent samples. Comparison of observed proportions was performed using Fisher’s exact test. Statistical analysis was conducted using SPSS 18 Statistical Software (SPSS Inc, Chicago, IL, USA) and significance was set at p less than 0.05.
Results
No differences were observed between the treatment and control groups in terms of VAS pain scores (range, 0 = no pain to 10 = worst pain) at rest, movement, and walking. No differences in VAS at Day 2 (worst phase of postoperative pain and inflammation) or equianalgesic doses of morphine and tramadol were observed (Table 2).
No differences in functional results up to 6 weeks including ROM, straight leg raising, walking without aid, swelling, and hematoma were found between both groups (Table 3) except for active flexion at 6 weeks after surgery, for which the control group outperformed the treatment group (120° versus 114°; p = 0.0235). No differences in length of stay were observed. No differences in blood loss and inflammatory tests were found (Table 4). All patients undergoing any type of cryotherapy expressed their subjective feeling of pain reduction during the treatment. In the cTreatment group, 30% of patients reported too much noise to be able to sleep while continuing their treatment overnight. Three patients reported a generalized cold feeling and one discontinued treatment for that reason.
Discussion
Cryotherapy, if effective, could play a major role in fast track rehabilitation after knee arthroplasty by its reduction of inflammation, pain, and swelling. Advanced cryotherapy devices are supposed to be even more efficient because they guarantee a steady low temperature during a fixed time. In this RCT, the superiority of advanced cryotherapy devices versus cold packs for enhanced recovery after knee arthroplasty was studied.
The weakness of this study is that neither the skin temperature nor the intraarticular temperature was measured to confirm effective cooling [18]. However, the cTreatment device contains a microchip that checks the cPad (Waegener, Beerse, Belgium) temperature continuously and therefore guarantees a controlled temperature. This was never intended to be an invasive study to measure intraarticular temperature changes, but to observe the clinical outcomes of advanced cryotherapy devices compared with classic cold packs. A second weakness is that the advanced cryotherapy devices were used only during hospitalization and not after discharge. Su et al. [24] reported the potential advantage of home use of a cryopneumatic device. In the current study the length of stay was 5 days and cooling could have been beneficiary after leaving the hospital, however home treatment with an advanced cryotherapy device was not possible in the actual study setting.
A final weakness is that our study was performed in a highly specialized arthroplasty section and because of the advanced anesthesia and surgical technique, local infiltration analgesia, and preventive multimodal pain management [15], a Type II error could exist and subtle differences in outcome would be unnoticed. Cooling reduces the tissue metabolic rate and relieves inflammation by suppressing enzymatic activity and prevention of secondary tissue damage and reduces muscle tissue spasms. The less invasive subvastus approach might have interfered here with the need for cryotherapy [16, 22]. A post hoc power analysis showed that with the subtle VAS difference of 0.5 points at Day 2 between both groups the required sample size would have been 363 subjects in each group to show a difference based on a two-sided statistical test. However, looking at the important financial cost of cTreatment compared with cold packs, the effect size should be high enough to obtain results in all types of surgical conditions.
The strength of this study was that one surgeon, using the same surgical approach, same implants, and same anesthesia and multimodal pain protocol, performed all surgeries. The cTreatment device was already in use for more than a year in the ward leading to paramedical expertise. An important criticism of cryotherapy has been that different temperatures, frequencies, and treatment protocols were compared [1, 2, 20]. Cryopneumatic therapy was compared with no cooling [19, 21, 22], with cold packs [12, 24], with compression [9], and with epidural analgesia [14, 16]. In this study we compared an advanced cryotherapy device with cold packs. An important factor of adding an advanced cryotherapy device to a multimodal pain protocol would be to reduce the consumption of morphine and the unpleasant side effects of this drug like nausea, vomiting, constipation, and morphine-induced hyperalgesia [10, 24]. Su et al. [24] observed lower morphine consumption in their multicenter RCT (509 mg versus 680 mg) up to 2 weeks postoperatively for a cryopneumatic device. Adie et al. [1, 2] found in their systematic review and meta-analysis of 11 prospective RCTs on cryotherapy, no benefits for pain, analgesia use, transfusion, swelling, or length of stay and only small benefits for blood loss and early ROM. No difference in VAS or morphine consumption was observed in the current RCT. A decreased nerve conduction velocity resulting in better pain tolerance can explain the local anesthetic effect of intense cooling [3]. However, this is merely a temporary measure that disappears after ice removal and moreover the nociceptive damage and the peripheral sensitization have already occurred at a distance from the cooled area [11], potentially explaining why no difference in analgesic consumption was observed.
Su et al. [24] did not observe any difference for ROM, swelling, or functional testing between a cryopneumatic device (GameReady) and ice packs. This also was observed by Holmström and Härdin [14] for the CryoCuff and by Adie et al. [2] in their systematic review. However Morsi [19] observed better outcomes in two-stage bilateral TKAs. In the current study lower knee flexion at 6 weeks postoperatively was observed that could be explained by the many hours patients remained in extension with the cryotherapy pad the first days after surgery.
Rice et al. [21] observed better quadriceps muscle control after 20 minutes of icing of the knee in healthy subjects (n = 16), but this was not confirmed in a clinical study by Holm et al. [13] after TKA. In the current study, quadriceps function was clinically evaluated by straight leg raising and walking without aid, but no differences were observed between both study groups.
Gibbons et al. [9] found no difference between cooled compression (Cryo/Cuff) and compression only (Robert Jones bandage) for pain scores, analgesia, range of movement, or complications. However, the length of stay for patients who received the Cryo/Cuff was 2 days longer. They found an advantage only for the Cryo/Cuff for visible blood loss. The same was observed by others [17, 25], but contradictory findings have been published [12, 14]. In the current study, no drains were used and total blood loss was measured as a decrease in hemoglobin level at Day 4 postoperatively to include the hidden blood loss. No differences in decrease in hemoglobin as a substitute for blood loss were observed in either group. A reduction of allogeneic blood transfusion would be an important factor to make advanced cryotherapy cost effective. In this study no transfusion was necessary in either group as was observed by Gibbons et al. [9]. Cryotherapy could have an immediate reduction of bleeding by vasoconstriction and compression but later lead to cold-induced vasodilatation and interfere with secondary hemostasis. There is also some evidence that application of ice locally impairs hemostasis [7]. This has been seen in humans and in animal models showing prolonged bleeding time, decreased platelet aggregation, increased clotting time, and increased clot formation time [7]. Hemostasis and coagulation are biochemical reactions that are most efficient at basal body temperature. In the current study no difference in blood loss was seen, possibly because tranexamic acid and local infiltration techniques with adrenaline were used [10, 26]. No difference in hematomas was observed.
Kullenberg et al. [16] and Holmström and Härdin [14] reported that cold compression was more efficient than epidural analgesia with better ROM, higher hemoglobin levels, and shorter hospital stay. Hospital stay was similar for both groups in the current study.
The skin is not the target tissue during cryotherapy but is most affected by it. Frostbite typically begins to occur at temperatures of approximately 10° C, with altered cutaneous sensation and decreased tissue oxygenation because of vasoconstriction. Prolonged cooling at less than 5° C can be dangerous. Necrosis occurs because of additional vasoconstriction, thrombosis, shunting of blood flow, and devitalization of the skin flap because of surgery. In a cryotherapy protocol at least 20 minutes of cessation should be permitted between 2-hour cryotherapy sessions [6].
Frostbite after cryotherapy can be a serious issue leading to major soft tissue problems, often necessitating additional surgery, with potential contamination of the implant. If the capsule is not affected and these wounds are impossible to close, local fasciocutaneous flaps or skin grafting often are necessary, which can lead to reduced mobility of the knee [6]. No serious adverse effects were found in either group; however, precautions were taken to avoid frostbite [5]. In the cTreatment group, 30% of patients reported too much noise to be able to sleep while continuing their treatment overnight. Three patients reported a generalized cold feeling and one discontinued treatment for that reason.
The key findings of this RCT were that advanced cryotherapy devices, representing prolonged continuous cooling, do not offer any clinical advantage for patients undergoing knee arthroplasty in a fast track setting compared with cold packs despite a substantially higher cost. The price for a cTreatment is USD 520. Because none of the stated goals appeared to have been achieved with advanced cryotherapy, the increased costs associated with this intervention cannot be substantiated.
References
Adie S, Kwan A, Naylor JM, Harris IA, Mittal R. Cryotherapy following total knee replacement. Cochrane Database Syst Rev. 2012;9:CD007911.
Adie S, Naylor JM, Harris IA. Cryotherapy after total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2010;25:709–715.
Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med. 2007;41:365–369; discussion 369.
Barry S, Wallace L, Lamb S. Cryotherapy after total knee replacement: a survey of current practice. Physiother Res Int. 2003;8:111–120.
Brown WC, Hahn DB. Frostbite of the feet after cryotherapy: a report of two cases. J Foot Ankle Surg. 2009;48:577–580.
Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28:376.e5–7.
Forsyth AL, Zourikian N, Valentino LA, Rivard GE. The effect of cooling on coagulation and haemostasis: should ‘Ice’ be part of treatment of acute haemarthrosis in haemophilia? Haemophilia. 2012;18:843–850.
Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–944.
Gibbons CE, Solan MC, Ricketts DM, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001;25:250–252.
Gibbs DM, Green TP, Esler CN. The local infiltration of analgesia following total knee replacement: a review of current literature. J Bone Joint Surg Br. 2012;94:1154–1159.
Grosu I, Lavand’homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2013 Nov 8 [Epub ahead of print].
Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143–146.
Holm B, Husted H, Kehlet H, Bandholm T. Effect of knee joint icing on knee extension strength and knee pain early after total knee arthroplasty: a randomized cross-over study. Clin Rehabil. 2012;26:716–723.
Holmström A, Härdin BC. Cryo/Cuff compared to epidural anesthesia after knee unicompartmental arthroplasty: a prospective, randomized and controlled study of 60 patients with a 6-week follow-up. J Arthroplasty. 2005;20:316–321.
Kehlet H, Thienpont E. Fast-track knee arthroplasty: status and future challenges. Knee. 2013;20(suppl 1):S29–33.
Kullenberg B, Ylipaa S, Soderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21:1175–1179.
Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174–178.
Martin SS, Spindler KP, Tarter JW, Detwiler KB. Does cryotherapy affect intraarticular temperature after knee arthroscopy? Clin Orthop Relat Res. 2002;400:184–189.
Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17:718–722.
Radkowski CA, Pietrobon R, Vail TP, Nunley JA 2nd, Jain NB, Easley ME. Cryotherapy temperature differences after total knee arthroplasty: a prospective randomized trial. J Surg Orthop Adv. 2007;16:67–72.
Rice D, McNair PJ, Dalbeth N. Effects of cryotherapy on arthrogenic muscle inhibition using an experimental model of knee swelling. Arthritis Rheum. 2009;61:78–83.
Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19:334–337.
Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty: correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86:561–565.
Su EP, Perna M, Boettner F, Mayman DJ, Gerlinger T, Barsoum W, Randolph J, Lee G. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 suppl A):153–156.
Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopedics. 1998;21:59–61.
Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on blood loss and transfusion rate in primary total knee arthroplasty. J Arthroplasty. 2013;28:1080–1083.
Acknowledgments
We thank Vanessa Briart PT, from Saint Luc University hospital for prospective functional outcome data collection, Bertin Wasamba MD, from Saint Luc University hospital for Excel file data collection, Pat Viroux PT and Hans Van Buynder PT from Waegener (Beerse, Belgium) for advice regarding cryotherapy and the study protocol, and Levi Dewaegenaere CEO, of Waegener (Beerse, Belgium) for the free cPad delivery.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Thienpont, E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty?. Clin Orthop Relat Res 472, 3417–3423 (2014). https://doi.org/10.1007/s11999-014-3810-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-014-3810-8