Abstract
Background
Larger femoral heads are commonly presumed to improve joint stability and hip biomechanics; some studies have suggested they may hasten recovery of a normal gait. To our knowledge, no gait analysis studies have compared different size head diameters in THA.
Questions/purposes
We compared (1) spatiotemporal gait parameters, (2) kinematic and kinetic gait parameters, and (3) Harris hip scores in patients undergoing THA randomized to receive a 28-, 36-, or ≥ 42-mm bearing couple. We hypothesized a larger femoral head would restore an earlier, more physiologic gait pattern.
Methods
This randomized, blinded study involved 60 patients who received the same cementless THA except for the size of the bearing. Inclusion criteria were primary hip arthritis, female sex, and age between 55 and 70 years. Exclusion criteria were other problems influencing walking ability. The patients were randomized into three groups of 20 each (28- and 36-mm ceramic-on-crosslinked polyethylene, ≥ 42-mm metal-on-metal). All patients underwent the same postoperative rehabilitation protocol. Gait evaluation using an optoelectronic system was performed preoperatively and at 2 and 4 months postoperatively.
Results
With the numbers available, no differences in spatiotemporal gait parameters, kinematic or kinetic gait parameters, or Harris hip scores emerged among the three groups. All variables assessed at 4 months postoperatively showed improvements across all groups, but the differences among them were not significant.
Conclusions
The hypothesis that a larger femoral head results in improved early gait performance was not supported by this study.
Level of Evidence
Level I, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
THA is a cost-effective operation that reduces pain and improves functionality and quality of life in patients with hip arthritis. Prostheses with a smaller head diameter (22–28 mm) were used in the past to reduce frictional torque and wear [2]. With the advent of new materials designed to reduce wear (crosslinked polyethylene) [8, 13, 21] and hard bearings to reduce wear and frictional torque (ceramic-on-ceramic, metal-on-metal) [11, 17, 18, 22], the number of THAs with larger diameter heads has increased in recent years. Larger heads may offer distinct advantages, including reduced risk of dislocation and component impingement, as well as increased ROM in some cases [1, 6, 10, 15, 20]. Furthermore, larger femoral heads have been theorized to improve joint biomechanics and perhaps better approximate a more normal-feeling hip [10].
Several studies using gait analysis to compare functional outcome after conventional THA and resurfacing hip arthroplasty (RHA) [7, 14, 16, 19] have suggested gait pattern after surgery may be altered, with a reduction in mean gait velocity [14], step length, stance phase duration [12, 16], hip abduction moment [14, 16], and hip flexion moment [16], in addition to abnormal loading as evinced by ground reaction force (GRF) patterns [12]. In a recent study in patients allocated to receive RHA or large-head THA, Lavigne et al. [7] reported, while both groups demonstrated fast recovery and normalization of most gait parameters at 3 to 6 months, there were no differences in clinical and gait parameters between the two groups. To our knowledge, no studies have, to date, compared by means of gait analysis the same prosthesis in which the only difference is femoral head diameter.
In the present study, we used gait analysis to compare (1) spatiotemporal gait parameters, (2) kinematic and kinetic gait parameters, and (3) Harris hip scores in patients undergoing THA randomized to receive a 28-, 36- or ≥ 42-mm femoral head. We hypothesized a larger femoral head would restore an earlier, more physiologic gait pattern.
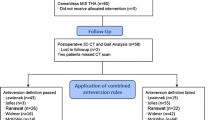
Patients and Methods
The local ethical committee approved the study design and protocol. We performed a prospective, randomized, blinded study in 60 female patients who underwent THA with three different femoral head diameters at our institute between February 2007 and December 2009. The three surgeons involved in the study implanted the three different head diameters according to a computer-generated randomization sequence. The patients, the evaluators at the gait laboratory, and the physician (LB) who performed the clinical evaluations were all blinded to the size of the head implanted. We included in the study female patients between the ages of 55 and 70 years, patients with unilateral primary hip osteoarthritis, and patients with a trumpet-shaped femoral canal suitable for the same cementless tapered femoral stem. We excluded patients whose underlying diagnoses were developmental dysplasia of the hip, Legg-Calvé-Perthes disease, or other pathologies altering hip anatomy; patients with disorders that affect ambulation; an American Society of Anesthesiology (ASA) score of greater than 2; previous lower limb orthopaedic surgery; and leg length discrepancy of greater than 1 cm. The 60 patients we enrolled represented 11% of all THAs during the period of study and 18% of our cementless THAs. The patients were randomly allocated into three study groups of 20 patients each according to prosthesis head size: 28 mm (G1), 36 mm (G2), and ≥ 42 mm (range, 42–48 mm) (G3). Four patients were lost to followup as they were unavailable for gait analysis. We excluded one patient who developed postoperative heterotopic ossification, with loss of hip motion requiring bone removal. No other major complications occurred. We analyzed only the remaining 55 patients who completed the study protocol (19 patients in G1; 17 patients in G2; 19 patients in G3) (Table 1). The three groups were similar for age and BMI.
All patients received a cementless acetabular cup Delta-PF (Lima Corp, San Daniele del Friuli, Italy) and a cementless stem Versys® ET (Zimmer, Inc, Warsaw, IN, USA). The bearing couple for the smaller head sizes (28 and 36 mm) was ceramic on crosslinked polyethylene, while metal-on-metal couplings were employed in the ≥ 42-mm group. The same surgical team, composed of three surgeons from the same department (LZ, RGC, CP), performed the surgeries through a posterolateral approach with transosseus repair in two layers of the posterior capsule and the external rotator muscles.
All patients underwent the same postoperative rehabilitation protocol with the same rehabilitation team.
A preoperative (PRE), a 2-month (POST 2), and a 4-month (POST 4) postoperative gait and clinical analysis were required as followup in the study.
All patients were clinically evaluated pre- and postoperatively. One observer who did not participate in the surgery (LB) examined all patients. Hip function was assessed according to the Harris hip score [5]. Pre- and postoperative ROM (flexion/extension, adduction/abduction, internal/external rotation) was measured with the patient in the supine position, using a goniometer according to the American Academy of Orthopaedic Surgeons guidelines [4].
Computerized gait assessment was performed using an optoelectronic system with passive markers (EliteClinic; BTS Bioengineering, Milan, Italy). The EliteClinic system allows for computerized three-dimensional recording of motion captured by six cameras at a sample rate of 100 Hz. The kinetic variable was measured using a dynamometric force platform (Kistler, GmbH, Winterthur, Switzerland). Passive markers were placed on anatomic landmarks on the patient’s body as described by Davis et al. [3]. Patients were instructed to walk barefoot several times at natural speed.
For each patient, the data of at least six trials per leg were collected from which the data from three trials per leg were extracted to define the same gait pattern (for kinematics and kinetics analysis) with the same gait speed. These trial data sets were considered for analysis of the following parameters. Spatiotemporal parameters included mean gait velocity (meters per second), cadence (steps per minute), duration of stance phase (as % of gait cycle), and stride length (meters). Kinematic parameters included hip ROM in three planes of motion, maximum flexion and extension, maximum adduction and abduction, and maximum internal and external rotation. Kinetic parameters included the peaks of flexion/extension, adduction/abduction, and intra/extrarotation moment (normalized to the patient’s weight). The first and second peaks and the minimum value between the two peaks of the GRF (normalized to the patient’s weight) were calculated to investigate the patient’s ability during the gait cycle. The parameters were computed for each patient and the mean values were then calculated for the three groups in the pre- and postoperative analyses. The gait data were compared against a normative database, maintained at our Motion Analysis Laboratory, to obtain a physiologic reference of the indexes, which served as a healthy control group age- and sex-matched to the three study groups.
Pre- and postoperative clinical scores and gait analysis parameters were compared using two-way ANOVA; the differences were analyzed, including the prosthesis head size and the timing of the assessment. The Shapiro-Wilk test was the normality test. A p value of less than 0.05 was considered significant.
Results
There were no differences among the three groups in any of the spatiotemporal parameters measured at baseline and postoperative evaluations (Fig. 1). All groups showed an improvement in mean gait velocity 4 months after surgery (G1: p = 0.003 POST 4 versus PRE, p = 0.003 POST 4 versus POST 2; G2: p = 0.014 POST 4 versus PRE, p = 0.044 POST 4 versus POST 2; G3: p < 0.001 POST 4 versus PRE, p < 0.001 POST 4 versus POST 2) (Fig. 1A). All groups showed increased stance duration at 2 months after surgery, which then decreased at 4 months after surgery compared with the preoperative values (G1: p = 0.009 POST 4 versus POST 2; G3: p = 0.01 POST 4 versus POST 2, p = 0.028 POST 4 versus PRE) (Fig. 1B). The patients who received a 28- or ≥ 42-mm femoral head showed increased stride length at 4 months after surgery compared with the preoperative and 2-month postoperative measurements (G1: p = 0.008 POST 4 versus POST 2, p = 0.005 POST 4 versus PRE; G3: p = 0.008 POST 4 versus POST 2, p < 0.001 POST 4 versus PRE) (Fig. 1C).
Graphs show the spatiotemporal gait parameters for the three groups: (A) gait velocity, (B) stance phase duration, and (C) stride length. Stance time and stride length refer to the surgically treated limb. Values are shown as mean ± SD. There are no differences among the groups in any of the spatiotemporal parameters measured at baseline and postoperative conditions. However, there are differences between baseline and postoperative in gait velocity (all groups), stance time (G1, G3), and stride length (G1, G3).
There were no differences among the groups in the preoperative kinematic and kinetic gait parameters, and these parameters were not influenced by prosthesis head size (Table 2). Hip extension during gait improved at the 4-month evaluation in the 28- and ≥ 42-mm groups compared to the preoperative analysis (p < 0.001 and p = 0.003, respectively). Hip extension moment improved at 4 months in the 28-mm group (p = 0.002 POST 4 versus PRE; p = 0.005 POST 4 versus POST 2). Before surgery, all patients showed a lower and postponed first peak of the GRF, a lower second peak, and a higher minimum value between the two peaks of force compared with the GRF pattern of the normality database (defined as the healthy controls) kept at the Motion Analysis Laboratory (Fig. 2). There was an effect of surgical treatment on the GRF mainly in the time of the first peak. Before surgery, the biphasic pattern of the GRF was lost compared with the pattern of the healthy subjects, which moved toward a normal pattern at 4 months after surgery (Fig. 3).
Graphs show the mean pattern of the vertical component of the ground reaction vector during the stance phase of the surgically treated leg for (A) G1, (B) G2, and (C) G3. The force pattern of the control group is from the data of a group of healthy subjects extracted from the database of the gait acquisition software. At baseline, all patients showed a lower and postponed first peak of the GRF, a lower second peak, and a higher minimum value between the two peaks of force. BW = body weight.
Graphs show the mean pattern of the vertical component of the ground reaction vector during the stance phase of the surgically treated leg for the three groups at (A) 2 and (B) 4 months postoperatively. The baseline biphasic pattern of the GRF was lost compared with the pattern of the healthy subjects, but it moved toward a normal pattern postoperatively. BW = body weight.
The mean Harris hip score at 4 months was higher (p < 0.001) for all three groups than the preoperative score (Fig. 4), with no differences among the groups in both the baseline and postoperative scores. Two-month postoperative improvements (p < 0.001) in ROM were observed in all three groups, with no differences among them (Table 3). Although leg length discrepancy can affect gait performance, no patient in this series showed a leg length discrepancy of more than 1 cm at the postoperative clinical evaluation.
Discussion
Larger femoral heads are commonly considered to restore better joint stability and biomechanics, with enhanced functionality during gait. Faster recovery of gait and a feeling of more normal walking have been reported. We evaluated how gait parameters may be influenced by head diameter at short-term followup after THA. Our hypothesis was that a larger head could restore an earlier, more physiologic gait pattern. This hypothesis was not supported by our study, however. We found no clinically important differences among the three groups in gait analysis parameters at 2 or 4 months after surgery.
This study has a number of limitations. One is the short followup period. However, the major part of gait recovery is known to occur within the early period [9, 16], and our aim was to evaluate the early recovery of gait after THA with different head diameters. The second limitation is the relatively small number of patients evaluated in each group, which could reduce our statistical power and our ability to identify a difference if indeed one were to have been present. On a related note, if the clinical outcome were to have been suboptimal in a few patients, in such a small study, the gait results could be skewed in favor or against one particular group. To minimize the likelihood this occurred, we did exclude the one patient who developed a complication (heterotopic ossification) unrelated to head size, which would clearly have affected the gait result. Even so, for these reasons, in a small pilot study, we cannot exclude the possibility that some gait-related or clinical differences existed among the groups tested. Finally, gait analysis is the most objective tool for evaluating gait recovery [7, 14, 16, 19]. To our knowledge, this is the first prospective, randomized study to compare, by means of gait analysis in a homogeneous cohort of patients, THA procedures in which the only variable was the implant head size.
Among previous studies comparing THA and RHA, Mont et al. [14], in a nonrandomized study published in 2007, noted, after RHA, patients appear to have a near-normal gait performance; however, no preoperative data were given. In 2009, Shrader et al. [19], in another nonrandomized study comparing two groups of seven patients each, found a greater improvement in gait pattern at 3 months after RHA than after THA. Confirming our findings, Lavigne et al. [7], in a randomized, double-blind study involving two groups of 21 patients allocated to receive large-head THA or RHA, found no differences in gait parameters between the groups at 3, 6, and 12 months after surgery. More recently, Petersen et al. [16], in a prospective, randomized study evaluating the gait characteristics of 11 patients with THA and 11 patients with RHA, found no differences in gait parameters between the two patient groups, except for hip abduction moment, which improved more in the patients with THA. This difference was attributed to the less invasive surgical procedure for THA.
In our study, no differences among the three groups were found at preoperative assessment or at either 2 or 4 months. On the postoperative evaluations, however, gait parameters improved in comparison with the preoperative conditions in all groups. Significant improvements in spatiotemporal parameters were observed at assessment 4 months after THA. Specifically with regard to the kinematic and kinetic analysis, a significant improvement in all groups was found after 4 months in the maximum hip intrarotation moment and the GRF pattern. The clinical results confirmed the gait findings of the study. Indeed, no differences in either the Harris hip score or the passive hip ROM emerged among the three groups. As compared with the baseline condition, improvements were noted at 2 and 4 months after surgery.
In this patient cohort, we demonstrated by means of gait analysis improvement in gait parameters after THA with 28-, 36-, and ≥ 42-mm bearing couples compared with preoperative values. Our study was randomized, and in it, all patients were treated using the same surgical technique and postoperative rehabilitation protocol, and the same types of prostheses were used by the same surgeons. The three patient groups were homogeneous for age and sex. With the numbers available, our results do not support the primary hypothesis that a larger femoral head diameter will improve gait recovery compared to a smaller one. We suggest our findings could be useful as pilot data for further studies including a larger sample size and longer followup.
References
Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2456–2463.
Charnley J. Total hip replacement by low friction arthroplasty. Clin Orthop Relat Res. 1970;72:7–21.
Davis RB, Õunpuu S, Tybursky D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587.
Greene WB, Heckman JD. The Clinical Measurement of Joint Motion. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1994.
Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755.
Jameson SS, Lees D, James P, Serrano-Pedraza I, Partington PF, Muller SD, Meek RM, Reed MR. Lower rates of dislocations with increased femoral head size after primary total hip replacement: a five-year analysis of NHS patients in England. J Bone Joint Surg Br. 2011;93:876–880.
Lavigne M, Therrien M, Nantel J, Roy A, Prince F, Vendittoli PA. The John Charnley Award. The functional outcome of hip resurfacing and large-head THA is the same: a randomized, double-blind study. Clin Orthop Relat Res. 2010;468:326–336.
Lee JH, Lee BW, Lee BJ, Kim SY. Midterm results of primary total hip arthroplasty using highly cross-linked polyethylene: minimum 7-year follow-up study. J Arthroplasty. 2011;26:1014–1019.
Lindemann U, Becker C, Unnewehr I, Muche R, Aminin K, Dejnabadi H, Nikolaus T, Puhl W, Huch K, Dreinhöfer KE. Gait analysis and WOMAC are complementary in assessing functional outcome in total hip replacement. Clin Rehabil. 2006;20:413–420.
Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty? Clin Orthop Relat Res. 2011;469:1547–1553.
Lusty PJ, Tai CC, Sew-Hoy RP, Walter WL, Walter WK, Zicat BA. Third-generation alumina-on-alumina ceramic bearings in cementless total hip arthroplasty. J Bone Joint Surg Am. 2007;89:2676–2683.
McCrory JL, White SC, Lifeso RM. Vertical ground reaction forces: objective measures of gait following hip arthroplasty. Gait Posture. 2001;14:104–109.
Meftah M, Ebrahimpour PB, He C, Ranawat AS, Ranawat CS. Preliminary clinical and radiographic results of large ceramic heads on highly cross-linked polyethylene. Orthopedics. 2011;34:133–137.
Mont MA, Seyler TM, Ragland PS, Starr R, Erhart J, Bhave A. Gait analysis of patients with resurfacing hip arthroplasty compared with hip osteoarthritis and standard total hip arthroplasty. J Arthroplasty. 2007;22:100–108.
Peters CL, McPherson E, Jackson JD, Erickson JA. Reduction in early dislocation rate with large-diameter femoral heads in primary total hip arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):140–144.
Petersen MK, Andersen NT, Mogensen P, Voight M, Søballe K. Gait analysis after total hip replacement with hip resurfacing implant or Mallory-head Exeter prosthesis: a randomised controlled trial. Int Orthop. 2011;35:667–674.
Randelli F, Banci L, D’Anna A, Visentin O, Randelli G. Cementless Metasul metal-on-metal total hip arthroplasties at 13 years. J Arthroplasty. 2012;27:186–192.
Saito S, Ishii T, Mori S, Hosaka K, Ootaki M, Tokuhashi Y. Long-term results of Metasul metal-on-metal total hip arthroplasty. Orthopedics. 2010;11:33.
Shrader MW, Bhowmik-Stoker M, Jacofsky MC, Jacofsky DJ. Gait and stair function in total and resurfacing hip arthroplasty: a pilot study. Clin Orthop Relat Res. 2009;467:1476–1484.
Smith TM, Berend KR, Lombardi AV Jr, Emerson RH Jr, Mallory TH. Metal-on-metal total hip arthroplasty with large heads may prevent early dislocation. Clin Orthop Relat Res. 2005;441:137–142.
Thomas GE, Simpson DJ, Mehmood S, Taylor A, McLardy-Smith P, Gill HS, Murray DW, Glyn-Jones S. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93:716–722.
Yeung E, Bott PT, Chana R, Jackson MP, Holloway I, Walter WL, Zicat BA, Walter WK. Mid-term results of third-generation alumina-on-alumina ceramic bearings in cementless total hip arthroplasty: a ten-year minimum follow-up. J Bone Joint Surg Am. 2012;94:138–144.
Acknowledgments
The authors thank Claudio Pagnuzzato MD as orthopaedic surgeon, Michele Corbella MD for his contribution as a member of the surgical team, and Christine Champlon MSEng of the Motion Analysis Laboratory for assistance in gait acquisition and data processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
During the study period, the institution of the authors received funding from Lima Corp (San Daniele del Friuli, Italy). Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Zagra, L., Anasetti, F., Bianchi, L. et al. No Difference in Gait Recovery After THA With Different Head Diameters: A Prospective Randomized Study. Clin Orthop Relat Res 471, 3830–3837 (2013). https://doi.org/10.1007/s11999-013-2926-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-013-2926-6