Abstract
Background
The effect of the extent of osteonecrosis on the survival of hip resurfacing for osteonecrosis of the femoral head (ONFH) has not been well documented, but is a potentially important variable in the decision to perform resurfacing.
Questions/purposes
We examined (1) the relationship between the volume of osteonecrosis in the femoral head before surgery and the extent of the residual necrotic bone after femoral head machining, (2) how the extent of the residual necrotic bone relative to the resurfaced femoral head (after femoral head machining) affected the survival of total hip resurfacing for patients with ONFH, and (3) how the extent of the necrotic bone relative to the entire femoral head (before femoral head machining) affected the survival and clinical outcome scores of patients who underwent total hip resurfacing.
Methods
Thirty-three patients (39 hips) who underwent hip resurfacing were reviewed after a mean followup of 8 years. The extent of osteonecrosis in the femoral head and residual osteonecrosis in the implant bony bed after femoral head machining were estimated using a three-dimensional MRI-based templating system.
Results
There was a statistically significant difference in the extent of osteonecrosis before and after femoral head machining, although the two were well correlated (r = 0.97). The mean percentage of osteonecrosis in the implant bony bed after femoral head machining was 5% smaller than that relative to the entire femoral head (range, −9% to 15%). There were no significant differences in implant survival between groups with small and large osteonecrosis classified by either the total amount of osteonecrosis before surgery or residual osteonecrosis after femoral head machining.
Conclusion
The extent of osteonecrosis in the femoral head significantly decreased after femoral head machining. Neither the residual osteonecrosis volume in the implant bony bed after femoral head machining nor the total amount of osteonecrosis before femoral head machining had significant influence on the survival of hip resurfacing.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Total hip resurfacing arthroplasty has some advantages over conventional THA, including a minimized risk of postoperative dislocation using larger femoral heads [7], lower wear of metal-on-metal articulation [19], and no surgical loss of the metaphyseal and diaphyseal femoral bone [1]. Therefore, a hip resurfacing arthroplasty may be suitable for some patients with osteonecrosis of the femoral head (ONFH) because of their younger ages and high activity levels [2]. However, some authors [5, 18] do not recommend hip resurfacing for ONFH, because the residual necrotic bone remaining after hip resurfacing could result in collapse of the remaining femoral head beneath the femoral implant and/or loosening of the component. Even with a technique to curette the residual necrotic bone and replace it with cement, a finite element analysis study [26] reported that the extent of cement replacing necrotic bone correlates with the strain at the bone cement interface. However, some authors [2, 11, 16] support hip resurfacing for ONFH even when some necrotic bone remains, but it is unclear how much the size of the lesion before resurfacing or the residual necrotic bone in the implant bony bed after resurfacing could clinically affect the stability of the femoral components.
Some surgeons assess the percentage volume of necrotic bone relative to the entire femoral head to determine whether hip resurfacing is indicated [20, 25]. Measurements of the volume of necrotic bone relative to the entire femoral head may correlate with the volume of the cement mantel, which replaces the necrotic bone. However, some of the volume of the necrotic bone is removed by machining of the femoral head, and the volume removed depends on the lesion size relative to the femoral head and the location of the lesion. Therefore, the size of necrotic bone relative to the entire femoral head may not represent the size of residual necrotic bone in the implant bony bed. No previous study, however, has measured the extent of the residual necrotic bone after hip resurfacing and correlated it to the outcomes of hip resurfacing for ONFH. Three-dimensional (3-D) reconstruction of preoperative MRI enables us to preoperatively measure the extent and location of the necrotic bone [15, 27, 31]. Moreover, by superimposing computer models of hip resurfacing implants on 3-D MRI by using 3-D templating software, the size of the residual necrotic bone after hip resurfacing can be quantitatively measured.
Therefore, we examined: (1) the relationship between the volume of osteonecrosis in the femoral head before surgery and the extent of the residual necrotic bone after femoral head machining; (2) how the extent of the residual necrotic bone after femoral head machining affected the survival and clinical outcome scores (Merle d’Aubigne-Postel and WOMAC™) of hip resurfacing for patients with ONFH; and (3) how the extent of the necrotic bone relative to the entire femoral head, ie, before femoral head machining, affected the survival and clinical outcome scores.
Patients and Methods
Institutional review board approval was obtained for this retrospective study (#11140). Metal-on-metal resurfacing arthroplasties for patients with ONFH were performed on 43 hips of 37 patients using the Birmingham Hip™ Resurfacing System (Smith & Nephew, Inc, Memphis, TN, USA), from February 1998 to December 2007. This system is composed of a hydroxyapatite-coated Porocast™ cobalt-chromium cup for cementless fixation and a cobalt-chromium femoral component for cemented fixation [8, 17]. Of the 37 patients with ONFH, one patient (one hip) was lost to followup, and three patients (three hips) died of unrelated causes at 2, 4, and 6 years after surgery; thus, 33 patients (39 hips) were available for inclusion in the study after a mean followup of 8 years (range, 2–13 years) (Table 1).
The radiologic stage was assessed using the radiologic staging system revised in 2001 by the Japanese Investigation Committee (JIC) [30] and the Association Research Circulation Osseous (ARCO) international staging system [29]. In the JIC and ARCO radiologic staging system, Stage 1 indicates there are no specific findings of osteonecrosis observed on radiographs. Stage 2 indicates there is demarcating sclerosis without collapse of the femoral head. Stage 3 indicates collapse of the femoral head, including the crescent sign, is seen without joint-space narrowing. Stage 3 is subdivided into stages 3A (less than 3 mm of collapse) and 3B (3 mm or more of collapse) in the JIC staging system. Stage 4 indicates osteoarthritic changes are seen. The location of osteonecrosis was evaluated according to the 2001 revised JIC classification system [30], which also was adopted in the ARCO classification system [29]. The location of osteonecrosis consists four types (A, B, C1, and C2) and is based on the extent of a lesion of low-signal-intensity or normal fat signal intensity demarcated by a low-signal-intensity band in the central coronal section of the femoral head as observed on T1-weighted images. Type A lesions occupy the medial 1/3 or less of the weightbearing portion. Type B lesions occupy the medial 1/3 to 2/3 Type C1 and Type C2 lesions occupy more than the medial 2/3 of the weightbearing portion, but, whereas Type C2 lesions extend laterally to the acetabular edge, type C1 lesions do not. The volume of osteonecrosis was evaluated according to the ARCO classification system [29], which is the same as the Steinberg classification system [28]. Hips were divided into three groups based on lesion volume: Group A, less than 15% of femoral head involvement; Group B, 15% to 30%; and Group C, greater than 30%. Hips in Stage 4 cannot be classified because of severe femoral head deformity (Table 2).
Contraindications for hip resurfacing for patients with ONFH at our institution included hips diagnosed with poor proximal bone quality of Type C according to the classification system of Dorr et al. [14], hips with osteonecrosis extending beyond the femoral neck, renal impairment, or histories of metal sensitivity.
All procedures were performed through a posterolateral approach with the patient in the lateral position. The acetabulum was reamed up to a 1-mm smaller size. Acetabular cups were impacted into the acetabulum by the press-fit technique aimed at 40° abduction and 15° anteversion by the radiographic definition. On the femoral side, a guide wire was passed from the surface of the femoral head, aiming for a slightly valgus alignment so the femoral stem axis was aligned to the medial femoral cortex. After femoral head machining, all of the necrotic bone was removed by curettage, burr, and irrigation until normal or dense white reactive bone was identified. Anchoring holes were made over the normal bone into the dome and chamfer areas. Finally, the femoral component was half-filled with cement (Surgical Simplex®, Stryker®, Kalamazoo, MI, USA) 2 minutes after mixing. A suction cannula was placed into the lesser trochanter to prevent elevation of intraosseous pressure during insertion of the femoral component. Full weightbearing was allowed immediately after the surgeries in all cases, regardless of osteonecrosis volume.
Clinical and radiographic followup was performed immediately after surgery, then at 3, 6, and 12 months, and annually thereafter. Clinical assessments were performed preoperatively and at the most recent followup using the Merle d’Aubigne and Postel hip score system [9]. The WOMACTM self-assessment questionnaire was administered at the most recent followup [6].
The inclination angle of the acetabular component, the femoral neck-shaft angle (N-S angle), and the stem-shaft of the femur angle (S-S angle) were measured on the immediate postoperative radiographs [3]. The position of the femoral component was defined as valgus (S-S angle was > N-S angle by 5°), varus (S-S angle < N-S angle by 5°), and neutral (when otherwise) [21]. Radiographs at the final examination evaluated radiolucency and osteolysis around the acetabular cup using the zone classification of DeLee and Charnley [10]. Loosening of the acetabular component was considered when there was a progressive radiolucent line of 2 mm or wider into a new zone or when a progressive migration occurred [32]. Radiolucency and migration of the femoral component was evaluated using the classification of femoral component fixation by Pollard et al. [23]. A Type 0 classification indicates there is no change. The Type 1 classification is divided into three subtypes according to the progression of the sclerotic and lucent lines toward the femoral head. The Type 1a classification occurs when the sclerotic line is confined to the curved tip of stem. The Type 1b classification occurs when the sclerotic line is confined to the distal 1 cm of the shaft of the stem. The Type 1c classification occurs when there is a sclerotic line with or without asymmetric lucent lines, extending proximally beyond the distal 1 cm of the shaft. The Type 2 classification is a migration into varus with asymmetric lucent lines. The Type 2 classification had been reported to be likely to fail [4]. Loosening of the femoral component was considered when there was subsidence of the femoral component by more than 5 mm or a change of stem-shaft angle by greater than 5° [24].
All patients underwent 3-D MR scans of bilateral hips using a 1.0-Tesla MRI system (Signa Horizon LX 1.0T; General Electric Medical Systems, Waukesha, WI, USA) to estimate the extent of the necrotic lesion before surgery. Two types of sequence were used. The one was a 3-D spoiled, gradient-recalled echo sequence (SPGR) with a repetition time (TR) of 7.9 ms, echo time (TE) of 3.08 ms, and flip angle of 30°. The other was a fat-suppressed SPGR sequence (FS-SPGR; TR/TE/flip angle: 23.7 ms/3.08 ms/30°). In segmentation of the osteonecrosis area, SPGR images were used in 33 of the 39 hips. FS-SPGR images were used in six hips because it was difficult to delineate the boundary of the lesions owing to bone marrow edema observed on the SPGR images. The boundary of the lesions on the FS-SPGR images was observed as an intermediate-intensity band even in cases with severe bone marrow edema [27]. The areas of osteonecrosis were measured by segmenting low or normal intensity areas surrounded by the low-intensity band on SPGR images or intermediate-intensity band on FS-SPGR images using an image-processing program (Image J version 1.44q, National Institutes of Health, Bethesda, MD, USA). We defined the necrotic lesion boundary as the outer margin of the low-intensity band on SPGR images or intermediate-intensity band on FS-SPGR because histologically, the low intensity band on SPGR images and the intermediate-intensity band on FS-SPGR images represent two layers of reparative tissue that cannot be separated on MR images. One is a fibrous vascular tissue layer which is mechanically very weak. The other is a sclerotic bone layer that consists of thickened trabecular bone with appositional bone formation and repaired viable bone marrow [27]. To avoid underestimation of the mechanically weak lesion, we included the outer margin of the low-intensity band on SPGR images or intermediate-intensity band on FS-SPGR images in the necrotic lesion.
Three-dimensional MRI-based templating was performed using our original templating digital software to estimate the residual osteonecrosis volume after femoral head machining. This templating can show multiple planar reconstructed (MPR) images through any orthogonal plane. The oblique coronal and oblique sagittal MPR views through the femoral neck axis were reconstructed. The plane consisting of the femoral neck axis and the center of the femoral medullary canal at 15 cm distal from the tip of the greater trochanter was defined as the oblique coronal plane of the femoral neck (Fig. 1A), and the plane perpendicular to the oblique coronal plane though the neck axis was defined as the oblique sagittal plane of the femoral neck (Fig. 1B). Virtual implantation was performed referencing postoperative radiographs, including the stem-shaft angle, the distance of the femoral component center from the tip of the greater trochanter on the AP radiograph, and the stem anteversion related to the femoral neck on the lateral radiograph.
The center of the femoral head was defined by fitting the sphere to the size of the subchondral bone of the noncollapsed part of the femoral head (thin white circle). Next, the center of the femoral neck was defined by fitting the two concentric spheres to the AP and superoinferior inner cortexes of the femoral neck at its isthmus (dashed circles). The line passing through the center of the femoral neck, and the femoral head center was defined as the femoral neck axis (white line). The center of the proximal femur was defined as the center of the femoral medullary canal at 15 cm distal from the tip of the greater trochanter (thick white circle). (A) The plane consisting of the femoral neck axis and the center of the proximal femur was defined as the oblique coronal plane of the femoral neck. (B) The plane perpendicular to the oblique coronal plane though the neck axis was defined as the oblique sagittal plane of the femoral neck.
The residual osteonecrosis volume after femoral head machining was calculated by the summation of the residual osteonecrosis areas in the serial oblique coronal planes with a pitch of 2 mm [15]. The volume of the femoral head after surface machining was calculated by the summation of the resurfaced femoral head areas on serial oblique coronal planes. The volume percentage of residual osteonecrosis in the prepared femoral head then was calculated (Fig. 2).
(A) The areas of residual osteonecrosis were measured by segmenting areas of low-intensity band. (B) The areas of the resurfaced femoral head were measured by the bony area in the femoral component. (C) The residual osteonecrosis volumes and the resurfaced femoral head volume were calculated by the summation of their areas on serial coronal planes.
The volume percentage of osteonecrosis relative to the entire femoral head was calculated by a similar method. The entire femoral head volume was calculated from fitting the sphere to the size of the subchondral bone of the noncollapsed part of the femoral head.
To investigate how much the total amount of osteonecrosis in the entire femoral head represented the extent of the residual necrotic bone after femoral head machining, we evaluated the difference and correlation between the volume percentage of residual osteonecrosis relative to the machined femoral head and the volume percentage of the total amount of osteonecrosis relative to the entire femoral head using the Wilcoxon t-test and Pearson’s correlation test, respectively. We classified patients, based on the percentage volume of residual osteonecrosis, into a small group (< 25%) and a large group (≥ 25%) to evaluate the effect of residual osteonecrosis on clinical scores and radiographic outcomes. Eighteen of 39 hips were in the small group (< 25%) and 21 were in the large group (≥ 25%) in the estimation of the residual osteonecrosis volume. There were no significant differences in age, gender, BMI, followup period, implant cup size, or femoral component size between the two groups (Table 3). Continuous variables (Merle d’Aubigne-Postel score, WOMACTM score, cup inclination angle, neck-shaft angle, and stem-shaft angle) were compared using the Mann-Whitney U test. Categorical variables to include femoral component position in comparison to the neck-shaft angle, classification of radiolucency, and migration of the femoral component were compared using Fisher’s exact probability test. Cumulative survival rates were calculated by using the Kaplan-Meier method with failure for loosening of the component as the end point. The log-rank test was used to compare survival probabilities between the groups. In addition, we also investigated the effect of the total amount of osteonecrosis on the clinical score and radiographic outcome by classification based on the percentage volume of osteonecrosis relative to the entire femoral head, dividing them into a small group (< 35%) and a large group (≥ 35%). We chose the 35% cutoff because previous studies reported good clinical results for hip resurfacing in patients with osteonecrosis smaller than 35% of the entire femoral head [20, 25]. Twenty-three of 39 hips were classified in the small group (< 35%) and 16 in the large group (≥ 35%) in the estimation of osteonecrosis volume relative to the entire femoral head. There were no significant differences in age, gender, BMI, followup period, implanted cup size, or femoral component size between the two groups (Table 4).
Results
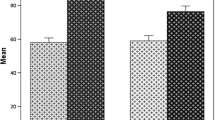
The volume percentage of residual osteonecrosis in the implant bony bed after femoral head machining was significantly smaller than the volume percentage relative to the entire femoral head (p < 0.001, Wilcoxon t-test), but the two parameters were well correlated (r = 0.97, p < 0.001, Pearson’s correlation coefficient) (Fig. 3). The mean volume percentage of residual osteonecrosis after femoral head machining was 29% (range, 9%–77%). The mean percentage volume of osteonecrosis relative to the entire femoral head was 34% (range, 14%–62%). The mean difference between the volume percentage of osteonecrosis in the entire femoral head and that of the residual osteonecrosis in the machined femoral head was 5% (range, −9% to 15%).
There was no difference in the survival rate between the small-lesion (< 25%) and large-lesion (≥ 25%) groups in terms of the volume percentage of residual osteonecrosis, using radiographic loosening of the component as the end point (p = 0.97; log-rank test). Furthermore there were no differences in the Merle d’Aubigne-Postel and WOMACTM scores at last followup, and no differences in terms of radiographic estimates of femoral component fixation using the criteria of Pollard et al. [23] between the groups (Table 5). There were no radiolucencies and no osteolysis in any zone around the acetabular components in either group. Femoral component loosening was observed in one hip in the small-lesion group and in one hip in the large-lesion group. The hip that showed femoral component loosening in the presence of a large lesion (39%) showed radiographic lucencies around the stem at 7 years and progressed to femoral loosening with varus displacement at 10 years. The other hip that had loosening develop was in the small-lesion group (12%); loosening in this hip was diagnosed at 7 months and it was revised at 10 months. There were no other revisions for any reason in this series.
There was no significant difference in the survival rate between the small-lesion (< 35%) and large-lesion (≥ 35%) groups in terms of osteonecrosis volume relative to the entire femoral head, using radiographic loosening of the component as the end point (p = 0.72; log-rank test). Furthermore, there were no differences between the small- and large-lesion groups in terms of the Merle d’Aubigne-Postel and WOMACTM scores at last followup, and no difference in terms of radiographic estimation of femoral component fixation using the criteria of Pollard et al. [23] (Table 6).
Discussion
In the current study, we hypothesized that the extent of residual osteonecrosis in the implant bony bed after femoral head machining would be significantly different than the amount of osteonecrosis that was present in the femoral head before surgery. We also hypothesized that the extent of residual osteonecrosis in the implant bony bed might affect the survival of hip resurfacing arthroplasty for patients with ONFH. The volume percentage of osteonecrosis in the implant bony bed after femoral head machining was significantly smaller than that relative to the entire femoral head, although the two showed a high correlation. This indicated that we could predict the extent of residual osteonecrosis in the femoral bony bed by measuring the osteonecrosis in the entire femoral head; however, in our study, there were no significant differences in clinical score, femoral component fixation, or implant survival between the small and large osteonecrosis groups classified by either the total amount of osteonecrosis before surgery or residual osteonecrosis after femoral head machining. This suggests that the extent of osteonecrosis was not a risk factor for revision or radiographic failure of hip resurfacing.
One possible limitation was a selection bias in terms of the size of osteonecrosis, because we excluded patients with larger osteonecrosis extending beyond the femoral neck junction, as it has been reported that the strain concentration was highest in the superior femoral neck adjacent to the distal rim of the femoral component [22]. Thus, we could not suggest the possible upper limit of osteonecrosis volume that would be indicated for hip resurfacing. The FDA listed the extent of osteonecrosis involvement of greater than 50% as a contraindication for hip resurfacing [12, 13]. Mont et al. [20] and Revell et al. [25] reported good clinical results for hip resurfacing in patients with osteonecrosis smaller than 35% of the entire femoral head; however, 16 hips in our series were observed to have lesion volumes larger than 35% of the entire femoral head, including two hips with lesion volumes larger than 60%, that showed good clinical results (Fig. 3). There were no significant differences in the clinical and radiologic outcomes between the lesion volume less than 35% and volume of 35% or more (Table 6). The upper limit of lesion volume therefore might be larger than many surgeons have considered. Ideally, a prospective study is necessary to eliminate patient-related biases, such as age, sex, bone quality, and related factors for osteonecrosis to determine the upper limit of the extent of necrosis acceptable for this procedure.
We think the cementing technique for fixation of the femoral component is a critical point for successful hip resurfacing in patients with ONFH, rather than the necrotic volume. The femoral component should be fixed on the cleaned, resurfaced femoral head. When the cement is fixed on necrotic tissue, such as yellowish and fibrous debris, this might lead to instability of the femoral component and result in loosening of the femoral component. Amstutz and LeDuff [2] also reported that necrotic bone must be completely removed down to the underlying normal bone or reparative bone to confirm proper component fixation and durability. They reported that the defect size after removal of residual osteonecrosis had no significant effect on the clinical and radiologic results of hip resurfacing after a mean followup of 7.6 years. We removed all the necrotic tissue until only normal or dense white reactive bone was observed. Moreover, we made anchoring holes over this normal bone or dense white reactive bone into the dome and the chamfer area. The effect of the cement anchoring technique on stability of the femoral component resurfacing could not be assessed in this study; however, we believe that these cementing techniques brought about favorable midterm hip resurfacing results for the patients with ONFH.
The total amount of osteonecrosis in the femoral head before surgery correlated well with the extent of necrotic bone that remained after femoral head machining, and the volume percentage of osteonecrosis in the femoral head decreased approximately 5% after femoral head machining. The amount of residual osteonecrosis volume in the implant bony bed and the total amount of osteonecrosis in the entire femoral head before surgery did not appear to affect survival of hip resurfacing for patients with ONFH at a mean followup of 8 years (range, 2–13 years).
References
Amstutz HC, Grigoris P, Dorey FJ. Evolution and future of surface replacement of the hip. J Orthop Sci. 1998;3:169–186.
Amstutz HC, Le Duff MJ. Hip resurfacing results for osteonecrosis are as good as for other etiologies at 2 to 12 years. Clin Orthop Relat Res. 2010;468:375–381.
Back DL, Dalziel R, Young D, Shimmin A. Early results of primary Birmingham hip resurfacings: an independent prospective study of the first 230 hips. J Bone Joint Surg Br. 2005;87:324–329.
Baker RP, Pollard TC, Eastaugh-Waring SJ, Bannister GC. A medium-term comparison of hybrid hip replacement and Birmingham hip resurfacing in active young patients. J Bone Joint Surg Br. 2011;93:158–163.
Beaule PE, Amstutz HC, Le Duff M, Dorey F. Surface arthroplasty for osteonecrosis of the hip: hemiresurfacing versus metal-on-metal hybrid resurfacing. J Arthroplasty. 2004;19(8 suppl 3):54–58.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840.
Burroughs BR, Hallstrom B, Golladay GJ, Hoeffel D, Harris WH. Range of motion and stability in total hip arthroplasty with 28-, 32-, 38-, and 44-mm femoral head sizes. J Arthroplasty. 2005;20:11–19.
Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184.
D’Aubigne RM, Postel M. Function al results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475.
DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32.
De Smet KA. Belgium experience with metal-on-metal surface arthroplasty. Orthop Clin North Am. 2005;36:203–213, ix.
Department of Health and Human Services. Food and Drug Administration. Birmingham Hip Resurfacing (BHR) System-P040033. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf4/p040033a.pdf. Accessed June 15, 2012.
Department of Health and Human Services. Food and drug Administration. Cormet Hp Resurfacing System-P0500016. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf5/p050016a.pdf. Accessed June 15, 2012.
Dorr LD, Faugere MC, Mackel AM, Gruen TA, Bognar B, Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone. 1993;14:231–242.
Kishida Y, Nishii T, Sugano N, Nakanishi K, Sakai T, Miki H, Ochi T, Yoshikawa H. Measurement of lesion area and volume by three-dimensional spoiled gradient-echo MR imaging in osteonecrosis of the femoral head. J Orthop Res. 2003;21:850–858.
Lian YY, Pei FX, Yoo MC, Cheng JQ, Fatou CY. Changes of the bone mineral density in proximal femur following total hip resurfacing arthroplasty in osteonecrosis of femoral head. J Orthop Res. 2008;26:453–459.
McMinn D, Daniel J. History and modern concepts in surface replacement. Proc Inst Mech Eng H. 2006;220:239–251.
McMinn DJ, Daniel J, Ziaee H, Pradhan C. Indications and results of hip resurfacing. Int Orthop. 2011;35:231–237.
Migaud H, Jobin A, Chantelot C, Giraud F, Laffargue P, Duquennoy A. Cementless metal-on-metal hip arthroplasty in patients less than 50 years of age: comparison with a matched control group using ceramic-on-polyethylene after a minimum 5-year followup. J Arthroplasty. 2004;19(8 suppl 3):23–28.
Mont MA, Seyler TM, Marker DR, Marulanda GA, Delanois RE. Use of metal-on-metal total hip resurfacing for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(suppl 3):90–97.
Nishii T, Sugano N, Miki H, Takao M, Koyama T, Yoshikawa H. Five-year results of metal-on-metal resurfacing arthroplasty in Asian patients. J Arthroplasty. 2007;22:176–183.
Olsen M, Davis ET, Whyne CM, Zdero R, Schemitsch EH. The biomechanical consequence of insufficient femoral component lateralization and exposed cancellous bone in hip resurfacing arthroplasty. J Biomech Eng. 2010;132:081011.
Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip: a five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88:592–600.
Ramakrishnan R, Jaffe WL, Kennedy WR. Metal-on-metal hip resurfacing radiographic evaluation techniques. J Arthroplasty. 2008;23:1099–1104.
Revell MP, McBryde CW, Bhatnagar S, Pynsent PB, Treacy RB. Metal-on-metal hip resurfacing in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(suppl 3):98–103.
Sakagoshi D, Kabata T, Umemoto Y, Sakamoto J, Tomita K. A mechanical analysis of femoral resurfacing implantation for osteonecrosis of the femoral head. J Arthroplasty. 2010;25:1282–1289.
Sakai T, Sugano N, Nishii T, Haraguchi K, Ochi T, Ohzono K. MR findings of necrotic lesions and the extralesional area of osteonecrosis of the femoral head. Skeletal Radiol. 2000;29:133–141.
Steinberg ME, Bands RE, Parry S, Hoffman E, Chan T, Hartman KM. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999;367:262–271.
Stulberg BN. Editorial comment. Clin Orthop Relat Res. 1997;334:2–5.
Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–605.
Takao M, Sugano N, Nishii T, Tanaka H, Masumoto J, Miki H, Sato Y, Tamura S, Yoshikawa H. Application of three-dimensional magnetic resonance image registration for monitoring hip joint diseases. Magn Reson Imaging. 2005;23:665–670.
Udomkiat P, Dorr LD, Wan Z. Cementless hemispheric porous-coated sockets implanted with press-fit technique without screws: average ten-year followup. J Bone Joint Surg Am. 2002;84:1195–1200.
Acknowledgments
We thank Hidenobu Miki MD, PhD, Nobuo Nakamura MD, PhD and Kenji Ohzono MD, PhD for helpful advice and support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Nakasone, S., Takao, M., Sakai, T. et al. Does the Extent of Osteonecrosis Affect the Survival of Hip Resurfacing?. Clin Orthop Relat Res 471, 1926–1934 (2013). https://doi.org/10.1007/s11999-013-2833-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-013-2833-x