Abstract
Background
Although 7% to 38% of revision total knee arthroplasties (RTKAs) are attributable to prosthetic knee infections, controversy exists regarding the best surgical approach while reducing the risk of extensor mechanism complications and the reinfection rate.
Questions/purposes
We compared The Knee Society Score© (KSS), incidences of complications, maximum knee flexion, residual extension lag, and reinfection rate in patients with prosthetic knee infections treated with two-stage RTKAs using either the tibial tubercle osteotomy (TTO) or the quadriceps snip (QS) for exposure at the time of reimplantation.
Methods
We prospectively followed 81 patients with chronic prosthetic knee infections treated between 1997 and 2004. Patients were randomized to receive a TTO or QS for exposure at the time of reimplantation. All patients had the same rehabilitation protocol. The minimum followup was 8 years (mean, 12 years; range, 8–15 years).
Results
Patients in the TTO group had a higher mean KSS than the QS group (88 versus 70, respectively). Mean maximum knee flexion was greater in the TTO group (113° versus 94°); with a lower incidence of extension lag (45% versus 13%). We observed no differences in reinfection rate between groups.
Conclusions
We found the TTO combined with an early rehabilitation protocol associated with superior KSS did not impair extensor mechanism function or increase the reinfection rate. We believe a two-stage RTKA with TTO is a reasonable approach for treating prosthetic knee infections.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection is a leading cause for revision total knee arthroplasty (RTKA) [11, 12]. Studies have shown that 6.6% to 38% of RTKAs are attributable to prosthetic knee infections [5, 11, 23, 24, 31, 41, 43, 55, 58, 76, 95, 96]. Although some [56, 63] prefer it, a two-stage procedure for chronic prosthetic knee infection requires temporary cement spacers to preserve limb length and reduce soft tissue retraction. Static spacers have been suggested [7, 15, 28, 30] to be more stable than mobile spacers when these knees have substantial bone loss. In either case subsequent exposure for reimplantation is more difficult owing to soft tissue shortening [2, 20, 29, 30, 33, 38, 49, 57, 89, 98], with an increased risk of extensor mechanism rupture, delayed wound healing, and reinfection [64, 79, 104].
Many exposures have been developed to preserve the integrity of the extensor mechanism in primary and revision procedures [13, 27, 62, 73, 79, 105]. In 1943, Coonse and Adams [20] described a proximal release of the extensor mechanism, called the VY quadricepsplasty. Although it provides a wide exposure, prolonged postoperative immobilization is required, with a high incidence of extension lag [83, 92] and avascular necrosis of the patella [60, 85]. Insall [52] modified this technique, extending the incision through the vastus lateralis to preserve the inferior lateral geniculate vessels, calling it the patellar turndown. Later, Garvin et al. [36] developed the quadriceps snip (QS) (Fig. 1), a 45°-angle cut in the vastus lateralis, and in a review of 16 patients, including 10 with RTKAs, rated 10 patients as having excellent results and six as having good results using the Hospital for Special Surgery scoring system, without extensor mechanism impairment and the ROM improved in all knees by an average of 30°. The tibial tubercle osteotomy (TTO) (Fig. 2) was described by Dolin [25, 26], and later by Whiteside [99] and Whiteside and Ohl [101]. Although TTO provides excellent exposure of the joint and allows lengthening and extensor realignment, it was associated with increased complication rates, including anterior knee pain, nonunion, tibial metaphyseal fracture, tubercle avulsion fracture, extension lag, and hardware-related pain, regardless whether screws or cerclage wires were used for fixation [6, 102]. The function and morbidity related to these exposures is unclear.
We therefore asked whether a TTO or QS would result in a superior Knee Society Score© (KSS) [53] in two-stage RTKAs for prosthetic knee infections, incidence of complications, postoperative maximum knee flexion, residual extension lag, and reinfection rates.
Patients and Methods
Ninety patients, treated between 1997 and 2004 for two-stage RTKAs for chronic prosthetic knee infections, were followed prospectively. A diagnosis of chronic prosthetic knee infection, at more than 6 weeks after TKA, [8, 40, 54, 71, 77, 93], defined by at least three of the following, was the inclusion criteria (Table 1): (1) unexplained pain with no radiographic evidence of implant malpositioning; (2) 10 mg/L or greater C-reactive protein (CRP) without preexisting inflammatory joint disease [4, 75, 86]; (3) 30 mm/hour or greater erythrocyte sedimentation rate (ESR) without preexisting inflammatory joint disease [4, 75, 86]; (4) radiographic implant loosening and/or periosteal osteogenesis and/or progressive nonfocal osteolysis without implant malpositioning [90]; (5) sinus or fistula communicating with prosthesis; (6) abnormal leukocytes labeled technetium-99 m bone scan (LeukoScan®, Immunomedics GmbH, Darmstadt, Germany) [61]; (7) a positive culture of synovial fluid collected preoperatively; (8) five or more polymorphonuclear cells in at least five high-power fields in periprosthetic tissue samples, collected on removal of primary implant [9, 32, 91]; (9) a positive synovial fluid cell count (more than 2000 polymorphonuclear cells with greater than 64% polymorphonuclear leukocytes) without preexisting inflammatory joint disease [75, 91]. A high American Society of Anesthesiologists (ASA) score [59] excluded four patients. They were treated with antibiotics and pain management. Computer-generated randomization populated the QS or TTO groups. No differences in demographics, functions, or serology differences were noted between them before primary implant removal (Table 2). Of the 90 patients recruited, nine were unavailable for final evaluations: five in the QS group (four died of unrelated reasons, one was lost to followup); four in the TTO group (two died of unrelated reasons, two lost to followup). Forty-two patients in the QS group and 39 in the TTO group were available with final followups ranging from 8 to 15 years. The groups also had similar indications for primary TKA (Table 3).
The sample size to detect at least a 20-point difference (two-sided, p = 0.05) in the KSS between the two groups at final followup [87], with an assumed SD of ± 25, an effect size of 0.8, and power of 80% was 35 subjects per group. Forty-five patients were recruited per group to compensate for 15% to 20% dropout.
Informed consent was obtained from patients, who were blinded at the time of primary implant removal. The study was approved by the institutional review board. Antibiotics were discontinued at least 4 weeks before primary implant removal.
Similar surgical procedures were performed by the senior authors (MM, SZ, FI) including use of tourniquet, type of cement spacer, and implant (P.F.C.® Sigma® TC3 Revision Knee System; DePuy®, Warsaw, IN, USA). The joint was exposed with a standard medial parapatellar arthrotomy through the previous skin incision, expanded for sinus excision. The components were removed in the same sequence. Scarred and necrotic tissue was débrided. We collected six tissue samples for microbiologic cultures: one from the suprapatellar pouch, one from each gutter, and one from each bone-implant interface. Additional samples were collected to determine the number of polymorphonuclear cells in the periprosthetic tissue. In 77 cases (38 in the QS group; 39 in the TTO group) five or more cells were found in at least five high power fields [9, 32, 91] (Table 1). Prophylactic antibiotic therapy with 2 g intravenous cefazolin was started just after tissue sampling and another gram was administered every 8 hours on the first postoperative day. A vancomycin-loaded (2 g per 20 g cement) static cement spacer (Simplex™ T, Stryker®, Kalamazoo, MI, USA) with tobramycin was molded intraoperatively.
At discharge, patients were prescribed intravenous antibiotics by an infectious disease specialist (EZ) based on microbiologic cultures (Table 4) and antibiograms. Patients with negative cultures received empiric antibiotic therapy (levofloxacin, 500 mg/day plus rifampicin, 600 mg/day, and teicoplanin, 800 mg/day) for 4 weeks. Laboratory tests (blood cell count, CRP, and ESR) were obtained at 7, 15, 30, and 60 days after surgery. Kidney and liver functions were monitored at 3-day intervals.
A standard postoperative protocol was followed (Table 5). At 60 days, all patients with no clinical signs of infection, normal ESR and CRP underwent LeukoScan® imaging. Patients with negative LeukoScan® imaging were offered RTKA.
Second-stage surgeries were performed according to the general principles described by Bourne and Crawford [10], under spinal anesthesia and sedation, through the original skin incision, with a tourniquet and on intravenous antibiotic prophylaxis of 2 g cefazolin. A medial parapatellar arthrotomy was performed with excision of scar tissue from the suprapatellar pouch and the gutters. The cement spacer was removed. Pulsatile lavage (Inter-pulse lavage system, Stryker, Newbury, UK) of the joint cavity was performed with 1000 mL sterile 0.9% normal saline solution, with no antibiotic or antiseptic. A drain was left in each gutter (for 48 hours) and the skin was closed with a Proximate® Px Skin Stapler (Ethicon, Johnson & Johnson Medical spa; New Brunswick, NJ, USA).
In the QS group, (n = 42) (Fig. 1), the rectus tendon was divided proximally and laterally at a 45°-angle at the apical end of the standard incision [36, 104] (Fig. 1). The patella was everted with knee flexion. After lateral release and excision of additional scar tissue, the knee was gently flexed to its limit and the position maintained for 1 minute to stretch the envelope. After implantation of the final components, the rectus tendon was repaired with a modified Mason-Allen knot [37], using a nonabsorbable suture (Ethibond No. 2, Ethicon, Somerville, MA, USA). Tension in the repaired quadriceps mechanism and patellar tracking were verified by cycling the knee through ROM according to the no-thumb technique to exclude patellar clunk [1, 35, 74]. A lateral release was required in 20 patients.
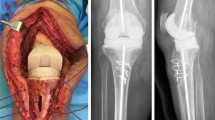
In the TTO group, (n = 39), the skin incision was extended on the proximal anteromedial tibial shaft for 10 cm. An 8- to 10-cm osteotomy of the tibial tubercle was made using an oscillating saw (Fig. 2) with an initial mediolateral cut just scoring the inner side of the lateral cortex, to be completed with osteotomes [94]. Before the saw cut, a step-cut was done at the proximal end of the osteotomy to prevent proximal migration [69, 87]. The distal end of the osteotomy was tapered to reduce anterior cortical stress risers [87] (Fig. 2). The lateral periosteal hinge and muscle attachments were left intact to preserve blood supply. The osteotomy was elevated laterally, rather than proximally [99]. During closure, two wires were passed from the lateral edge of the tibial tubercle into the medial tibial cortex (Fig. 3). A third wire was passed proximally through the lateral edge of the osteotomized tibial tubercle to impact it under the transverse step-cut osteotomy and prevent proximal migration. Lateral drill holes were placed proximal to the medial drill holes, angling 45° to the shaft, pulling the osteotomy distally [94]. After placement of the stem, the three wires were tightened onto the medial shaft of the tibia (Fig. 4), compressing the osteotomy to the cortical bone, to resist lateral dislocation during flexion. The no-thumb technique was used to check patellar tracking and exclude patellar clunk [1, 35, 74]. A lateral release was performed in 23 patients. Severe patella baja was corrected in 19 patients by freeing the patellar tendon insertion from the proximal end of the tubercle which was removed using a rongeur, reducing the tibial tubercle proximally (Fig. 5). No adjunctive fixation was required in either group.
This radiograph shows the proximally repositioned tibial tubercle used to treat patella baja. The patient, a 53-year-old man, previously had a Schatzker Type 4 tibial plateau fracture. He was treated at an outside institution with open reduction and internal fixation. He had severe posttraumatic knee arthritis develop and 5 years after the fracture, was treated with one-stage hardware removal and TKA. A prosthetic knee infection was diagnosed 6 months after the TKA and he was referred to our institute where he was treated with a two-stage RTKA. Despite the distal gap at the osteotomy site the patient did not have pain at the osteotomy site at final followup.
The same rehabilitation protocol was used in both groups (Table 5).
Postoperative followups were performed by one of four observers (MLP, GMMM, GR, IH) not associated with the surgery (Table 5). Major procedure-related complications were defined as nonunion of the osteotomized fragment [69, 99, 103], progressive proximal displacement or migration of the osteotomized fragment greater than 5 mm [16, 19, 44, 69, 99, 103], avulsion fracture of the osteotomized fragment [16, 19], and fracture of the tibial metaphysis [88, 99].
Four independent observers with no clinical and surgical contact with the patients (MLP, GMMM, GR, IH) examined all postoperative radiographs. The lateral radiograph was used to assess healing (presence of bridging callus [16, 34, 47, 70]) of the osteotomy fragment and proximal displacement (greater than 5-mm gap between the distal end of the fragment and the tibia) [16]. The integrity of the wires and progressive radiolucencies between the prosthesis and the host bone also were assessed [90]. Interobserver correlation coefficient was 0.87.
Pain at the osteotomy site [106], delayed wound healing, and superficial skin necrosis [44, 88] not requiring surgical management were considered minor complications. Infection was considered to have recurred if at any evaluation patients had at least two of the following: (1) unexplained pain and discomfort with no implant malpositioning observed on radiographs; (2) CRP of 10 mg/L or greater without preexisting inflammatory joint disease [4, 75, 86]; (3) ESR of 30 mm/hour or greater without preexisting inflammatory joint disease [4, 75, 86]; (4) radiographic evidence of implant loosening, periosteal bone formation, and/or progressive nonfocal osteolysis without malpositioning [90]; or (5) a sinus tract or fistula communicating with the prosthesis.
Continuous variables (age, BMI, number of comorbidities, interval from TKA to prosthetic knee infection and from prosthetic knee infection to RTKA, ESR, C-reactive protein, number of débridement and cement spacer exchange procedures performed before RTKA, maximum knee flexion, KSS, followup time) were expressed as an arithmetic mean ± SD with minimum and maximum ranges. The Mann-Whitney U test also was used to compare difference in the KSS between the groups at final followup and to compare continuous variables between them (age, BMI, number of comorbidities, interval from TKA to prosthetic knee infection and from prosthetic knee infection to RTKA, ESR, CRP, number of débridement and cement spacer exchange procedures performed before RTKA, maximum knee flexion, followup time). Demographic differences were evaluated using the independent t-test and Fisher’s exact test. Fisher’s exact test and chi-square tests were used to check nominal variables (reinfection, major complications, stiffness requiring manipulation under anesthesia, and extension lag) between groups. Statistical analysis was performed using SPSS 16 (SPSS Inc, IBM, Armonk, NY, USA).
Results
Both groups showed clinical improvement; patients in the QS group had a lower (p = 0.017) mean KSS with respect to patients in the TTO group at the last followup: 70 ± 41 versus 88 ± 43, respectively (Table 6).
The incidence of general complications was similar in the two groups. Two patients in the QS group had pulmonary embolism and three (two in the QS group and one in the TTO group) had deep venous thrombosis (Table 6). All complications resolved with medical treatment. All patients with a TTO had radiographic evidence of callus formation on the lateral view (Fig. 5). No patients had symptomatic proximal migration or avulsion fracture of the fragment, metaphyseal tibial fractures, or breakage of the fixation wires (Table 7). Eleven patients reported pain on the tibial tubercle at the 6 months observation; eight underwent hardware removal and were free of pain 1 year after surgery. The remaining three patients declined hardware removal and continued to experience mild to moderate pain over the fixation wires. The remaining 28 patients in the TTO group were free of pain at the TTO site at the 6-month followup and remained free of pain at last observation. Three patients in the QS group (7%) and three in the TTO group (8%) underwent manipulation for persistent knee stiffness after the second followup 2 months after surgery (Table 6). All six patients gained greater than 90° ROM with no complications related to the procedure.
Mean postoperative maximum knee flexion was greater (p = 0.003) for the TTO group than the QS group (113° versus 94°, respectively). A greater percentage (p = 0.005) of patients in the QS group had a residual extension lag: 45% versus 13% (Table 6).
We observed no differences in the reinfection rate between the two groups at last followup (Table 4), and no patient had rupture of the extensor mechanism. Three patients (7%) in the QS group and two (5%) in the TTO group had recurrences of infection; they were treated with joint débridement and positioning of new antibiotic-loaded bone cement spacers. In all cases, the same surgical exposure that was used for the reimplantation procedure was used for prosthesis removal. After the infections resolved, two patients in the QS group and two in the TTO group underwent new reimplantations and remained free of infection at their last followup. The remaining patient in the QS group required three additional surgical débridements and antibiotic-loaded bone cement spacer exchange. This patient had delayed wound healing requiring a gastrocnemius flap, and he finally underwent a knee arthrodesis with a press-fit intramedullary nail [51]. The patient remained free of infection at the last observation (Table 6) (Fig. 5).
Discussion
Although 25% of all RTKAs performed are for infection [11] and the two-stage RTKA is the accepted standard for a prosthetic knee infection [56, 63], controversy exists regarding which surgical exposure provides superior clinical results with the lowest risk of extensor mechanism disruption and reinfection [79, 104]. One previous prospective study [6] of QS and TTO in patients who had RTKA found similar KSS clinical scores for both groups, better ROM in the QS group, and less extension lag in the TTO group; the authors reported no prosthetic knee infections. We therefore compared function, complications, and reinfection rates in chronic prosthetic knee infections treated with a two-stage RTKA using either a TTO or a QS.
Our study has several limitations. First, the equal rate of recurrent infection between groups could be a Type II error. To the best of our knowledge, in 1997 when the current study was designed, no prospective comparative study on reinfection rates in patients treated with a two-stage RTKA for a prosthetic knee infection was reported in literature. The a priori power analysis was based on the question whether a TTO or QS would result in a superior KSS [53] of at least 20 points in two-stage RTKAs for prosthetic knee infections. Two-stage RTKAs for prosthetic knee infections have reported rates of infection control between 80% and 96% [17, 39, 42, 45, 46, 50, 64, 68, 84, 100]. If a mean reinfection rate is 10% and a difference of 5% would be clinically meaningful, 450 patients per group would reach a power of at least 80% with a CI of 95%. Considering the 0.7% to 2% incidence of prosthetic knee infections [65, 66, 72, 78, 82], this would be unrealistic. Second, a comparison of all the surgical exposures described [97, 104], was not performed for same reason. Third, different fixation techniques of the tibial tubercle [6, 26, 94, 99, 101, 102, 106] were not performed, however, cadaveric studies have shown no differences in failure rate [14, 21, 22]. The current study can be compared with published studies on the basis of patient demographics (Table 8), clinical results (Table 9), and complications (Table 7).
Our patients in the TTO group had superior KSS, although 11 patients in the TTO group required hardware removal because of painful cerclage wires.
In contrast to previous reports [3, 6, 16, 69, 94, 99, 106], we observed no complications related to the TTO. We identified no extensor mechanism ruptures with either QS or TTO. Probably the most proximal cerclage wire through the tubercle fragment provides additional resistance against proximal migration of the fragment, while the two more distal cerclage wires reduce the stress riser effect of additional holes drilled through the tubercle.
A two-stage RTKA for a prosthetic knee infection requires an interim antibiotic-loaded bone cement spacer to be held in situ until the infection is resolved, with a potential for joint stiffness and extensor mechanism dysfunction. There were no differences in the manipulation rate between groups and patients who underwent manipulation gained a mean maximum knee flexion of 93° ± 14° (range, 88°–100°) without complications. Furthermore, the TTO group showed lower residual extension lags and higher knee flexion compared with the QS group with the same postoperative rehabilitation. Our findings suggest that TTO with early rehabilitation does not impair extensor mechanism function.
The reinfection rates of the two groups were similar. The two patients in the TTO group who had reinfections underwent new two-stage RTKAs with repeat TTOs and remained free of infection at last followup with no radiographic evidence of nonunion, a result consistent with previous reports [16, 69, 99]. Our study confirmed that repeat TTO in a RTKA does not impair bone healing potential [19, 99].
Our data suggest that TTO provides a superior KSS than QS in two-stage RTKAs in prosthetic knee infections, with comparable complications. The findings confirm those reported by Mendes et al. [69] that TTO is an efficacious alternative for surgical exposure in two-stage RTKAs for prosthetic knee infections regarding clinical results, healing potential of the osteotomized fragment, and complication rates.
References
Aglietti P, Baldini A, Buzzi R, Indelli PF. Patella resurfacing in total knee replacement: functional evaluation and complications. Knee Surg Sports Traumatol Arthrosc. 2001;9(suppl 1):S27–33.
Anderson JA, Sculco PK, Heitkemper S, Mayman DJ, Bostrom MP, Sculco TP. An articulating spacer to treat and mobilize patients with infected total knee arthroplasty. J Arthroplasty. 2009;24:631–635.
Arredondo J, Worland RL, Jessup DE. Nonunion after a tibial shaft fracture complicating tibial tubercule osteotomy. J Arthroplasty. 1998;13:958–960.
Austin MS, Ghanem E, Joshi A, Lindsay A, Parvizi J. A simple, cost-effective screening protocol to rule out periprosthetic infection. J Arthroplasty. 2008;23:65–68.
Barrack RL, Engh G, Rorabeck C, Sawhney J, Woolfrey M. Patient satisfaction and outcome after septic versus aseptic revision total knee arthroplasty. J Arthroplasty. 2000;15:990–993.
Barrack RL, Smith P, Munn B, Engh G, Rorabeck C. The Ranawat Award: Comparison of surgical approaches in total knee arthroplasty. Clin Orthop Relat Res. 1998;356:16–21.
Booth RE Jr, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:57–60.
Borden LS, Gearen PF. Infected total knee arthroplasty: a protocol for management. J Arthroplasty. 1987;2:27–36.
Bori G, Soriano A, García S, Gallart X, Mallofre C, Mensa J. Neutrophils in frozen section and type of microorganism isolated at the time of resection arthroplasty for the treatment of infection. Arch Orthop Trauma Surg. 2009;129:591–595.
Bourne RB, Crawford HA. Principles of revision total knee arthroplasty. Orthop Clin North Am. 1998;29:331–337.
Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51.
Buller LT, Sabry FY, Easton RW, Klika AK, Barsoum WK. The preoperative prediction of success following irrigation and debridement with polyethylene exchange for hip and knee prosthetic joint infections. J Arthroplasty. 2012;27:857–864.e1–4.
Cadambi A, Engh GA. Use of a semitendinosus tendon autogenous graft for rupture of the patellar ligament after total knee arthroplasty: a report of seven cases. J Bone Joint Surg Am. 1992;74:974–979.
Caldwell PE, Bohlen BA, Owen JR, Brown MH, Harris B, Wayne JS, Jiranek WA. Dynamic confirmation of fixation techniques of the tibial tubercle osteotomy. Clin Orthop Relat Res. 2004;424:173–179.
Calton TF, Fehring TK, Griffin WL. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin Orthop Relat Res. 1997;345:148–154.
Chalidis BE, Ries MD. Does repeat tibial tubercle osteotomy or intramedullary extension affect the union rate in revision total knee arthroplasty? A retrospective study of 74 patients. Acta Orthop. 2009;80:426–431.
Chiang ER, Su YP, Chen TH, Chiu FY, Chen WM. Comparison of articulating and static spacers regarding infection with resistant organisms in total knee arthroplasty. Acta Orthop. 2011;82:460–464.
Choi HR, Burke D, Malchau H, Kwon YM. Utility of tibial tubercle osteotomy in the setting of periprosthetic infection after total knee arthroplasty. Int Orthop. 2012;36:1609–1613.
Choi HR, Kwon YM, Burke DW, Rubash HE, Malchau H. The outcome of sequential repeated tibial tubercle osteotomy performed in 2-stage revision arthroplasty for infected total knee arthroplasty. J Arthroplasty. 2012;27:1487–1491.
Coonse GK, Adams JD. A new operative approach the knee joint. Surg Gynecol Obstet. 1943;77:344–347.
Davis K, Caldwell P, Wayne J, Jiranek WA. Mechanical comparison of fixation techniques for the tibial tubercle osteotomy. Clin Orthop Relat Res. 2000;380:241–249.
Deane CR, Ferran NA, Ghandour A, Morgan-Jones RL. Tibial tubercle osteotomy for access during revision knee arthroplasty: Ethibond suture repair technique. BMC Musculoskelet Disord. 2008;9:98.
Deburge A, Guepar. Guepar hinge prosthesis: complications and results with two years’ follow-up. Clin Orthop Relat Res. 1976;120:47–53.
Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77:761–766.
Dolin MG. Osteotomy of the tibial tubercle in total knee replacement: a technical note. J Bone Joint Surg Am. 1983;65:704–706.
Dolin MG. Osteotomy of the tibial tubercle during total knee replacement: a report of twenty-six cases. J Bone Joint Surg Am. 1990;72:790.
Emerson RH Jr, Head WC, Malinin TI. Extensor mechanism reconstruction with an allograft after total knee arthroplasty. Clin Orthop Relat Res. 1994;303:79–85.
Emerson RH Jr, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138.
Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46.
Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis: the Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16.
Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315–318.
Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–1813.
Freeman MG, Fehring TK, Odum SM, Fehring K, Griffin WL, Mason JB. Functional advantage of articulating versus static spacers in 2-stage revision for total knee arthroplasty infection. J Arthroplasty. 2007;22:1116–1121.
Frost HM. The biology of fracture healing: an overview for clinicians. Part I. Clin Orthop Relat Res. 1989;248:283–293.
Fukunaga K, Kobayashi A, Minoda Y, Iwaki H, Hashimoto Y, Takaoka K. The incidence of the patellar clunk syndrome in a recently designed mobile-bearing posteriorly stabilised total knee replacement. J Bone Joint Surg Br. 2009;91:463–468.
Garvin KL, Scuderi G, Insall JN. Evolution of the quadriceps snip. Clin Orthop Relat Res. 1995;321:131–137.
Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76:371–380.
Goldstein WM, Kopplin M, Wall R, Berland K. Temporary articulating methylmethacrylate antibiotic spacer (TAMMAS): a new method of intraoperative manufacturing of a custom articulating spacer. J Bone Joint Surg Am. 2001;83(suppl 2 pt 2):92–97.
Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res. 2011;469:985–993.
Gristina AG, Kolkin J. Current concepts review: total joint replacement and sepsis. J Bone Joint Surg Am. 1983;65:128–134.
Haasper C, Kendoff D, Gebauer M, Gehrke T, Klauser W. [Revision of unconstrained total knee arthroplasty: a technical analysis][in German]. Z Orthop Unfall. 2012;150:290–295.
Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements: prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812.
Haenle M, Skripitz C, Mittelmeier W, Skripitz R. Economic impact of infected total knee arthroplasty. ScientificWorldJournal. 2012;2012:198515.
Halder AM. [Tibial tubercle osteotomy][in German]. Oper Orthop Traumatol. 2012;24:85–94.
Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term follow-up of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39.
Hanssen AD, Trousdale RT, Osmon DR. Patient outcome with reinfection following reimplantation for the infected total knee arthroplasty. Clin Orthop Relat Res. 1995;321:55–67.
Heppenstall R. Fracture healing. In: Heppenstall RB, ed. Fracture Treatment and Healing. Philadelphia, PA: WB Saunders. 1980:35–64.
Hirschmann MT, Hoffmann M, Krause R, Jenabzadeh R-A, Arnold MP, Friederich NF. Anterolateral approach with tibial tubercle osteotomy versus standard medial approach for primary total knee arthroplasty: does it matter? BMC Musculoskelet Disord. 2010;11:167.
Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–131.
Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;321:45–54.
Iacono F, Bruni D, Lo Presti M, Raspugli G, Bondi A, Sharma B, Marcacci M. Knee arthrodesis with a press-fit modular intramedullary nail without bone-on-bone fusion after an infected revision TKA. Knee. 2012;19:555–559.
Insall JN. Surgical approaches to the knee. In Insall JN, Windsor RE, Scott WN, Kelley MA, Aglietti P. Surgery of the Knee. Ed 2, Vol 1. New York, NY: Churchill Livingstone; 1993;135–148.
Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14.
Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098.
Jämsen E, Huotari K, Huhtala H, Nevalainen J, Konttinen YT. Low rate of infected knee replacements in a nationwide series: is it an underestimate? Acta Orthop. 2009;80:205–212.
Jämsen E, Stogiannidis I, Malmivaara A, Pajamäki J, Puolakka T, Konttinen YT. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77.
Jones RE, Huo MH. The infected knee: all my troubles now. J Arthroplasty. 2006;21(4 Suppl 1):50–53.
Julin J, Jämsen E, Puolakka T, Konttinen YT, Moilanen T. Younger age increases the risk of early prosthesis failure following primary total knee replacement for osteoarthritis: a follow-up study of 32,019 total knee replacements in the Finnish Arthroplasty Register. Acta Orthop. 2010;81:413–419.
Keats AS. The ASA classification of physical status: a recapitulation. Anesthesiology. 1978;49:233–236.
Kelly MA, Clarke HD. Stiffness and ankylosis in primary total knee arthroplasty. Clin Orthop Relat Res. 2003;416:68–73.
Klett R, Kordelle J, Stahl U, Khalisi A, Puille M, Steiner D, Bauer R. Immunoscintigraphy of septic loosening of knee endoprosthesis: a retrospective evaluation of the antigranulocyte antibody BW 250/183. Eur J Nucl Med Mol Imaging. 2003;30:1463–1466.
Leopold SS, Greidanus N, Paprosky WG, Berger RA, Rosenberg AG. High rate of failure of allograft reconstruction of the extensor mechanism after total knee arthroplasty. J Bone Joint Surg Am. 1999;81:1574–1579.
Lombardi AV Jr, Karnes JM, Berend KR. A motion maintaining antibiotic delivery system. J Arthroplasty. 2007;22(4 suppl 1):50–55.
Macheras GA, Kateros K, Galanakos SP, Koutsostathis SD, Kontou E, Papadakis SA. The long-term results of a two-stage protocol for revision of an infected total knee replacement. J Bone Joint Surg Br. 2011;93:1487–1492.
Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;46:471–478.
Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Osmon DR. Prosthetic joint infection diagnosed postoperatively by intraoperative culture. Clin Orthop Relat Res. 2005;439:38–42.
Maruyama M. Tibial tubercle osteotomy in revision total knee arthroplasty. Arch Orthop Trauma Surg. 1997;116:400–403.
Meek RM, Masri BA, Dunlop D, Garbuz DS, Greidanus NV, McGraw R, Duncan CP. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the PROSTALAC articulating spacer. J Bone Joint Surg Am. 2003;85:1888–1892.
Mendes MW, Caldwell P, Jiranek WA. The results of tibial tubercle osteotomy for revision total knee arthroplasty. J Arthroplasty. 2004;19:167–174.
Morgan J, Leighton R. Radiographic appearance of fracture healing. In: Morgan J, Leighton R, eds. Radiology of Small Animal Fracture Management. Philadelphia, PA: WB Saunders; 1995:34–43.
Morrey BF, Westholm F, Schoifet S, Rand JA, Bryan RS. Long-term results of various treatment options for infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:120–128.
Mortazavi SM, Molligan J, Austin MS, Purtill JJ, Hozack WJ, Parvizi J. Failure following revision total knee arthroplasty: infection is the major cause. Int Orthop. 2011;35:1157–1164.
Nazarian DG, Booth RE Jr. Extensor mechanism allografts in total knee arthroplasty. Clin Orthop Relat Res. 1999;367:123–129.
Ogata K, Ishinishi T, Hara M. Evaluation of patellar retinacular tension during total knee arthroplasty: special emphasis on lateral retinacular release. J Arthroplasty. 1997;12:651–656.
Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RS, Barrack RL. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res. 2008;466:2628–2633.
Pedersen AB, Mehnert F, Odgaard A, Schrøder HM. Existing data sources for clinical epidemiology: The Danish Knee Arthroplasty Register. Clin Epidemiol. 2012;4:125–135.
Petty W, Bryan RS, Coventry MB, Peterson LF. Infection after total knee arthroplasty. Orthop Clin North Am. 1975;6:1005–1014.
Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715.
Rand JA, Morrey BF, Bryan RS. Patellar tendon rupture after total knee arthroplasty. Clin Orthop Relat Res. 1989;244:233–238.
Ries MD, Richman JA. Extended tibial tubercle osteotomy in total knee arthroplasty. J Arthroplasty. 1996;11:964–967.
Ritter MA, Carr K, Keating EM, Faris PM, Meding JB. Tibial shaft fracture following tibial tubercle osteotomy. J Arthroplasty. 1996;11:117–119.
Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53.
Scott RD, Siliski JM. The use of a modified V-Y quadricepsplasty during total knee replacement to gain exposure and improve flexion in the ankylosed knee. Orthopedics. 1985;8:45–48.
Singer J, Merz A, Frommelt L, Fink B. High rate of infection control with one-stage revision of septic knee prostheses excluding MRSA and MRSE. Clin Orthop Relat Res. 2012;470:1461–1471.
Smith PN, Parker DA, Gelinas J, Rorabeck CH, Bourne RB. Radiographic changes in the patella following quadriceps turndown for revision total knee arthroplasty. J Arthroplasty. 2004;19:714–719.
Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683.
Stern SH, Insall JN. Posterior stabilized prosthesis: results after follow-up of nine to twelve years. J Bone Joint Surg Am. 1992;74:980–986.
Tabutin J, Morin-Salvo N, Torga-Spak R, Cambas PM, Vogt F. Tibial tubercule osteotomy during medial approach to difficult knee arthroplasties. Orthop Traumatol Surg Res. 2011;97:276–286.
Thabe H, Schill S. Two-stage reimplantation with an application spacer and combined with delivery of antibiotics in the management of prosthetic joint infection. Oper Orthop Traumatol. 2007;19:78–100.
Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88:149–155.
Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562.
Trousdale RT, Hanssen AD, Rand JA, Cahalan TD. V-Y quadricepsplasty in total knee arthroplasty. Clin Orthop Relat Res. 1993;286:48–55.
Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523.
van den Broek CM, van Hellemondt GG, Jacobs WC, Wymenga AB. Step-cut tibial tubercle osteotomy for access in revision total knee replacement. Knee. 2006;13:430–434.
Vanhegan IS, Morgan-Jones R, Barrett DS, Haddad FS. Developing a strategy to treat established infection in total knee replacement: a review of the latest evidence and clinical practice. J Bone Joint Surg Br. 2012;94:875–881.
Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: Long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop Relat Res. 2006;452:28–34.
Wall SJ, Rose DM, Khanuja HS, Sutter EG, Knight TA, Belkoff SM, Mears SC. Biomechanical comparison of extensile exposures in total knee arthroplasty. Orthopedics. 2010;33:387.
Wentworth SJ, Masri BA, Duncan CP, Southworth CB. Hip prosthesis of antibiotic-loaded acrylic cement for the treatment of infections following total hip arthroplasty. J Bone Joint Surg Am. 2002; 84(suppl 2):123–128.
Whiteside LA. Exposure in difficult total knee arthroplasty using tibial tubercle osteotomy. Clin Orthop Relat Res. 1995;321:32–35.
Whiteside LA, Nayfeh TA, LaZear R, Roy ME. Reinfected revised TKA resolves with an aggressive protocol and antibiotic infusion. Clin Orthop Relat Res.2012;470:236–243.
Whiteside LA, Ohl MD. Tibial tubercle osteotomy for exposure of the difficult total knee arthroplasty. Clin Orthop Relat Res.1990;260:6–9.
Wolff AM, Hungerford DS, Krackow KA, Jacobs MA. Osteotomy of the tibial tubercle during total knee replacement: a report of twenty-six cases. J Bone Joint Surg Am. 1989;71:848–852.
Young CF, Bourne RB, Rorabeck CH. Tibial tubercle osteotomy in total knee arthroplasty surgery. J Arthroplasty. 2008;23:371–375.
Younger AS, Duncan CP, Masri BA. Surgical exposures in revision total knee arthroplasty. J Am Acad Orthop Surg. 1998;6:55–64.
Zanotti RM, Freiberg AA, Matthews LS. Use of patellar allograft to reconstruct a patellar tendon-deficient knee after total joint arthroplasty. J Arthroplasty. 1995;10:271–274.
Zonnenberg CB, Lisowski LA, van den Bekerom MP, Nolte PA. Tuberositas osteotomy for total knee arthroplasty: a review of the literature. J Knee Surg. 2010;23:121–129.
Acknowledgments
We thank Eleonora Zamparini MD, specialist in infective diseases, for help in providing the most adequate antibiothic therapy to all patients involved in the study, and Mirco Lo Presti MD, Giulio Maria Marcheggiani Muccioli MD, Giovanni Raspugli MD, and Ibrahim Hakkawi MD, who blindly performed clinical evaluations and radiographic measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Rizzoli Orthopaedic Institute, Bologna University, Bologna, Italy.
About this article
Cite this article
Bruni, D., Iacono, F., Sharma, B. et al. Tibial Tubercle Osteotomy or Quadriceps Snip in Two-stage Revision for Prosthetic Knee Infection? A Randomized Prospective Study. Clin Orthop Relat Res 471, 1305–1318 (2013). https://doi.org/10.1007/s11999-012-2763-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-012-2763-z