Abstract
Background
Management of pelvic ring injuries using minimally invasive techniques may be desirable if reduction and stability can be achieved. We present a new technique, the anterior pelvic bridge, which is a percutaneous method of fixing the anterior pelvis through limited incisions over the iliac crest(s) and pubic symphysis.
Description of Technique
An incision is made over each anterior iliac crest and a 6- to 8-cm incision is centered over the symphysis. Either a locking reconstruction plate or a spinal rod is placed through a subcutaneous tunnel overlying the external oblique fascia in the subcutaneous tissue, and fixation into the iliac crest and pubis is achieved to effect stability.
Methods
A randomized controlled trial comparing anterior pelvic external fixation (APEF) versus anterior pelvic internal fixation (APIF) for unstable pelvic ring injuries was begun in October 2010. Patients with unstable pelvic ring injuries were enrolled and followed with respect to fracture reduction, surgical pain, complications, and functional outcome scores.
Results
As of January 2012, 23 patients met inclusion; however, 12 patients refused participation because of the possibility of external fixation, leaving 11 patients (four male, seven female) enrolled. At 6-month followup, there was a single pin tract infection in the APEF cohort and no complications or pain in the APIF cohort.
Conclusions
This clinical experience lends support to the use of a new minimally invasive technique to stabilize the anterior pelvis, particularly given the resistance on the part of patients to consider external fixation.
Level of Evidence
Level II, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There have been recent descriptions of treating pelvic ring injuries using minimally invasive techniques [2, 6, 13, 31]. External fixation methods for pelvic ring injuries are well described [5, 10, 15, 19, 23, 25, 30]. Anterior ring external fixation has been described as a way to complement posterior ring fixation [11, 20, 27]. In these cases, the surgeon desires complimentary fixation to render greater stability to the pelvic ring.
For unstable pelvic ring injuries, Goldstein et al. [7] prioritized reduction and fixation of the posterior pelvic lesion(s) followed by a decision as to whether to enhance fixation of the ring with some anterior construct. If the posterior pelvic structures are intact, most would advocate only anterior fixation [18]. Anterior fixation may be achieved by open reduction and internal fixation (ORIF) [1], percutaneous transramus fixation [23, 28], or external fixation [8, 10].
Cole et al. [2, 16] recently described a new method in which subcutaneous plates are placed through small incisions over the ilium and fixed to the contralateral ilium or pubic symphysis or both. We termed these subcutaneous techniques of anterior pelvic internal fixation (APIF) “the anterior pelvic bridge.” Our early experience was reported in a retrospective comparative cohort investigation studying differences between the APIF technique and anterior pelvic external fixation (APEF) with anterior superior iliac spine (ASIS) pins for unstable pelvic ring injuries or plate placement in the case of APIF. In that study, 48 patients (24/group) with comparable demographic data and fracture patterns were reviewed for wound infections, ability to maintain fracture reduction, surgical site pain, and other complications [2]. One patient had a complication in the APIF group versus nine in the APEF group. Complications in the APEF group included pin site infection (n = 6) and loss of fixation from Schanz pin loosening (n = 4). Four of the pin site infections were superficial and resolved with oral antibiotics, while the other two required hospitalization and parenteral antibiotics. No further surgery was required. The findings of that pilot study seemed compelling, and coupled with the subjective reports by surgeons of greater patient satisfaction, a prospective randomized controlled trial comparing the two techniques was begun in October 2010.
Our purposes here are to describe this new technique and its application and our early clinical experience with the randomized controlled trial.
Surgical Technique
The following techniques represent two different variations of the pelvic bridge, one using locking reconstruction plates and screws (Synthes USA, Paoli, PA) and the other using an occipitocervical fusion plate-rod hybrid implant (Synthes USA) with pedicle screws for fixation. The indication for this anterior pelvic bridge method is simply an unstable pelvic ring for which a surgeon desires anterior pelvic stability. This technique may be used as an option to any other form of anterior pelvic fixation (external fixation, percutaneous ramus screws, ORIF). The method requires adequate posterior ring stability. It can be used for patients who have residual instability anteriorly after the posterior pelvis has either been fixed or verified to be stable as assessed by stressing the pelvis intraoperatively under fluoroscopy [26]. In patients who have a pure symphyseal lesion, we recommend formal ORIF of the pubic symphysis and not the use of the anterior pelvic bridge because ORIF of the pubic symphysis allows for a single incision at the midline, an anatomic reduction of the pubis, and greater stability with less implant. The contraindications are (1) severe soft tissue issue precluding anterior pelvic incisions, including prior urologic or general surgery wounds or wounds from trauma; and (2) injuries requiring rapid stabilization in a hemodynamically unstable patient.
After we established the posterior pelvis was stable or the posterior tension band was intact, the patient was positioned supine on an imaging table for preparing and draping. One method was to use 3.5-mm locking reconstruction plates that were percutaneously applied and spanned the iliac crest of the injured side to the contralateral pubic tubercle. The plates used had 14, 16, or 18 holes depending on the patient size and location of the lesions. Precontouring of the plates was performed preoperatively on a Sawbones® pelvis (Pacific Research Laboratories Inc, Vashon, WA, USA) so that there was a span of at least two holes over the anterior crest and four holes over the pubic symphysis (Fig. 1). Unilateral or bilateral application of the plates was performed depending on the presence of unilateral or bilateral anterior pelvic lesions. If bilateral plates were implanted, a plate was fixed to each side of the midline, overlapping the length of the pubic tubercles (usually two to three holes past midline) at the pubic symphysis. In the case of unilateral plate application, we made a 3-cm incision from the ASIS posterior toward the gluteus tubercle and a separate 6- to 8-cm incision centered over the symphysis pubis (Fig. 2). A subcutaneous tunnel was created between the two incisions, superficial to the external oblique fascia, inguinal ligament, and rectus sheath. The contoured reconstruction plate was then passed (Fig. 2, inset; Fig. 3) and aligned so that the plate spanned from the anterior iliac crest to the contralateral pubic tubercle. Care was taken to contour the plate slightly anteriorly over the inguinal region. First, the plate was loosely attached to the iliac crest approximately 2 cm posterior to the ASIS with a 3.5-mm cortical screw. If necessary, Schanz pins in either one or both ilia can be used to effect reduction. Often however, the reduction has been achieved by virtue of the priority to reduce and fix the posterior ring first anatomically. The 3.5-mm cortical screws were then placed to approximate the plate to the bone. One or two 3.5-mm locking screws were then placed in the iliac crest and into the pubic tubercles (Fig. 4). In the case of bilateral plate application, a second contoured reconstruction plate was loosely applied from the opposite iliac crest to the contralateral pubic tubercle, overlapping in the symphyseal region with the first plate. A 3.5-mm cortical screw was then placed through one of the overlapping holes into the pubic tubercles. After the cortical screws were tightened, the construct was completed by placing 3.5-mm locking screws into the remaining parasymphyseal screw holes (Fig. 5). A C-arm was used during this procedure to evaluate and establish the reduction of the pelvis and was also helpful in verifying plate placement and screw vectors, particularly over the parasymphyseal area, though it was less necessary in experienced hands.
Skin incisions are demonstrated for the anterior pelvic bridge technique using locking reconstruction plates. Three incisions are required to place bilateral reconstruction plates: one incision over each ASIS and a transverse Pfannenstiel incision centered over the symphysis pubis. Important neurovascular structures are shown, including the LFCN coursing inferiorly to the inguinal ligament and the femoral nerve overlying the psoas muscle. Insertion of the precontoured reconstruction plates can be performed by hand after carefully creating a subcutaneous tunnel above the external oblique muscle with a cob or periosteal surfer (inset). The plate slides over the area of the conjoint tendon, well anterior to the posterior neurovascular structures. The posterior pelvic ring must be either stable or stabilized as the primary consideration of treatment for pelvic ring injuries. Reprinted with permission and copyright 2012 by Wolters Kluwer Health from Cole PA, Gauger EM, Anavian J, Ly TV, Morgan RA, Heddings AA. Anterior Pelvic External Fixator Versus Subcutaneous Internal Fixator in the Treatment of Anterior Ring Pelvic Fractures. J Orthop Trauma. 2012 February 21 [Epub ahead of print].
An APIF reconstruction plate is shown in relation to surrounding anatomic structures. The spermatic cord and round ligament (not shown) are visualized directly through the Pfannenstiel incision and can be easily avoided during implant placement. m = muscle; n = nerve. Reprinted with permission and copyright 2012 by Wolters Kluwer Health from Cole PA, Gauger EM, Anavian J, Ly TV, Morgan RA, Heddings AA. Anterior Pelvic External Fixator Versus Subcutaneous Internal Fixator in the Treatment of Anterior Ring Pelvic Fractures. J Orthop Trauma. 2012 February 21 [Epub ahead of print].
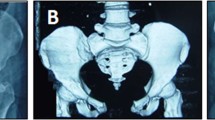
(A) Preoperative AP and postoperative (B) AP, (C) inlet, and (D) outlet pelvic radiographs show placement of bilateral S1 and S2 iliosacral screws and the anterior pelvic bridge. Two 16-hole reconstruction plates were precontoured on a Sawbones® pelvic model before surgery. Four holes overlapped in the midline for additional stability.
The second method of applying this technique was similar in that fixation spanned from the iliac crest of the injured side to the contralateral pubic tubercle and also could be applied unilaterally or bilaterally. However, instead of using a pelvic reconstruction plate, the construct included an occipitocervical spinal implant called a plate-rod, which is a four-hole titanium plate that transitions to a 4.0-mm rod (Fig. 6). In the case of unilateral plate-rod application, a 3-cm incision was made over the anterior iliac crest ending at the ASIS and a separate 6- to 8-cm incision was centered over the symphysis pubis. A subcutaneous tunnel was created between the two incisions, superficial to the external oblique fascia, inguinal ligament, and rectus sheath just as in the first method. The plate-rod was passed through the subcutaneous tunnel, secured to the iliac crest with screws, and anchored to a 4.5-mm polyaxial pedicle screw placed in the contralateral pubic tubercle down into the inferior ramus. If bilateral plate-rod application was used, a plate-rod was applied to each side, overlapping in the middle at the level of the symphysis. The two rods were then connected with one or more crossconnector joints if the surgeon desired (Fig. 7). Any excess rod length on either side of the midline was cut in situ with a rod cutter as necessary. At least two screws were placed into the anterior column down the crest. The rod segment of the implant spanned across the parasymphyseal region for alignment of pedicle screws as desired.
A bilateral plate-rod fixator is demonstrated on an oblique view of a Sawbones® pelvic model. The four-hole plate over the iliac crest transitions to a 4.0-mm rod that is contoured to span across the symphysis pubis. Cortical screws are used to anchor the plate to the iliac crest. 4.5-mm polyaxial pedicle screws are inserted in the pubic tubercles and are placed slightly offset from each other in the anterior-posterior plane to prevent interference with each other and to allow for connection. A crossconnecting joint is applied that further increases the stability of the construct (inset).
Preoperative (A) inlet and (B) outlet radiographs illustrate initial pelvic ring injury with left superior and inferior rami fractures and a concomitant pubic root fracture. Postoperative (C) inlet and (D) outlet pelvic radiographs show left iliosacral screw fixation and bilateral plate-rod pelvic bridge fixation with crossconnector at the midline and pedicle screws into the parasymphyseal region. Though the left-sided parasymphyseal pedicle screw appears to go into fracture, the surgeon drilled, measured, and concluded worthwhile fixation was achieved.
Postoperatively, the patients remained touchdown weightbearing for 6 weeks after their procedure during which there were no restrictions to hip motion. At 6 weeks, patients were assessed for fracture consolidation and generally allowed to weightbear. The hardware was removed at approximately 6 to 12 weeks postsurgery, utilizing the same incisions for implantation. While both implants are feasible for placement, we have found hardware removal is easier with the rods, given that scar tissue grows through the plate holes. However, the occipitocervical plate rod is more expensive, and therefore now this decision is up to surgeon preference. Removal of implants is our current practice given the lack of long-term outcomes after this surgery. Implants could become difficult to remove or may irritate or compress soft tissues for example. After hardware removal, patients were allowed full weightbearing as tolerated.
Patients and Methods
In October 2010, we established a randomized, controlled trial comparing the two fixation methods. Inclusion criteria for the study were unstable pelvic ring injuries, in which a surgeon desired anterior pelvic fixation, in patients older than 17 years. Patients were excluded if they (1) had substantial soft tissue injury precluding safe anterior pelvic incisions, (2) had pure symphysis disruption, or (3) or were pregnant. After eligible patients agreed to enroll in the study, an envelope containing a randomly assigned fixation method was opened by the surgeon. As of January 2012, 23 patients were eligible for the study. Twelve patients were not enrolled for the following reasons: eight patients refused external fixation and insisted on internal fixation despite substantial exhortation to participate in the trial based on the gold standard track record of external fixation; two patients underwent subsequent spinal surgery for burst fractures with planned prone positioning, and thus the surgeon deemed external fixation to be inferior; two patients simply failed to be randomized properly; and three were excluded due to severe degloving or bladder injuries requiring surgical incisions. The eight patients who refused randomization expressed they did not want external fixation because of its obtrusiveness and visibility and a perception of increased discomfort. Of the 11 patients who have been successfully enrolled in the trial, five were randomized to APEF and six to APIF. Two of the six randomized to the APIF group were labeled as intention to treat because after posterior fixation the surgeon assessed pelvic stability fluoroscopically and deemed anterior surgical fixation unnecessary. The mean followup was 5.1 months (range, 6–54 weeks).
We analyzed the AP, inlet, and outlet radiographs for displacement and had the patients complete two surveys: an SF-36v2 and a questionnaire assessing pain, numbness, sexual dysfunction, and activity at the standard clinical followup visits (2 weeks, 6 weeks, 12 weeks, 6 months, 1 year). We also tracked patients for the following complications: nonunion, malreduction, implant failure, wound infection, surgical site pain, deep pelvic pain, difficulty voiding, sexual dysfunction, need for revision surgery, delayed ambulation, deep vein thrombosis, and lateral femoral cutaneous nerve (LFCN) or perineal dysesthesia.
For our power calculation, we made three assumptions: (1) APIF would have less associated morbidity than APEF, (2) the chance of APIF outperforming APEF for each of the listed categories of complication was roughly constant across each type, and (3) the complications we investigated comprised the vast majority of negative outcomes for this condition. These assumptions justified the use of a one-sided exact binomial test. We used simulation (performed in the software R, R Foundation for Statistical Computing, Vienna, Austria [21]) to determine the chance of correctly rejecting the null under various alternative hypotheses. With an alpha of 5% and predicting 20% loss to followup, we estimated 86 subjects would yield 80% power to detect a 10-percentage point difference across all types of complications.
Results
One of five patients in the APEF group returned to the operating room postoperatively for a pin site infection that was recalcitrant to oral antibiotics and local pin care. No other infections have occurred in the APEF cohort. Of the seven patients who have completed 6 months of followup, three of the APEF group reported surgical site pain. There have been no complications or complaints of anterior surgical site pain thus far in the APIF group at the 6-month followup visit. No further analysis of functional outcome or patient satisfaction results in the early phase of this trial can be performed at this time due to inadequate numbers and followup.
Discussion
Potential benefits of minimally invasive anterior surgical pelvic fixation may include reduced blood loss, soft tissue complications, and infection, as well as faster rehabilitation of the patient with better pain control. Our purposes were to describe a new form of minimally invasive pelvic fixation and to give a preliminary report of a randomized controlled trial.
Our trial is only in its initial stages but provides a good basis for context of this technique paper. Because of a limited number of patients enrolled and minimal followup, we only reported early complications and patient-reported pain. Second, many patients’ perception and dislike of external pelvic fixators became apparent through the beginning of this trial. Despite investigators motivated to enhance study enrollment and in the context of an ethical discussion with patients about options, eight patients refused the study due to refusal of external fixation. It is conceivable, if not likely, this type of patient self-selection will create a bias in the study results, but it might stand to reason this bias would eliminate patients potentially most adverse to external fixation outcomes, at least from the point of view of patient satisfaction and perhaps pain.
Limits to the anterior pelvic bridge technique are inherent as well. Due to the lack of direct visualization, some anatomic structures are theoretically at risk for injury during hardware placement, including the LFCN, femoral artery, femoral vein, femoral nerve, genitofemoral nerve, ilioinguinal nerve, iliohypogastric nerve, and the round ligament in females or the spermatic cord in males. However, a cadaveric study on the proximity of these structures to a subcutaneously placed APIF found no risk to these anatomic structures in this technique (Fig. 3) [16]. Other possible unstudied disadvantages could include implant costs or increased surgical operating room time.
However, there are several potential advantages regarding the use of an anterior pelvic bridge compared to external fixation. First, the hardware is placed entirely under the skin; therefore, the surgical wounds are closed primarily. Second, there is no or minimal hardware prominence, in contradistinction to an external fixator, and fewer difficulties with clothing wear, sitting in a chair, sexual intercourse, skin impingement, and surgical site pain [13, 31]. Other drawbacks to external fixation include pin tract infections, fixator loosening, limited surgical access to the abdomen, and reoperations [14, 15, 17, 23, 24, 30].
Pelvic external fixation has been used to rapidly stabilize an unstable pelvic ring injury and decrease pelvic volume in an attempt to provide hemostasis in the setting of pelvic hemorrhage [8, 22, 29]. In this context, it is applied for provisional stability with the goal of decreasing pelvic volume. In these circumstances, we have used a pelvic external fixator for the early postinjury provisional phase and then converted to the use of a pelvic bridge when rendering definitive fixation.
Subcutaneous plating and spinal rod methods for stabilizing the posterior pelvic ring injury have been studied and reported as reasonable alternatives for posterior fixation of unstable pelvic ring injuries [3, 12]. Another percutaneous, subcutaneous method of anterior pelvic fixation was described by Kuttner et al. [13] in a study utilizing a pedicle screw and spinal rod technique. In that study, they retrospectively reviewed a series of 22 patients treated with their “subcutaneous cross-over internal fixator.” Nineteen patients were available for postoperative followup at an average of 2.5 years. They reported excellent/good, moderate, and poor results in 31.6%, 63.2%, and 5.3% of their patients, respectively. Their complications included one wound infection, one loosening of a pedicle screw, and seven (36.8%) transient LFCN palsies. The LFCN palsy likely occurred during placement or removal of hardware in the supraacetabular region where the nerve is at greater risk for injury [4, 5, 9]. Two studies from the United States using the same technique as Kuttner et al. [13] were recently published using spinal rods and pedicle screws placed in the anterior inferior iliac spines (AIIS) [6, 31]. Both reported two patients with neuropraxias and complications with the subcutaneous fixator causing discomfort at the abdominal crease. The placement of pedicle screws at the AIIS requires incisions directly over the AIIS with pedicle screws placed in a high-risk zone for the LFCN [4, 5, 9, 13].
The pelvic bridge technique described herein has other potential advantages over this method, which renders fixation only to each ilium at the AIIS via pedicle screws and a single rod. For example, it is mechanically advantageous to add fixation to the pubic symphysis in the midline, particularly when gaining purchase into the side of an intact hemipelvis (contralateral tubercle). Additionally, a surgeon can stay on the same side of a unilateral injury, such as a sacral fracture with ipsilateral ramus fractures, thus not dissecting or disturbing an uninvolved side of the pelvis.
In conclusion, the anterior pelvic bridge is a novel method of fixation for the anterior pelvis to be used in the treatment armamentarium of unstable pelvic ring injuries. The pelvic bridge does not fall into the category of ORIF or external fixation but rather into a new genre of minimally invasive fixation techniques for the anterior pelvis. The goal of this method is to provide a surgical option that is less insulting to the patient, is associated with fewer complications, and adequately addresses stability in a way that complements the primary posterior pelvic ring stability. Early clinical reports suggest advantages that include fewer wound complications, less surgical site pain, and unobstructed access to the abdomen for any non-pelvic-related interventions.
References
Barei DP, Shafer BL, Beingessner DM, Gardner MJ, Nork SE, Routt ML. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68:949–953.
Cole PA, Gauger EM, Anavian J, Ly TV, Morgan RA, Heddings AA. Anterior Pelvic External Fixator Versus Subcutaneous Internal Fixator in the Treatment of Anterior Ring Pelvic Fractures. J Orthop Trauma. 2012 February 21 [Epub ahead of print].
Dienstknecht T, Berner A, Lenich A, Nerlich M, Fuechtmeier B. A minimally invasive stabilizing system for dorsal pelvic ring injuries. Clin Orthop Relat Res. 2011;469:3209–3217.
Doklamyai P, Agthong S, Chentanez V, Huanmanop T, Amarase C, Surunchupakorn P, Yotnuengnit P. Anatomy of the lateral femoral cutaneous nerve related to inguinal ligament, adjacent bony landmarks, and femoral artery. Clin Anat. 2008;21:769–774.
Gansslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27(suppl 1):SA13–SA20.
Gardner MJ, Mehta S, Mirza A, Ricci WM. Anterior Pelvic Reduction and Fixation Using a Subcutaneous Internal Fixator. J Orthop Trauma. 2011 October 28 [Epub ahead of print].
Goldstein A, Phillips T, Sclafani SJ, Scalea T, Duncan A, Goldstein J, Panetta T, Shaftan G. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26:325–333.
Gylling SF, Ward RE, Holcroft JW, Bray TJ, Chapman MW. Immediate external fixation of unstable pelvic fractures. Am J Surg. 1985;150:721–724.
Haidukewych GJ, Kumar S, Prpa B. Placement of half-pins for supra-acetabular external fixation: an anatomic study. Clin Orthop Relat Res. 2003;411:269–273.
Kellam JF. The role of external fixation in pelvic disruptions. Clin Orthop Relat Res. 1989;241:66–82.
Kim WY, Hearn TC, Seleem O, Mahalingam E, Stephen D, Tile M. Effect of pin location on stability of pelvic external fixation. Clin Orthop Relat Res. 1999;361:237–244.
Korovessis P, Stamatakis M, Baikousis A. Posterior stabilization of unstable sacroiliac injuries with the Texas Scottish Rite Hospital spinal instrumentation. Orthopedics. 2000;23:323–327.
Kuttner M, Klaiber A, Lorenz T, Fuchtmeier B, Neugebauer R. [The pelvic subcutaneous cross-over internal fixator] [in German]. Unfallchirurg. 2009;112:661–669.
Lindahl J, Hirvensalo E, Bostman O, Santavirta S. Failure of reduction with an external fixator in the management of injuries of the pelvic ring: long-term evaluation of 110 patients. J Bone Joint Surg Br. 1999;81:955–962.
Mason WT, Khan SN, James CL, Chesser TJ, Ward AJ. Complications of temporary and definitive external fixation of pelvic ring injuries. Injury. 2005;36:599–604.
Moazzam C, Heddings A, Moodie P, Cole P. Anterior Pelvic Subcutaneous Internal Fixator Application: An Anatomic Study. J Orthop Trauma. 2012 February 14 [Epub ahead of print].
Palmer S, Fairbank AC, Bircher M. Surgical complications and implications of external fixation of pelvic fractures. Injury. 1997;28:649–653.
Papakostidis C, Kanakaris NK, Kontakis G, Giannoudis PV. Pelvic ring disruptions: treatment modalities and analysis of outcomes. Int Orthop. 2009;33:329–338.
Pohlemann T, Bosch U, Gansslen A, Tscherne H. The Hannover experience in management of pelvic fractures. Clin Orthop Relat Res. 1994;305:69–80.
Ponsen KJ, Joosse P, Van Dijke GA, Snijders CJ. External fixation of the pelvic ring: an experimental study on the role of pin diameter, pin position, and parasymphyseal fixator pins. Acta Orthop. 2007;78:648–653.
R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009.
Riemer BL, Butterfield SL, Diamond DL, Young JC, Raves JJ, Cottington E, Kislan K. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35:671–675; discussion 676–677.
Routt ML Jr, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop Relat Res. 2000;375:15–29.
Routt ML Jr, Simonian PT. Internal fixation of pelvic ring disruptions. Injury. 1996;27(suppl 2):B20–B30.
Routt ML Jr, Simonian PT, Swiontkowski MF. Stabilization of pelvic ring disruptions. Orthop Clin North Am. 1997;28:369–388.
Sagi HC, Coniglione FM, Stanford JH. Examination under anesthetic for occult pelvic ring instability. J Orthop Trauma. 2011;25:529–536.
Simonian PT, Routt ML Jr. Biomechanics of pelvic fixation. Orthop Clin North Am. 1997;28:351–367.
Starr AJ, Nakatani T, Reinert CM, Cederberg K. Superior pubic ramus fractures fixed with percutaneous screws: what predicts fixation failure? J Orthop Trauma. 2008;22:81–87.
Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br. 1988;70:1–12.
Tucker MC, Nork SE, Simonian PT, Routt ML Jr. Simple anterior pelvic external fixation. J Trauma. 2000;49:989–994.
Vaidya R, Colen R, Vigdorchik J, Tonnos F, Sethi A. Treatment of unstable pelvic ring injuries with an internal anterior fixator and posterior fixation: Initial clinical series. J Orthop Trauma. 2012;26:1–8.
Acknowledgments
The authors thank Aaron R. Jacobson DC for help with preparation of the manuscript and useful comments
Author information
Authors and Affiliations
Corresponding author
Additional information
The institution of one or more of the authors (TGH, BWH, PAC) has received, in any 1 year, funding for unrelated research from Synthes USA (Paoli, PA, USA) and Zimmer Inc (Warsaw, IN, USA). Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Hiesterman, T.G., Hill, B.W. & Cole, P.A. Surgical Technique: A Percutaneous Method of Subcutaneous Fixation for the Anterior Pelvic Ring: The Pelvic Bridge. Clin Orthop Relat Res 470, 2116–2123 (2012). https://doi.org/10.1007/s11999-012-2341-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-012-2341-4