Abstract
Background
Thrombin formation commences perioperatively in orthopaedic surgery and therefore some surgeons prefer preoperative initiation of pharmacologic thromboprophylaxis. However, because of the potential for increased surgical bleeding, the postoperative initiation of thromboprophylaxis has been advocated to reduce blood loss, need for transfusion, and bleeding complications. Trials on timing of thromboprophylaxis have been designed primarily to detect thrombotic events, and it has been difficult to interpret the magnitude of blood loss and bleeding events owing to lack of information for bleeding volume and underpowered bleeding end points.
Questions/purposes
We therefore asked whether there are differences in blood loss, transfusion requirements, and other postoperative clinical complications with preoperative versus postoperative start of thromboprophylaxis with dalteparin.
Methods
In a double-blind, randomized controlled trial, 80 patients undergoing primary cemented THA were allocated to dalteparin injections starting 12 hours before or 6 hours after surgery. Blood loss was measured by weighing sponges and drapes, volume in suction drains during surgery, and wound drains until removal 24 hours postoperatively. Hemoglobin and hematocrit were recorded at predefined times during and after surgery.
Results
We found no differences in blood loss (1081 mL ± 424 mL versus 1023 mL ± 238 mL), bleeding-related events (10% versus 17%), or number of patients who had transfusions (12 versus five) with preoperative and postoperative thromboprophylaxis, respectively. Other complications were few in both groups.
Conclusions
Our data suggest blood loss is similar with preoperative and postoperative initiation of dalteparin thromboprophylaxis, but indicate a trend toward fewer transfusion requirements which might favor postoperative start of thromboprophylaxis.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood loss in patients undergoing THA can be substantial and induce postoperative complications [20]. Surgical site bleeding and hematomas may cause nerve compression, prolonged wound drainage, infection, extended hospital stay, and extended rehabilitation [21, 22]. Anemia may aggravate vascular diseases [4], and homologous blood transfusions carry a small risk of infection, immunologic reactions, and fluid overload and may increase costs [19]. Surgical bleeding depends on the magnitude of surgery, the procedure, and patient-specific factors.
In THA, deep vein thrombosis (DVT) and other thrombin-driven events may originate during surgery and in a few patients nonfatal or fatal pulmonary embolisms (PE) develop. General chemical prophylaxis therefore has been recommended [10, 27]. Type of antithrombotic drug, dosage, and timing of the first dose may influence bleeding and development of thrombosis. Low-molecular-weight heparins (LMWHs) are widely used antithrombotics because of their favorable efficacy-to-safety profiles [9, 23], but the best timing of the first dose remains controversial [24, 28]. Preoperative initiation 12 hours before surgery has been based on the premise that DVT starts during surgery and that preoperative initiation is necessary to optimize antithrombotic effectiveness [17, 27]. In contrast, the premise for prophylaxis started after surgery has been to avoid the potential for increased bleeding complications. In clinical trials on antithrombotic regimens, venographically detected DVT has been a primary end point and bleeding a secondary underpowered outcome. Owing to various bleeding definitions, these trials have been criticized for underestimating the risk of bleeding and related complications [6, 7, 14].
We therefore asked if (1) there is a clinically important difference in total blood loss in THA between preoperative or postoperative start of thromboprophylaxis, and (2) there is a difference between the two regimens in transfusion requirements, incidence of bleeding events, and other complications detected up to 6 months after surgery.
Patients and Methods
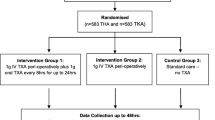
After this study was approved by the regional ethics committee, we prospectively randomized 80 patients 50 years or older who underwent cemented THA for primary osteoarthritis between March and June 2008. During that same time, we treated a total of 104 patients with primary cemented THAs for osteoarthritis. Exclusion criteria were allergy to LMWH, bleeding disorders, renal failure, hepatic disease, active treatment for malignancy, ongoing antithrombotic treatment, history of DVT or PE, and patients experiencing major operations, traumas, stroke, or cardiac infarction the last 3 months before surgery. All patients were routinely hospitalized the day before surgery. We excluded 10 patients from enrollment owing to antithrombotic treatment, five patients with a history of DVT or PE, and two patients with liver disease. Seven patients refused to participate in the study. This left 80 patients for study. None of the 80 patients was lost to followup 6 months after surgery, and data collection was completed for all participants.
We performed a power analysis based on two earlier studies in which a significant reduction in the number of patients who had transfusions showed a 30% reduction in total blood loss [3, 15]. We considered this reduction clinically relevant. The effect size was based on blood loss and transfusion requirements in two earlier studies [3, 15]. In a prospective controlled study on patients who had THAs, Johansson et al. [15], found a 27% reduction in total blood loss (355 mL) reduced (p = 0.009) the number of patients who had transfusions. In a retrospective study [3], we found a 30% reduction in total blood loss (370 mL) reduced (p = 0.006) the number of patients who received transfusions by 28%. We believe a reduced risk for being exposed to blood transfusions is clinically relevant. With 80% power (alpha = 0.05), at least 37 patients were required in each group. We randomized a total of 80 patients to compensate for patients who might withdraw consent.
There were no differences between patients who received preoperative or postoperative start of thromboprophylaxis in terms of demographics (Table 1). We found no difference in operative times and length of hospitalization (Table 2), and preoperative laboratory values also were similar (Table 3).
In the hospital’s written patient information, patients are advised to stop antiplatelet medication (ie, NSAIDs and high-dose aspirin) 1 week before surgery. A complete record of the patients’ medications during the study period was recorded.
We assigned patients to either 5000 IU dalteparin (Fragmin®; Pharmacia and Upjohn, Stockholm, Sweden) subcutaneously or placebo (saline) injected 12 hours before surgery. All patients had 5000 IU dalteparin subcutaneously 6 hours after surgery and each day until the 35th postoperative day. Randomization was prepared in blocks of 20. Treatment group assignment was concealed by the hospital staff. The syringes with 5000 IU dalteparin and placebo with the same volume in each syringe were prepared by a study nurse who otherwise was not engaged in the study, according to randomized strata. The injection was blinded to the investigator, hospital staff, and the patient. The study blinding was broken after all patients had completed 6 months of followup.
All patients received spinal anesthesia without hypotensive effect with 5 mg/mL bupivacaine (Marcain®; AstraZeneca, Södertälje, Sweden) injected at the lumbar level. Cephalothin (Keflin®; EuroCept Pharmaceuticals BV, Kortenhoef, The Netherlands) 2 g was administered within 30 minutes of the arthroplasty. An equivalent dose subsequently was given 3 hours, 9 hours, and 15 hours after surgery as prophylaxis against infection. Voluven® and Ringer’s acetate (Fresenius KABI, Bad Homburg, Germany) were used as plasma substitutes.
The operation was performed with the patient in the lateral position, using a standardized posterior approach where only the piriformis muscle was detached and with capsular repair at the end of the procedure. All procedures were performed by two surgeons with at least one being experienced in performing THAs. All patients received stem and cups (Exeter®; Stryker Orthopaedics, Mahwah, NJ, USA) embedded in Simplex® tobramycin bone cement (Stryker Howmedica, Limerick, UK).
Postoperative analgesia was administered according to a standard protocol consisting of paracetamol + codeine sulfate (Paralgin forte®; Weifa AS, Oslo, Norway) and ketobemidone (Ketorax®; Jenahexal Pharma, Jena, Germany). Closed postoperative drainage was used for 24 hours. All patients were mobilized on the first postoperative day, and a program for simple self-administrated exercises was provided by the physiotherapists during hospitalization. Walking with the use of crutches was advised 6 to 8 weeks after surgery. Regular outpatient physiotherapy was not recommended until 2 months after surgery. We did not allow concomitant mechanical prophylaxis against DVT.
Hemoglobin and hematocrit were measured during surgery, before the first postoperative injection of dalteparin, and on postoperative Days 1, 3, and 6. We recorded the number of blood transfusions and plasma substitutes. The primary outcome was the volume of blood loss measured by weighing sponges and drapes (1 mg = 1 mL), volume in suction drains during surgery, and wound drains until removal 24 hours postoperatively [20]. We also recorded the number of patients who received transfusions, consumption of units of allogeneic leukodepleted erythrocyte concentrate, and decrease in hemoglobin concentration postoperatively in the two groups. We used a standard protocol with transfusion thresholds where a hemoglobin level less than 8 g/dL triggered transfusion and patients with a level greater than 10 g/dL did not receive a transfusion. Hemoglobin level on its own may be a poor indicator of tissue hypoxia, and the decision to transfuse patients with hemoglobin between 8 and 10 g/dL will, to some extent, be influenced by other parameters such as concomitant disease, weight, age, and others [1]. RBCs were given in 300-mL units, and autologous blood was not used. We evaluated all patients on a daily basis during hospitalization for possible bleeding events, such as hematoma, ongoing excessive bleeding, prolonged wound drainage (greater than 7 days), infections, and other complications. Overall surgical complications were classified according to Dindo et al. [8].
If patients showed any clinical sign of thromboembolic events, such as respiratory distress, chest pain, unstable hemodynamics, and a swollen, red, painful leg, we performed objective tests, including ECG, blood gases, plain chest radiography, venography, and spiral CT, after a clinical examination. Routine ultrasound screening, venography, or CT was not performed. A clinical research file (CRF) (Appendix 1; supplemental materials are available with the online version of CORR) was completed on a daily basis during hospitalization and at 6 months’ followup.
The data are presented as mean, range, and 1 SD or 95% CI. Patient characteristics, blood loss, hemoglobin, hematocrit, fluid volume, and operation time were compared between the two groups using Student’s t-test. We used the Mann-Whitney U test to compare the number of blood transfusions. Chi-square and Fisher’s exact tests were used to compare frequencies. We used SPSS Statistics Version 17 (IBM Inc, Chicago, IL, USA) for all analyses.
Results
The total volumes of blood loss during surgery and until drain removal were similar (p = 0.460) in the preoperative and postoperative prophylaxis groups: 1081 mL ± 424 mL versus 1023 mL ± 238 mL, respectively (Table 2). Blood loss until the first postoperative injection of dalteparin (p = 0.202) or after the first postoperative injection and until removal of drains (p = 0.471) also was similar. We observed the same decrease in hemoglobin in the two groups (Table 3). Decreases in hemoglobin greater than 2.0 g/dL were measured for 82.5% versus 90% of patients during surgery and until the first postoperative injection and 92% versus 90% of patients during surgery and the day after surgery. There were no differences in hematocrit between the two groups at any time. Neither hemoglobin nor hematocrit had recovered to preoperative levels on Postoperative Day 6.
We found no difference in the total amount of transfusion requirements among the groups. More (p = 0.099) patients in the preoperative group received blood transfusions during hospitalization (12 of 40 versus five of 40). Both groups received a similar (p = 1.000) number of RBC units until the first postoperative injection of dalteparin (two with two units of packed red cells and two with one unit in both groups) (Table 4). Altogether, 27 units of RBCs were transfused in the preoperative versus 11 units in the postoperative group (p = 0.071). The volumes of colloids and fluids were the same.
In the preoperative group, four patients had bleeding-related events (Table 5). Three patients had wound hematomas, of which one was evacuated 5 days after surgery. One patient had wound drainage leading to prolonged hospitalization, but there were no positive bacteriologic cultures indicating infection. In the postoperative group, one patient had excessive bleeding after surgery, which was treated by surgical hemostasis. Four patients experienced hematomas and two had wound drainage without a positive culture that resulted in prolonged hospitalization. In the preoperative group, two patients had clinically suspected venous thrombosis not venographically confirmed. One patient with chest pain was examined by the cardiologist without obtaining any specific diagnosis. None had suspected PE during 6 months of followup. In the postoperative group, one patient had clinical and radiographically (spiral CT) confirmed PE 6 days after surgery and was treated according to protocol. The number of patients with other complications was low. In the preoperative group, one patient experienced a deep infection 3 months after surgery, and one patient dislocated the hip 2 months after surgery. In the postoperative group, one patient was admitted to another hospital with ileus, which spontaneously resolved. According to the classification of Dindo et al. [8] of surgical complications, three versus seven complications were Grade 1, 13 versus seven were Grade 2, and three versus zero were Grade 3 in the preoperative group versus the postoperative group.
Discussion
Pharmacologic thromboprophylaxis is recommended in major orthopaedic surgery but potentially may increase bleeding and transfusion requirements, which makes its use controversial [18]. LMWH has been associated with increased operative blood loss and transfusions [29], and to reduce bleeding and its side effects, the first injection has been postponed until after surgery. However, the scientific basis for such a change in practice is uncertain and needs further attention. In this double-blind, randomized study of patients undergoing THA, we compared preoperative with postoperative start of 5000 IU dalteparin. We asked if (1) there is a clinically important difference in total blood loss in THA between these two approaches of thromboprophylaxis, and (2) there is a difference between the two regimens in transfusion requirements, incidence of bleeding events, and other complications detected up to 6 months after surgery.
There are some limitations to this study. First, our sample size was small, but it was powered to detect a difference in total blood loss as the primary outcome of the study and with clinical relevance related to transfusion [3, 15]. Second, a proportion of blood loss after surgery is hidden. The volume of hematomas is difficult to estimate clinically or by ultrasonography, although several methods and indices for calculation of hidden blood loss have been proposed [20, 26]. However, any underestimation of such masked blood loss should be equally distributed randomly in groups. We recorded hemoglobin, hematocrit, and volume of fluids transfused at fixed times during and after surgery and found these recordings consistent across the two groups and followed the same pattern. Third, our transfusion guidelines leave a gray zone between upper and lower transfusion thresholds where transfusion decisions are based on numerous factors, including preoperative and postoperative hemoglobin levels, age, BMI, additional comorbidities, physicians preferences, and others [1, 2, 25]. However, as there were no differences in any parameters between the two groups, we consider the decision to transfuse to be equal in the two groups. Fourth, we did not use predefined classifications of bleeding events. Variability in reporting of these events makes it difficult to compare between trials. Therefore, we rather described them in clinical terms, and there were no major differences between our two groups.
Trials of thromboprophylactic agents have shown wide variation in bleeding definitions and recording of bleeding events, and together with lack of statistical power, this may have resulted in misleading interpretation of the findings for bleeding [7, 12, 14]. This inconsistency also makes it difficult to draw conclusions from meta-analyses and make recommendations. In The North American Fragmin Trial, 2500 IU dalteparin was given either 1 hour before or 6 hours after surgery and compared with warfarin initiated 12 to 24 hours postoperatively [13]. Different surgical procedures were included, ie, primary THA and revisions. Fewer radiographic DVTs were recorded for both dalteparin regimens compared with warfarin. Predefined bleeding events were similar in all groups, but the proportion of patients receiving transfusions was greater for the dalteparin groups, particularly for those receiving dalteparin preoperatively. Consequently, a 6-hour postoperative dalteparin regimen was recommended even if the study was underpowered to assess the trial-specified bleeding. In a retrospective study, we found a reduction in total blood loss during THA when the first dose of dalteparin was postponed from 12 hours before to 6 hours after surgery [3], but the clinical importance of this reduction was questioned. In the current study in which the same drug and dose were compared, we could not find differences in total blood loss during and after surgery or in decrease in hemoglobin or hematocrit at any time until discharge after approximately 1 week. Other parameters that could influence blood loss, such as operative time, type of surgery, age, and BMI, were similar in the two study groups and strengthen our observations.
Our overall incidence of bleeding complications was greater than reported by others [18], which could reflect the method of collecting and classifying data. Some researchers use a decrease in hemoglobin greater than 2 g/dL in the definition of major bleeding [11], and the rate of major bleeding frequently is reported as a safety outcome in trials of thromboprophylaxis [9, 13, 16, 23]. We found such a decrease in hemoglobin for the majority of patients until the first postoperative injection (83% versus 90%) and the day after surgery (93% versus 90%), which indicates a decrease in hemoglobin is a poor parameter for defining major bleeding in patients undergoing THA.
Many patients undergoing major orthopaedic surgery receive blood transfusions (Table 6). A transfusion frequency of 30% to 40% has been reported in a publication regarding new anticoagulants [16], and a review on transfusion decision-making reported between 16% and 50% of patients who had THAs received transfusions [1]. We found similar percentages: 30% with preoperative and 12.5% with postoperative thromboprophylaxis. Our study was not powered to show differences in transfusions and we observed no differences in total units transfused, frequency of transfusions, or number of patients who had transfusions. Parameters known to affect transfusion requirements, such as preoperative and postoperative hemoglobin level, American Society of Anesthesiologists physical status classification, weight, and age [1], were similar in both groups and should not influence the results. However, fewer patients received blood transfusions and the number of RBC units transfused was less in the postoperative group.
Thrombosis formation begins at the time of surgery in THA, and it follows that efforts to prevent the formation of thrombi should begin as early as possible. The timing of initiation of pharmacologic prophylaxis is a clinical decision that should consider the risk of venous thromboembolism and bleeding associated with antithrombotic therapy. We found no differences in blood loss when 5000 IU dalteparin was initiated 12 hours before or 6 hours after primary THA. However, we observed a trend toward fewer transfusions with postoperative start.
References
Barr PJ, Donnelly M, Cardwell C, Alam SS, Morris K, Parker M, Bailie KE. Drivers of transfusion decision making and quality of the evidence in orthopedic surgery: a systematic review of the literature. Transfus Med Rev. 2011;25:304–316.
Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10.
Borgen PO, Dahl OE, Reikeras O. Preoperative versus postoperative initiation of dalteparin thromboprophylaxis in THA. Hip Int. 2010;20:301–307.
Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, Noveck H, Strom BL. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
Colwell CW Jr, Chelly JE, Murkin JM, Stevens D, O`Keefe TJ, Hall R, Parvizi J. Randomized study of aprotinin effect on transfusions and blood loss in primary THA. Clin Orthop Relat Res. 2007;465:189–195.
Dahl OE, Ogren M, Agnelli G, Eriksson BI, Cohen AT, Mouret P, Rosencher N, Bylock A, Panfilov S, Andersson M. Assessment of bleeding after concomitant administration of antiplatelet and anticoagulant agents in lower limb arthroplasty. Pathophysiol Haemost Thromb. 2006;35:428–434.
Dahl OE, Quinlan DJ, Bergqvist D, Eikelboom JW. A critical appraisal of bleeding events reported in venous thromboembolism prevention trials of patients undergoing hip and knee arthroplasty. J Thromb Haemost. 2010;8:1966–1975.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213.
Francis CW, Pellegrini VD Jr, Totterman S, Boyd AD Jr, Marder VJ, Liebert KM, Stulberg BN, Ayers DC, Rosenberg A, Kessler C, Johanson NA. Prevention of deep-vein thrombosis after total hip arthroplasty: comparison of warfarin and dalteparin. J Bone Joint Surg Am. 1997;79:1365–1372.
Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133(6 suppl):381S–453S.
Graafsma YP, Prins MH, Lensing AW, de Haan RJ, Huisman MV, Buller HR. Bleeding classification in clinical trials: observer variability and clinical relevance. Thromb Haemost. 1997;78:1189–1192.
Hull RD, Liang J, Brant R. Pooled analysis of trials may, in the presence of heterogeneity inadvertently lead to fragile conclusions due to the importance of clinically relevant variables being either hidden or lost when the findings are pooled. Thromb Res. 2010;126:164–165.
Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, Soderberg K, Holmqvist A, Mant M, Dear R, Baylis B, Mah A, Brant R. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. The North American Fragmin Trial Investigators. Arch Intern Med. 2000;160:2199–2207.
Hull RD, Yusen RD, Bergqvist D. State-of-the-art review: assessing the safety profiles of new anticoagulants for major orthopedic surgery thromboprophylaxis. Clin Appl Thromb Hemost. 2009;15:377–388.
Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–319.
Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–39.
Kakkar VV, Howe CT, Flanc C, Clarke MB. Natural history of postoperative deep-vein thrombosis. Lancet. 1969;2:230–232.
Lachiewicz PF. Comparison of ACCP and AAOS guidelines for VTE prophylaxis after total hip and total knee arthroplasty. Orthopedics. 2009;32(12 suppl):74–78.
Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br. 2008;90:1128–1136.
Liu X, Zhang X, Chen Y, Wang Q, Jiang Y, Zeng B. Hidden blood loss after total hip arthroplasty. J Arthroplasty. 2011;26:1100–1105.
Novicoff WM, Brown TE, Cui Q, Mihalko WM, Slone HS, Saleh KJ. Mandated venous thromboembolism prophylaxis: possible adverse outcomes. J Arthroplasty. 2008;23(6 suppl 1):15–19.
Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:33–38.
Planes A, Vochelle N, Mazas F, Mansat C, Zucman J, Landais A, Pascariello JC, Weill D, Butel J. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thromb Haemost. 1988;60:407–410.
Raskob GE, Hirsh J. Controversies in timing of the first dose of anticoagulant prophylaxis against venous thromboembolism after major orthopedic surgery. Chest. 2003;124(6 suppl):379S–385S.
Salido JA, Marin LA, Gomez LA, Zorrilla P, Martinez C. Preoperative hemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors. J Bone Joint Surg Am. 2002;84:216–220.
Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty: correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86:561–565.
Sharrock NE, Go G, Harpel PC, Ranawat CS, Sculco TP, Salvati EA. The John Charnley Award: thrombogenesis during total hip arthroplasty. Clin Orthop Relat Res. 1995;319:16–27.
Strebel N, Prins M, Agnelli G, Buller HR. Preoperative or postoperative start of prophylaxis for venous thromboembolism with low-molecular-weight heparin in elective hip surgery? Arch Intern Med. 2002;162:1451–1456.
Walsh M, Preston C, Bong M, Patel V, Di Cesare PE. Relative risk factors for requirement of blood transfusion after total hip arthroplasty. J Arthroplasty. 2007;22:1162–1167.
Warwick D, Bannister GC, Glew D, Mitchelmore A, Thornton M, Peters TJ, Brookes S. Perioperative low-molecular-weight heparin: is it effective and safe. J Bone Joint Surg Br. 1995;77:715–719.
Acknowledgments
We thank Nina Bøhler, study nurse, for valuable help in preparing the injections and conducting the block randomization in this controlled study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he, or a member of his immediate family, has no commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in the connection with this submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was performed at Martina Hansens Hospital, Baerum Postterminal, Norway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Borgen, P.O., Dahl, O.E. & Reikerås, O. Blood Loss in Cemented THA is not Reduced with Postoperative Versus Preoperative Start of Thromboprophylaxis. Clin Orthop Relat Res 470, 2591–2598 (2012). https://doi.org/10.1007/s11999-012-2320-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-012-2320-9