Abstract
Background
Continuing efforts have been made to develop minimally invasive surgery techniques for THA. One of the most commonly performed of these techniques is the mini-posterior approach. All reported series using this approach describe surgical detachment of the short external rotators of the hip. In 2008, Penenberg et al. described an innovative surgical technique that preserves the short external rotators. We present the results of a single-incision modification of this technique in 135 patients.
Description of Technique
This technique is based on preservation of all of the short external rotators of the hip with the exception of the piriformis or conjoined tendon. This single-incision technique required the development of specialized instrumentation for exposure and reaming of the acetabulum. The specialized retractors also successfully minimized trauma to the skin and subcutaneous tissue.
Methods
For the 135 patients undergoing THA with this technique, we analyzed demographic and operative data. We recorded complications, evaluated postoperative clinical function using the Harris hip score, and assessed cup abduction angle, cup anteversion, and stem alignment on radiographs. Minimum followup was 14 months (mean, 22 months; range, 14–33 months).
Results
There were no dislocations, no sciatic nerve palsies, no wound complications, and low transfusion rates (8%). The postoperative Harris hip score averaged 96.5 (range, 87–100). Overall acetabular cup abduction angle averaged 41° (range, 21°–49°) and anteversion averaged 21° (range, 15°–27°). Four percent and 2% of femoral components were inserted into more than 2° varus and 2° valgus alignment, respectively.
Conclusions
This technique shows promise as an alternative tissue-sparing method for minimally invasive THA.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There have been continuing efforts during the past decade to develop minimally invasive surgery techniques for performing THA [2, 6, 9, 10, 18, 24, 30, 31]. The purported benefit of minimally invasive THA compared with traditional THA remains controversial [2, 3, 6, 13, 23, 26]. Proponents of minimally invasive THA cite improved patient satisfaction, less blood loss, shorter hospital stays, and early improved function [2, 3, 6, 13, 23, 26]. However, in the evolution of minimally invasive THA, there has been justified concern regarding the potential for increased complications and the possibility of compromising an already very successful operative procedure [16, 19, 32]. Some reports of complications associated with minimally invasive THA techniques include component malposition, femoral shaft fractures, sciatic nerve palsies, femoral nerve palsy, catastrophic blood loss, significant muscle trauma, and death [1, 8]. However, there are reported series of minimally invasive THA with low complication rates and excellent clinical outcomes [2, 10, 23].
Several authors have suggested the potential benefits of minimally invasive THA are not in the smaller size of the incision but in the limited surgical dissection performed [2, 15]. However, reports that purport to provide less surgical tissue trauma do not always result in improved clinical outcomes or patient satisfaction [21]. This could be because some so-called minimally invasive surgical techniques may actually cause more muscle damage than more traditional surgical approaches [30].
One of the most commonly performed minimally invasive surgical techniques for THA is the mini-posterior approach [3, 10, 20, 21]. All reported series of mini-posterior approaches describe surgical detachment of the short external rotators of the hip. The clinical effect of surgical detachment of the short external rotators of the hip is not known. However, there may be a clinical benefit to leaving some of the short external rotators intact as this represents less surgical dissection and potential improvement in hip stability and postoperative recovery. There are several published reports of THA using modified posterior approaches that preserve some of the short external rotators [15, 17, 23]. It is possible the preservation of these anatomic structures may contribute to postoperative hip stability compared with traditional posterior or mini-posterior approaches. In addition, preservation of these structures may result in improved postoperative gait mechanics, but further study is required to determine this. Penenberg et al. [23] reported excellent results using a posterosuperior approach for THA, including 0% dislocation, 0% nerve injury, and 0% wound complication rates. This technique uses a straight reamer through a percutaneous second incision to facilitate acetabular instrumentation and component placement. These excellent results may be attributable in part to the innovative surgical technique, which includes preservation of some of the short external rotators of the hip.
We describe a posterosuperior rotator-preserving arthroplasty performed through a single incision using specially designed acetabular instrumentation, and report our intraoperative variables, early complications, Harris hip scores, and postoperative radiographic results using this technique.
Surgical Technique
The patient was positioned in the lateral decubitus position anteriorly on the operative table (Fig. 1). The anterior pelvis positioner was adjustable, which facilitated additional intraoperative adduction of the hip while instrumenting the proximal femur.
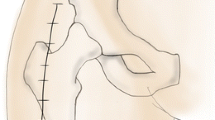
The initial incision was made from the posterosuperior corner of the greater trochanter, extending proximally in line with the fibers of the gluteus maximus (Fig. 2). An 8-cm incision was of satisfactory length in the majority of the cases. After the initial skin incision was made, self-retaining retractors were placed into the subcutaneous tissue and additional soft tissue dissection was done down to the gluteus maximus fascia.
The gluteus maximus fascia then was incised sharply. This exposed the muscle fibers deep to the fascia, which were bluntly divided longitudinally with a Cobb elevator. The deep fascia layer of the gluteus maximus muscle was gently divided before exposure of the pericapsular fat.
The pericapsular fat then was resected using electrocautery (Video 1. Supplemental materials are available with the online version of CORR). The piriformis tendon was palpable in the superior aspect of the wound deep to the gluteus medius muscle. A 90°-bent Hohmann retractor then was placed over the piriformis tendon (Fig. 3). The piriformis tendon was detached as close to its insertion as possible [23]. The piriformis tendon frequently was conjoined to the internal obturator tendon at its insertion [27]. In these cases, the internal obturator tendon and the piriformis tendon were reflected and repaired together using the conjoined tendon. The capsule then was incised in the inferior aspect of the wound, and the capsulotomy extended posterior and superior to the superior acetabulum. This created a superior and inferior capsular leaflet for later capsular repair.
After the capsulotomy was performed, the hip was dislocated by flexing, adducting, and internally rotating the leg (Video 2. Supplemental materials are available with the online version of CORR). After dislocation, a 90°-bent Hohmann and a thin curved Hohmann were used to expose the femoral neck. An initial cut was made perpendicular to the long axis of the femoral neck. This cut was made at the subcapital level at the junction between the inferior-most aspect of the femoral head and the superior-most aspect of the femoral neck. After this osteotomy, the head fragment was removed. Electrocautery was used to expose the superior portion of the remaining femoral neck. A second saw cut was made, and then the neck fragment was removed (Video 3. Supplemental materials are available with the online version of CORR). Typically, the neck fragment measured 3 to 4 mm; however, this was usually thinner in varus necks and thicker in valgus necks. The thickness of this neck fragment was based on preoperative templating.
To expose the acetabulum, a sharp curved Hohmann was placed over the anterior acetabular rim. A second 90°-bent Hohmann then was placed at the inferior margin of the acetabulum. This was placed either inside or outside the inferior capsule tissue. A 90°-hand-held retractor then was placed in the superior aspect of the wound to delineate the margin between the superior labrum and the superior capsule. A pituitary rongeur was used to grasp the labrum, which was resected from the superior rim of the acetabulum, preserving the superior capsule for later closure (Video 4. Supplemental materials are available with the online version of CORR). After this, a 90°-sharp Hohmann was placed deep to the superior capsule and then was gently tapped into the superior acetabulum. Finally, an optional fourth retractor was placed on the posterior aspect of the acetabulum. This was a double-bent Hohmann retractor. The acetabulum could now be clearly seen (Fig. 4). Soft tissue on the medial aspect of the acetabulum was removed. Any additional labrum was resected at this time.
The initial acetabulum was reamed using a straight reamer and was reamed medially. The amount of medial reaming performed was based on preoperative radiographic assessment of the medial osteophyte. After straight reaming to the desired depth, the remaining reaming was performed with the angled acetabular reamer (Video 5. Supplemental materials are available with the online version of CORR).
The angled acetabular reamer had a 55°-reamer angle (Greatbatch Medical, Clarence, NY, USA) (Fig. 5). This correlated to a 40°-cup abduction angle and 15° pelvic tilt. This reamer had a short neck to prevent impingement on the external obturator muscle and inferior capsule. Sequential reaming was then performed with the angled reamer up to 1 mm less than the cup size. The acetabulum was irrigated, and a curved cup impactor (Fig. 6) was used to impact the acetabular component into place (Fig. 7) (Video 6. Supplemental materials are available with the online version of CORR). Screws then could be placed at the discretion of the surgeon. Osteophytes were removed from the acetabular rim using an osteotome and a pituitary rongeur. The acetabular bearing surface then was impacted into place and all of the retractors were removed.
To prepare the femur, the leg was placed in 40° flexion, 40° adduction, and 40° internal rotation. A handheld right-angle retractor was placed in the inferior aspect of the wound to retract the quadratus muscle away from the proximal femur. A right-angle Hohmann retractor then was placed over the superior femoral neck. A third retractor was placed under the calcar to expose the proximal femur. A box chisel was used to remove the bone from the lateral neck. Hand reaming was performed to lateralize the proximal femoral canal. A power tapered reamer was used to enlarge the femoral neck region. Sequential broaching was performed with an offset handle (Fig. 8) to fill the proximal femur in the mediolateral dimension based on preoperative templating and intraoperative assessment. Trial components then were placed. The hip was reduced and limb length, offset, and stability were assessed (Video 7. Supplemental materials are available with the online version of CORR). Limb length was assessed using the distance from the tip of the greater trochanter to the top of the femoral prosthesis, based on preoperative templating [23]. The tip of the greater trochanter was used as the principle reference for the height of the prosthesis, since the lesser trochanter could not be seen or palpated during the procedure, as it was covered by the quadratus and external obturator muscles. The hip then was redislocated, the trial components removed, and the final implant seated (Video 8. Supplemental materials are available with the online version of CORR). The wound was copiously irrigated. The capsule then was repaired with a running Number 1 absorbable suture. The piriformis or conjoined tendon was repaired to the posterior fibers of the gluteus medius. The gluteus maximus fascia was repaired using a running Number 1 absorbable suture. The subcutaneous layer was closed using 2-0 absorbable suture and the skin was closed using staples. The soft tissue structures before capsular enclosure were anesthetized using 20 mL to 30 mL 0.25% Marcaine® (AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA) with epinephrine.
Pain management included the use of dexamethasone, ketorolac, metoclopramide, celecoxib, and pregabalin. A single dose of dexamethasone was given intravenously in the recovery room. The dose varied from 4 mg to 8 mg and was dependent on weight. Patients who weighed less than 55 kg were given 4 mg. Patients who weighed more than 100 kg were given 8 mg. All other patients were given 6 mg. Patients with a known history of diabetes were not given any dexamethasone. A single dose of ketorolac was given intravenously in the recovery room. Patients who weighed 75 kg or less were given 15 mg ketorolac, and patients who weighed more than 75 kg were given 30 mg. Patients with a known history of renal failure were not given any ketorolac. All patients were given pregabalin 50 mg orally twice daily. All patients were given one dose (10 mg) of metoclopramide intravenously in the recovery room. Patients who did not have a sulfa allergy were given celecoxib 200 mg orally twice daily beginning on the day of surgery. Acetaminophen 650 mg orally every 4 hours was used for mild breakthrough pain and a combination of hydrocodone bitartrate 5 mg and acetaminophen 325 mg was used for moderate breakthrough pain. For severe pain, there were no standing orders, and a separate assessment was made before administration of any intravenous narcotics. Treatment of severe breakthrough pain was necessary only for patients who were actively using narcotics immediately before surgery.
Patients and Methods
Permission was obtained from the institutional review board at our institution to perform this retrospective study. During an 18-month period, 135 THAs were performed (January 2009 through July 2010). All surgical procedures were performed by one surgeon (DJR). No patient was excluded on the basis of BMI or hip disease. This study included only patients who had a primary THA. Patients who had prior hip surgery were excluded from the study. The average age of the patients was 72 years (range, 45–92 years) (Table 1). Their average height was 167 cm (range, 129–195 cm), and average weight was 80.86 kg (range, 40–170 kg). The average BMI was 27.3 (range, 19.5–40.0). Ninety-two percent of patients had osteoarthritis, 5% had avascular necrosis, and 3% had rheumatoid arthritis. Fifty-two percent of surgeries involved the left hip and 48% the right hip. One hundred twenty-six patients had general anesthesia and nine had regional anesthesia. We reviewed patient data and clinical followup for an average of 22 months (range, 14–33 months).
All patients had a cementless acetabular cup (Trilogy®; Zimmer Inc, Warsaw, IN, USA) placed. All patients had one to three screws for acetabular fixation based on the intraoperative assessment by the surgeon. All acetabular cups had UHMWPE liners for use with a 32-mm head. No lipped liners were used. All patients had implantation of a forged titanium, tapered, collarless, cementless stem (ML Taper®; Zimmer). All hips used a 32-mm cobalt-chromium head.
All patients began ambulation, weightbearing as tolerated, on the day of surgery, if postoperative nausea or hypotension did not preclude this under the supervision of a licensed physical therapist. Patients initially began ambulating with the use of a walker, but attempted ambulation without any aids was done with each patient, based on a safety assessment by the physical therapist. All patients were given aspirin 325 mg orally three times daily for 2 weeks as venous thromboprophylaxis. If patients were receiving warfarin preoperatively, this was resumed postoperatively instead of aspirin.
We collected the following data for analysis: age, height, weight, BMI, preoperative diagnosis, estimated blood loss, blood replacement during hospitalization, length of incision, operative time, length of hospital stay, type of anesthesia, and disposition after discharge from the hospital. We recorded complications. Postoperative clinical function was assessed by the operative surgeon, using the Harris hip score. In addition, a radiographic analysis was performed by the operative surgeon, which assessed the cup abduction angle, cup anteversion, and stem alignment. Acetabular cup abduction angle and femoral component alignment were assessed using a standard AP radiograph. Acetabular anteversion was assessed using a cross-table lateral technique with the contralateral hip in maximal flexion.
Results
The average incision length was 9 cm (range, 8–15 cm) (Table 1). Although the majority of patients had a skin incision between 8 cm and 9 cm, the use of a longer incision in larger patients was not believed to compromise the surgical technique, as the short external rotators, excluding the piriformis or conjoined tendon, were preserved in all cases. Estimated blood loss averaged 354 mL (range, 250–800 mL). The transfusion rate was 8% (11 of 135 patients). Operative time averaged 57 minutes (range, 35–65 minutes). The average hospital stay was 1.98 days (range, 1–3 days). At discharge from the hospital, 91 patients went home and 44 went to a rehabilitation facility.
There were no sciatic nerve palsies. One patient had revision surgery to a modular neck design at 2 weeks postoperatively owing to excessive leg lengthening. The leg was longer than the patient was willing to accept and she elected to have a revision procedure using modular components. There was one intraoperative fracture, which required plating of the femur and restricted weightbearing postoperatively. One patient had revision surgery owing to inadequate offset and clinical subluxation. There were no deep or superficial wound infections requiring antibiotics or secondary surgical procedures. No patient had revision surgery owing to failure of the cup or stem ingrowth. There were no clinically significant deep venous thromboses and no pulmonary emboli. There were no dislocations, and there was no hip bursitis related to excessive offset.
The postoperative Harris hip score averaged 96.5 (range, 87–100).
The overall acetabular cup abduction angle averaged 41° (range, 21°–49°) and 21° anteversion (range, 15°–27°). The percentage of femoral components inserted into greater than 2° varus alignment was 4%, and all of these were in the first ½ of the study; 2% of the femoral components were in greater than 2° valgus.
Discussion
Our series reports a single-incision modification of the posterosuperior technique described by Penenberg et al. [23]. As with other minimally invasive approaches, the development of specialized instrumentation was required [12]. The use of a short-neck, high-angle reamer was necessary to perform this procedure through a single incision. In addition, specialized retractors were developed to achieve adequate surgical exposure and prevent trauma to the skin. As with the technique used by Penenberg et al. [23], our posterosuperior approach preserves the short external rotators of the hip, with the exception of the piriformis or conjoined tendon.
There are several limitations to this study. First, it is not a controlled prospective randomized study. Second, the operative surgeon performed the clinical and radiographic analyses; therefore, there may be intraobserver bias in the results. Finally, the followup of this group of patients is relatively short. Given these shortcomings, our results should be interpreted with caution. Nevertheless, we feel justified in presenting these preliminary results, as they compare favorably with those in other published studies using modified rotator-preserving posterior approaches [15, 17, 23].
Multiple studies have shown improvement in postoperative hip stability when the short external rotators are repaired after THA using a posterior approach [11, 14, 22, 29]. However, the failure rate of surgically repaired short external rotators after posterior THA is as much as 80% [28]. As a result, some authors have modified the posterior approach to preserve some of the short external rotators of the hip [15, 17, 23]. Khan et al. [15] reported excellent results in a series of 100 patients using a less-invasive modification of a standard posterior approach. This approach is similar to that reported by Kim et al. [17] in that it preserves the piriformis and the inferior portion of the quadratus. Specialized acetabular instrumentation was not required in either of these series, which used a posteroinferior rotator-preserving approach. However, Khan et al. [15] believed this approach might preclude the use of a canal-filling or nonshouldered femoral prosthesis, as insertion of these types of femoral prostheses might damage the piriformis tendon during the surgical procedure. Furthermore, they noted four patients were excluded from their study owing to iatrogenic injury to the piriformis muscle.
Rotator-preserving modifications to the standard posterior hip approach can be divided into a posteroinferior approach, which preserves the piriformis or conjoined tendon [15, 17], and a posterosuperior approach, which detaches and repairs the piriformis or conjoined tendon and preserves the more inferior short external rotators [23]. The advantage of the posterosuperior approach is that it does not restrict femoral stem options, and the potential disadvantage is that it requires specialized acetabular instrumentation. The advantage of the posteroinferior approach is that traditional acetabular instrumentation can be used, and the disadvantage is that it might limit femoral stem options. Either type of posterior rotator-preserving arthroplasty may have the potential to improve hip stability compared with a standard posterior THA, but additional study is required to determine this.
Included in our series were obese patients with a BMI of as much as 40. Although longer skin incisions were required in patients with higher BMI, the exposure and prosthetic implantation were completed successfully with preservation of the inferior short external rotators. We have no experience in using this technique in patients with severe hip deformities or with prior hip surgery; therefore, this technique should be used with extreme caution in these clinical circumstances. Some authors have recommended in situ osteotomy in cases of an incarcerated femoral head owing to protusio acetabulae or excessive osteophyte formation. We believe in situ osteotomy would be exceedingly difficult using this approach and recommend against its use when in situ osteotomy is required. As with any limited-exposure hip arthroplasty, precise knowledge of the surgical anatomy is essential, and extreme care during the surgical procedure is required to avoid iatrogenic injury to the proximate neurovascular structures.
Our transfusion rate was 8%, which is similar to that reported in the series of Penenberg et al. [23] (10%). These transfusion rates are lower than those reported elsewhere in the literature, which are as high as 59% using standard hip arthroplasty approaches [4]. However, neither our series nor that of Penenberg et al. [23] analyzed the transfusion events in light of preoperative hemoglobin and weight of the patient, which has been shown to have significant predictive value for transfusion events [25]. Khan et al. [15] found their less invasive technique resulted in less blood loss, but this did not result in a decrease in transfusion events [15]. Further study is required to determine whether this type of tissue-sparing arthroplasty results in decreased blood loss and decreased overall transfusion rates compared with a standard posterior hip arthroplasty.
The cup abduction angle in this study was highly variable (range, 21°–49°). This is consistent with a recent report in the literature that shows a much higher variability in cup position than previously reported [5]. Two patients required early revision in our series and one patient sustained an intraoperative fracture. One patient had revision surgery owing to a long leg postoperatively, and one had revision surgery owing to inadequate offset. The revision attributable to the long leg was in a 72-year-old woman with a height of 160 cm. The intraoperative limb length was not assessed correctly, and the patient had residual over-lengthening of 1.3 cm and was unsatisfied with the result. This patient required a revision procedure 2 weeks postoperatively to correct the limb length inequality. A second early revision was required owing to inadequate offset. A more detailed intraoperative determination of abductor tension and more careful assessment of the distance between the posterior border of the greater trochanter and the pelvis might have resulted in detection of the inadequate offset intraoperatively and potentially prevented the need for subsequent revision surgery. One patient with Dorr Type C bone sustained an intraoperative fracture of the femoral shaft. This was attributable to inadequate capsular exposure before dislocation. All three of these complications could have been attributable in part to limited exposure and unfamiliarity with the surgical technique and therefore may represent part of the learning curve associated with this technique.
There were no revisions for component malpositions in our series. There were no dislocations, sciatic nerve palsies, wound complications, or thromboembolic events. Use of ambulatory assistive devices was discontinued rapidly in most patients and there were no hip precautions used. Our results compare favorably with those of other published reports of minimally invasive hip arthroplasty [7, 10]. The surgical anatomy in this technique is familiar to surgeons using a posterior hip approach, and this approach does not preclude immediate conversion to a standard posterior hip approach for any reason. We believe this tissue-sparing technique shows potential promise as an alternative method of facilitating minimally invasive THA.
References
Bal BS, Haltom D, Aleto T, Barrett M. Early complications of primary total hip replacement performed with a two-incision minimally invasive technique. J Bone Joint Surg Am. 2005;87:2432–2438.
Berger RA, Duwelius PJ. The two-incision minimally invasive total hip arthroplasty: technique and results. Orthop Clin North Am. 2004;35:163–172.
Berry DJ, Berger RA, Callaghan JJ, Dorr LD, Duwelius PJ, Hartzband MA, Lieberman JR, Mears DC. Minimally invasive total hip arthroplasty: development, early results, and a critical analysis. J Bone Joint Surg Am. 2003;85:2235–2246.
Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10.
Callanan MC, Jarrett B, Bragdon CR, Zurakowski D, Rubash HE, Freiberg AA, Malchau H. The John Charnley Award. Risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res. 2011;469:319–329.
Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty: a prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89:1153–1160.
Duwelius PJ, Hartzband MA, Burkhart R, Carnahan C, Blair S, Wu Y, Grunkemeier GL. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop (Belle Mead NJ). 2010;39:2–6.
Fehring TK, Mason JB. Catastrophic complications of minimally invasive hip surgery: a series of three cases. J Bone Joint Surg Am. 2005;87:711–714.
Goldstein WM, Branson JJ, Berland KA, Gordon AC. Minimal-incision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(suppl 4):33–38.
Hartzband MA. Posterolateral minimal incision for total hip replacement: technique and early results. Orthop Clin North Am. 2004;35:119–129.
Hedley AK, Hendren DH, Mead LP. A posterior approach to the hip joint with complete posterior capsular and muscular repair. J Arthroplasty. 1990;(5 suppl):S57–S66.
Howell JR, Garbuz DS, Duncan CP. Minimally invasive hip replacement: rationale, applied anatomy, and instrumentation. Orthop Clin North Am. 2004;35:107–118.
Hungerford DS. Minimally invasive total hip arthroplasty: in opposition. J Arthroplasty. 2004;19(4 suppl 1):81–82.
Kao JT, Woolson ST. Piriformis tendon repair after total hip replacement. Orthop Rev. 1992;21:171–174.
Khan RJ, Fick D, Khoo P, Yao F, Nivbrant B, Wood D. Less invasive total hip arthroplasty: description of a new technique. J Arthroplasty. 2006;21:1038–1046.
Kim YH. Comparison of primary total hip arthroplasties performed with a minimally invasive technique or a standard technique: a prospective and randomized study. J Arthroplasty. 2006;21:1092–1098.
Kim YS, Kwon SY, Sun DH, Han SK, Maloney WJ. Modified posterior approach to total hip arthroplasty to enhance joint stability. Clin Orthop Relat Res. 2008;466:294–299.
Meneghini RM, Smits SA, Swinford RR, Bahamonde RE. A randomized, prospective study of 3 minimally invasive surgical approaches in total hip arthroplasty: comprehensive gait analysis. J Arthroplasty. 2008;23(6 suppl 1):68–73.
Ogonda L, Wilson R, Archbold P, Lawlor M, Humphreys P, O’Brien S, Beverland D. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes: a prospective, randomized, controlled trial. J Bone Joint Surg Am. 2005;87:701–710.
Pagnano MW, Trousdale RT, Meneghini RM, Hanssen AD. Patients preferred a mini-posterior THA to a contralateral two-incision THA. Clin Orthop Relat Res. 2006;453:156–159.
Pagnano MW, Trousdale RT, Meneghini RM, Hanssen AD. Slower recovery after two-incision than mini-posterior-incision total hip arthroplasty: a randomized clinical trial. J Bone Joint Surg Am. 2008;90:1000–1006.
Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop Relat Res. 1998;355:224–228.
Penenberg BL, Bolling WS, Riley M. Percutaneously assisted total hip arthroplasty (PATH): a preliminary report. J Bone Joint Surg Am. 2008;90(suppl 4):209–220.
Pour AE, Parvizi J, Sharkey PF, Hozack WJ, Rothman RH. Minimally invasive hip arthroplasty: what role does patient preconditioning play? J Bone Joint Surg Am. 2007;89:1920–1927.
Salido JA, Marin LA, Gomez LA, Zorrilla P, Martinez C. Preoperative hemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors [erratum in J Bone Joint Surg Am. 2002;84:79]. J Bone Joint Surg Am. 2002;84:216–220.
Sculco TP. Minimally invasive total hip arthroplasty: in the affirmative. J Arthroplasty. 2004;19(4 suppl 1):78–80.
Solomon LB, Lee YC, Callary SA, Beck M, Howie DW. Anatomy of piriformis, obturator internus and obturator externus: implications for the posterior surgical approach to the hip. J Bone Joint Surg Br. 2010;92:1317–1324.
Stähelin T, Drittenbass L, Hersche O, Miehlke W, Munzinger U. Failure of capsular enhanced short external rotator repair after total hip replacement. Clin Orthop Relat Res. 2004;420:199–204.
Suh KT, Park BG, Choi YJ. A posterior approach to primary total hip arthroplasty with soft tissue repair. Clin Orthop Relat Res. 2004;418:162–167.
Vail TP, Mariani EM, Bourne MH, Berger RA, Meneghini RM. Approaches in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(suppl 5):10–12.
Wenz JF, Gurkan I, Jibodh SR. Mini-incision total hip arthroplasty: a comparative assessment of perioperative outcomes. Orthopedics. 2002;25:1031–1043.
Woolson ST, Mow CS, Syquia JF, Lannin JV, Schurman DJ. Comparison of primary total hip replacements performed with a standard incision or a mini-incision. J Bone Joint Surg Am. 2004;86:1353–1358.
Acknowledgments
We thank Pete Bandel and R. Oliver Barrett for technical assistance and Nicole L. Ebarb for assistance with preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
One of the authors certifies that he (DJR), or a member of his immediate family, has or may receive payments or benefits, in any 1 year, an amount in excess of $100,000.00 from a commercial entity (Greatbatch Medical, Clarence, NY, USA) related to this work. One of the authors certifies that he (DJR), or a member of his immediate family, has patent/licensing arrangements with Greatbatch Medical.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1 (MPG 16148 kb)
Video 2 (MPG 17175 kb)
Video 3 (MPG 13326 kb)
Video 4 (MPG 15250 kb)
Video 5 (MPG 21128 kb)
Video 6 (MPG 23801 kb)
Video 7 (MPG 32174 kb)
Video 8 (MPG 15170 kb)
About this article
Cite this article
Roger, D.J., Hill, D. Minimally Invasive Total Hip Arthroplasty Using a Transpiriformis Approach: A Preliminary Report. Clin Orthop Relat Res 470, 2227–2234 (2012). https://doi.org/10.1007/s11999-011-2225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-011-2225-z