Abstract
Background
Patients with local recurrence of soft-tissue sarcomas have a poor overall survival. High-grade, soft-tissue sarcomas in deep locations may have a poorer prognosis regarding local recurrence than low-grade sarcomas or those located superficially. Although previous reports evaluated tumors at various depths, it is unclear what factors influence recurrence of deep, high-grade sarcomas.
Questions/purposes
We therefore determined whether possible risk factors (tumor size, location, histologic subtype, unplanned excision, local recurrence at presentation, metastasis at diagnosis, surgical procedure, surgical margin, and adjuvant treatments) influenced local recurrence of deep, high-grade, soft-tissue sarcomas.
Patients and Methods
We retrospectively reviewed 433 patients with deep, high-grade, soft-tissue sarcomas surgically treated between 1985 and 2005. For each patient, we reviewed tumor size, location, histologic subtype, unplanned excision, local recurrence at presentation, metastasis at diagnosis, surgical procedure, surgical margin, and adjuvant treatments and determined the effect of each prognostic variable on local recurrence. The minimum followup was 1 month (median, 51 months; range, 1–305 months).
Results
Forty-seven patients had local recurrence at a median of 10.7 months. Local recurrence at presentation, metastasis at diagnosis, and positive margins independently predicted local recurrence. No other factors independently predicted local recurrence.
Conclusions
Unplanned excisions did not increase the rate of local recurrence of deep, high-grade, soft-tissue sarcomas if treated appropriately. Aggressiveness of tumor represented by metastasis or local recurrence at presentation may be a risk for local recurrence.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients who develop a local recurrence from a soft-tissue sarcoma have a poor prognosis [1, 7, 8]. One study suggests the development of a local recurrence is the most important factor associated with decreased survival, and once patients develop a local recurrence, they are about three times more likely to die from disease compared to patients who do not develop a local recurrence [6]. Novais et al. [8] confirmed local recurrence independently predicts survival after adjusting for all major risk factors. Contemporary multimodal treatment of primary soft-tissue sarcoma reportedly reduces the incidence of local recurrence [1, 6]. However, when it occurs, local recurrence remains a devastating event for patients and usually entails substantial morbidity.
Sarcomas located deep in the fascia have a spectrum of diagnoses and prognoses different from those of subcutaneous sarcomas [5, 9, 12]. Subcutaneous sarcomas are typically smaller than deep tumors, probably because they are more easily detected, but can be of the highest grade of malignancy [12]. The prognosis for a deep sarcoma is reportedly worse than that for a subcutaneous sarcoma [5]. A later study confirmed the prognosis of a subcutaneous sarcoma is better than that for a deep sarcoma, but primarily because of the small size [9]. High-grade tumors have a higher rate of local recurrence than low-grade sarcomas [12]. The risk for local recurrence of deep, high-grade sarcomas likely differs from that of low-grade tumors or subcutaneous sarcomas.
Whether patients undergoing unplanned excision of their primary sarcoma have a higher risk of local recurrence is controversial [2, 4, 10, 13]. Several studies suggest unplanned excisions of soft-tissue sarcomas result in increased rates of local recurrence, mainly because of residual tumor [4, 10]. While patients with unplanned excisions tend to have superficial tumors, other reports show no adverse impact of unplanned excision on local recurrence [2, 13]. Additionally, whether local recurrence, when it occurs, lessens the chance of local control of the tumor is also controversial [6, 11, 16]. While several studies [6, 11] report patients who are referred after a local recurrence have an increased risk of developing a subsequent local recurrence after surgery, others suggest local recurrence after definitive surgery loses its prognostic importance on the development of a subsequent local recurrence [16]. The effect of local recurrence or unplanned excision on subsequent local recurrence in patients with deep, high-grade tumors may differ from recurrence of unplanned excision of subcutaneous or low-grade sarcomas; however, there is no large series specifically addressing these issues. We presume (1) positive margins after surgical resection are associated with an increase in local recurrence [3, 14], (2) presentation with local recurrence is a risk for subsequent local recurrence [6, 11], and (3) unplanned excision may be a risk factor for local recurrence in deep, high-grade soft-tissue sarcomas [2, 13].
We therefore determined whether possible risk factors (tumor size, location, histologic subtype, unplanned excision, local recurrence at presentation, metastasis at diagnosis, surgical procedure, surgical margin, and adjuvant treatments) influenced local recurrence of deep, high-grade, soft-tissue sarcomas.
Patients and Materials
We retrospectively reviewed the charts of 433 patients with deep, high-grade, soft-tissue sarcomas between 1985 and 2005. Since treatment strategies, including adjuvant therapies, have changed over the years, we excluded patients diagnosed before 1985. We considered a tumor to be in the extremity if it was at or beyond the shoulder or at or below the hip and considered other tumors to be in the trunk. We excluded patients with tumors located in the retroperitoneum but included those located on the chest wall or abdominal wall. In addition, we excluded tumors located superficial to the fascia and only considered tumors on or under the fascia as deep, soft-tissue sarcomas in the study. Lastly, we excluded low-grade tumors (Grade 1 in the Fédération Nationale des Centres de Lutte Contre le Cancer grading system [15]), desmoid tumors, and dermatofibrosarcoma protuberances. The median age of the 433 patients was 52 years (range, 1–99 years), and there were 243 males (56%) and 190 females (44%). No patients were lost to followup. The minimum followup was 1 month (median, 51 months; range, 1–305 months). We did not recall any patients specifically for this study; we obtained all data from medical records.
Demographic data collected included gender, age at the time of diagnosis, and duration of followup. Seventy-seven patients (18%) received unplanned excision elsewhere before the initial visit, and 79 (18%) were treated elsewhere and presented with local recurrence at the initial visit (Table 1). Fifty-four patients (12%) had metastasis at diagnosis. We recorded tumor characteristics according to tumor size (< 5 cm or ≥ 5 cm), site of primary tumor (trunk, extremity), and histologic subtype. We determined tumor size by the imaging studies at diagnosis, using maximal diameter measured on CT or MRI. If patients had unplanned excision elsewhere, we determined the tumor size by using images taken at other institutions, if available. We reviewed the pathology report when patients had tumors excised without preoperative imaging studies. Seventy-seven percent of patients (332 patients) presented with tumors 5 cm or larger, whereas 23% (111 patients) had tumors less than 5 cm (Table 2). Most patients had tumors located in an extremity (390 patients, 90%). A pathologist experienced in musculoskeletal pathology (RM) confirmed histologic diagnosis. The most frequent histologic subtype was malignant fibrous histiocytoma (171), followed by synovial sarcoma. Other tumors consisted of rare histologic subtypes, including spindle cell sarcoma, clear cell sarcoma, chondrosarcoma (extraskeletal myxoid chondrosarcoma and mesenchymal chondrosarcoma), hemangiopericytoma, hemangiosarcoma, lymphangiosarcoma, extraskeletal osteosarcoma, and undifferentiated sarcoma (Table 2).
The surgeons used preoperative radiotherapy when they presumed they could not achieve a wide surgical margin without sacrificing neurovascular structures or important organs.
The surgeons performed limb-sparing surgery in 386 patients (Table 3) and treated 47 patients with amputations proximal to the wrist or ankle. The operating surgeon and pathologist examined all surgical specimens. They evaluated surgical specimens in at least two perpendicular planes and sliced them where they thought the margin was closest to the tumor. We defined the margins as positive if tumor was present at the resection margin and as negative if tumor was not present at the margin regardless of the distance between the tumor and the margin. We defined local recurrence as the recurrence at the site of previous treatment. Patients who had surgical excision with positive margins underwent either radiation therapy or additional surgery. If patients developed tumors after these treatments, we considered them as local recurrence. Even when patients had positive margins, all tumors were at least marginally excised and there were no gross residual tumors. For patients who underwent definitive surgical resection of the primary tumor, we recorded the surgical margins (positive, negative). Of the 433 patients who underwent surgical resection or amputation, 365 patients had negative margins. Of the 47 patients treated by amputation, 41 patients had negative margins.
All patients underwent chest CT and physical examination with ultrasound every 3 months, and we obtained MRI when ultrasound was not sufficient to evaluate local recurrence or suspected local recurrence. We met with patients every 3 months for 5 years, every 6 months for 10 years, and then annually until 15 years after surgery. We evaluated patients by physical examination and did not obtain chest CT after 15 years. We recorded the surgical procedure used and adjuvant treatments administered. Also, we collected data regarding unplanned tumor excision elsewhere before the initial visit (yes or no), local recurrence at presentation, metastasis at diagnosis, surgical procedures (limb-sparing, amputation), surgical margins (positive, negative), chemotherapy (preoperative, postoperative), and radiotherapy (preoperative, postoperative). We recorded local recurrence of tumor with the time of failure and defined local control as the time between surgical resection or amputation and the detection of local recurrence of disease.
We examined the effect of each variable (size, location, histologic subtype, unplanned excision, local recurrence at presentation, and metastasis at diagnosis) and treatments (surgical procedure, surgical margins, chemotherapy, and radiotherapy) on event-free survival using Cox proportional-hazard models. We entered all variables significant at a p value of less than 0.05 in the univariable analysis into a multivariable model. A power analysis revealed a sample size of 433 patients and 47 patients with local recurrence would provide a power of 67% to detect a difference in survival outcomes with a two-sided alpha of 0.05. We used Stata® 10 statistical software (StataCorp, College Station, TX) to analyze all data.
Results
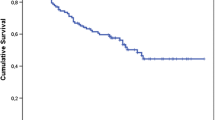
Forty-seven of the 433 patients who underwent surgical excision had local recurrences. The median time to development of local recurrence was 10.7 months (range, 1–77 months). Positive margin independently predicted local recurrence, with a rate ratio (RR) of 5.9 (95% confidence interval [CI] = 3.1–11.1) (Table 4). Local recurrence at presentation was associated with an increased rate of local recurrence, with a RR of 1.6 (95% CI = 1.2–2.2). Metastasis at diagnosis independently predicted local recurrence, with a RR of 2.8 (95% CI = 1.5–5.5). Size, tumor location, histologic subtype, and unplanned excision did not predict local recurrence after controlling for confounding variables. Adjuvant treatments (radiation therapy and chemotherapy) did not affect the rate of local recurrence.
Discussion
Patients with local recurrence of soft-tissue sarcomas have a poor overall survival. High-grade, soft-tissue sarcomas in deep locations may have a poorer prognosis regarding local recurrence than low-grade sarcomas or those located superficially. Although previous reports evaluate tumors at any depth, there are no large series focusing on deep, high-grade sarcomas. The risk of local recurrence of deep, high-grade sarcomas may differ from that of low-grade tumors or subcutaneous sarcomas. Therefore, we determined whether possible risk factors (tumor size, location, histologic subtype, unplanned excision, local recurrence at presentation, metastasis at diagnosis, surgical procedure, surgical margin, and adjuvant treatments) influence local recurrence for deep, high-grade, soft-tissue sarcomas.
We acknowledge several limitations in this study. First, there was a limited number of patients within each histologic subtype. We found the histologic subtype did not predict local recurrence, but the chance of a Type II error is substantial. However, our study is a relatively large series of high-grade, soft-tissue sarcomas located deep in the fascia, and 433 patients provided sufficient numbers to identify differences in other prognostic factors. Second, followup time for each patient varied. This is because we evaluated risk factors for local recurrence using Cox regression and person-time data; we included all patients seen within the study period to avoid selection bias. The median time of followup was 50.8 months, which should be sufficient because the median time to develop local recurrence is 10.7 months.
Positive margins reportedly increase the risk for local recurrence [3, 14]. In our study, positive margin was a major adverse prognostic factor, and patients who had a tumor excised with a positive margin had 5.9 times the local recurrence rate of those with negative margins. Most such patients underwent another tumor bed excision or radiation therapy. We do not routinely use preoperative radiotherapy for high-grade, soft-tissue sarcomas; we reserve preoperative radiation for cases where the surgeon could not achieve a wide surgical margin without sacrificing neurovascular structures. This could explain the observation that positive margins have a large impact on local recurrence in our series.
We found both local recurrence at presentation and metastasis at diagnosis predicted subsequent local recurrence. These findings are compatible with previous observations that patients referred after a local recurrence have an increased risk of developing a subsequent local recurrence [6, 11]. This finding supports the hypothesis that local recurrence is rarely the source of metastasis but rather the result of aggressive tumor biology, and both local recurrence at presentation and metastasis at diagnosis may be indicators of higher biologic tumor aggressiveness.
Interestingly, patients receiving an unplanned excision at an outside facility did not have a higher rate for local recurrence compared to those who receive initial surgical treatment at our hospital, even for deep, high-grade sarcomas. Patients with unplanned excisions had a tendency to have superficial tumors, and some reports show no adverse impact of unplanned excision on local recurrence [2, 13]. However, our series exclusively analyzed sarcomas located deep in the fascia. Our results suggest the local control after unplanned excisions was good if treated appropriately with tumor bed excision with or without radiotherapy, even when sarcomas were deeply located. Although we did not find a higher local recurrence rate for patients with unplanned excisions, we recommend deep tumors initially be adequately excised because if recurrence develops, subsequent treatment may require excising nerves, vessels, or other important organs, resulting in poor function.
In conclusion, we observed local recurrence at presentation, metastasis at diagnosis, and positive margins increased the rate of local recurrence in deep, high-grade soft-tissue sarcomas, whereas histologic subtype, tumor location, size, limb-sparing procedures, and unplanned excision were not risk factors for local recurrence in our patients with soft-tissue sarcomas.
References
Abatzoglou S, Turcotte RE, Adoubali A, Isler MH, Roberge D. Local recurrence after initial multidisciplinary management of soft tissue sarcoma: is there a way out? Clin Orthop Relat Res. 2010;468:3012–3018.
Arai E, Nishida Y, Tsukushi S, Wasa J, Ishiguro N. Clinical and treatment outcomes of planned and unplanned excisions of soft tissue sarcomas. Clin Orthop Relat Res. 2010;468:3028–3034.
Bell RS, O’Sullivan B, Liu FF, Powell J, Langer F, Fornasier VL, Cummings B, Miceli PN, Hawkins N, Quirt I. The surgical margin in soft-tissue sarcoma. J Bone Joint Surg Am. 1989;71:370–375.
Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90:203–208.
Collin C, Godbold J, Hajdu S, Brennan M. Localized extremity soft tissue sarcoma: an analysis of factors affecting survival. J Clin Oncol. 1987;5:601–612.
Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, Eilber FR. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226.
Gronchi A, Lo Vullo S, Colombo C, Collini P, Stacchiotti S, Mariani L, Fiore M, Casali PG. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251:506–511.
Novais EN, Demiralp B, Alderete J, Larson MC, Rose PS, Sim FH. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res. 2010;468:3003–3011.
Peabody TD, Monson D, Montag A, Schell MJ, Finn H, Simon MA. A comparison of the prognoses for deep and subcutaneous sarcomas of the extremities. J Bone Joint Surg Am. 1994;76:1167–1173.
Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res. 2008;466:3093–3100.
Rougraff BT, Davis K, Cudahy T. The impact of previous surgical manipulation of subcutaneous sarcoma on oncologic outcome. Clin Orthop Relat Res. 2005;438:85–91.
Rydholm A, Gustafson P, Rooser B, Willen H, Berg NO. Subcutaneous sarcoma: a population-based study of 129 patients. J Bone Joint Surg Br. 1991;73:662–667.
Sawamura C, Springfield DS, Marcus KJ, Perez-Atayde AR, Gebhardt MC. Factors predicting local recurrence, metastasis, and survival in pediatric soft tissue sarcoma in extremities. Clin Orthop Relat Res. 2010;468:3019–3027.
Singer S, Corson JM, Demetri GD, Healey EA, Marcus K, Eberlein TJ. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg. 1995;221:185–195.
Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F, Lagarde C. Soft-tissue sarcomas of adults: study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42.
Ueda T, Yoshikawa H, Mori S, Araki N, Myoui A, Kuratsu S, Uchida A. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J Bone Joint Surg Br. 1997;79:553–557.
Acknowledgment
The authors thank Rikuo Machinami, MD, PhD, for evaluation of pathologic specimens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Cancer Institute Hospital for Japanese Foundation for Cancer Research.
About this article
Cite this article
Sawamura, C., Matsumoto, S., Shimoji, T. et al. What Are Risk Factors for Local Recurrence of Deep High-grade Soft-tissue Sarcomas?. Clin Orthop Relat Res 470, 700–705 (2012). https://doi.org/10.1007/s11999-011-2017-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-011-2017-5